Abstract
Fucoid algae release gametes into seawater following an inductive light period (potentiation), and gamete expulsion from potentiated receptacles of Pelvetia compressa began about 2 min after a light-to-dark transition. Agitation of the medium reversed potentiation, with an exponential time course completed in about 3 h. Light regulated two signaling pathways during potentiation and gamete expulsion: a photosynthetic pathway and a photosynthesis-independent pathway in which red light was active but blue light was not. Uptake of K+ appears to have an important role in potentiation, because a 50% inhibition of potentiation occurred in the presence of the tetraethylammonium ion, a K+-channel blocker. A central role of anion channels in the maintenance of potentiation is suggested by the premature release of gametes in the light when receptacles were incubated with inhibitors of slow-type anion channels. An inhibitor of tyrosine kinases, tyrphostin A63, also inhibited potentiation. A model for gamete release from P. compressa is presented that proposes that illumination results in the accumulation of ions (e.g. K+) throughout the cells of the receptacle during potentiation, which then move into the extracellular matrix during gamete expulsion to generate osmomechanical force, resulting in gamete release.
Developmental and life history events in photosynthetic organisms often involve complex responses to natural variations in light intensity and quality. Light is processed in a variety of ways: either through the photosynthetic apparatus (Durnford and Falkowski, 1997, and refs. therein) or through other photoreceptors such as the phytochrome (Quail et al., 1995), cryptochrome (Ahmad and Cashmore, 1996), or rhodopsin families (Robinson et al., 1998). In lower plants and algae, light influences processes as diverse as cell differentiation (in cyanobacteria [Campbell et al., 1993]), photopolarization of zygotes (in fucoid algae [Robinson and Miller, 1997; Robinson et al., 1998]), and control of branching (in mosses [Ermolayeva et al., 1996]).
Natural populations of fucoid algae release gametes into SW in the light during periods of low water motion (Pearson and Brawley, 1996; Serrão et al., 1996). Gamete release is photosynthesis dependent, since blocking photosynthetic electron transport in the light with DCMU prevents gamete release (Serrão et al., 1996). Low water motion stimulates gamete release by limiting the inorganic carbon required for photosynthesis (Pearson et al., 1998). We demonstrated this with experiments in which addition of excess inorganic carbon to SW under calm conditions blocked gamete release; conversely, gamete release occurred in inorganic carbon-free SW independently of the hydrodynamic conditions (Pearson et al., 1998). The chances of successful external fertilization are increased by ensuring that gametes are released during relatively calm periods, when dilution will be slow.
Some of the key environmental factors controlling gamete release are known; however, we have little information about how these signals are transduced into downstream events resulting in gamete expulsion. Therefore, the aim of this study was to investigate the signaling pathway. Since oogonia and antheridia are released by being forced through pores in the subepidermal conceptacles of the reproductive tissue (receptacles), our hypothesis is that environmental signals ultimately result in ionic movements, leading to osmotic changes within the receptacles that stimulate gamete expulsion.
Ionic fluxes are involved in several osmomechanical processes in lower and higher plants. These include the K+- and Cl−-driven swelling and shrinking of motor cells that control leaf movements in several higher plants (Satter et al., 1988; Lee, 1990; Antkowiak and Engelmann, 1995). In guard cells, the best understood osmoregulatory system of higher plants, light-dependent ionic movements drive turgor changes caused by fluxes of K+ and the anions malate and Cl− (Assmann, 1993; Roelfsema and Prins, 1998). Membrane depolarizations are often an early event in signal transduction pathways involving ion channels, as in the phytochrome-mediated, Ca2+-dependent depolarizations involved in branching of the moss Physcomitrella patens (Ermolayeva et al., 1996) and in stomatal closure (Schroeder and Keller, 1992; Schroeder et al., 1993).
Guard cell anion channels are currently thought to be a central control mechanism in the signal transduction pathways for stomatal function, allowing sustained plasma membrane depolarization (Schroeder and Keller, 1992; Schroeder et al., 1993; Pei et al., 1997; for review, see Schroeder, 1995). Down-regulation of S-type anion channels is necessary during K+-driven stomatal opening, whereas sustained plasma membrane depolarization resulting from the opening of anion channels drives K+ efflux and stomatal closure (Schwartz et al., 1995). Recent studies have implicated phosphorylation and dephosphorylation events in the regulation of inward and outward K+ currents (Luan et al., 1993; Thiel and Blatt, 1994; Li et al., 1998) and anion channels (Schmidt et al., 1995; Pei et al., 1997) in guard cells. This suggested that it would be of interest to investigate the roles of K+ and anion fluxes and of phosphorylation and dephosphorylation in gamete release in fucoid algae. Furthermore, the inorganic carbon sensitivity of gamete release in fucoids shows intriguing functional parallels with the role of malate as a CO2 sensor and modulator of guard cell anion-channel activity (Hedrich and Marten, 1993; Hedrich et al., 1994). Therefore, we also performed experiments to investigate the effect of malate on gamete release.
There are two distinct phases in gamete release in the fucoid alga Pelvetia compressa (J. Agardh) De Toni (formerly P. fastigiata, Silva, 1996). First, receptacles become competent to release gametes following culture for ≥4 h under calm conditions in the light. This is referred to as potentiation in this report. Second, gamete expulsion is a rapid process (minutes) that is triggered by transferring receptacles to darkness (Jaffe, 1954). Gamete expulsion does not occur normally during potentiation under laboratory conditions unless old receptacles are used. The temporal and functional separation of potentiation and gamete expulsion in P. compressa makes it a useful model in which to study the mechanistic basis underlying these processes. On the basis of our results, we suggest that (a) light signals during potentiation are processed via two separate pathways: one sensed via photosynthetic electron transport and the other photosynthesis independent and possibly red-light dependent, (b) K+ uptake plays a role in potentiation and gamete expulsion may involve changes in anion-channel activities, and (c) phosphorylation events involving Tyr kinase(s) are involved in the signaling pathway for potentiation.
MATERIALS AND METHODS
Reproductive branches of the intertidal brown alga Pelvetia compressa (J. Agardh) De Toni were collected at Pigeon Point, California, and shipped overnight between layers of moistened paper in Styrofoam boxes that contained cool packs. For the experiments reported here, material was stored at 5°C in a cold room and used within 10 d and normally within 1 week. To minimize artifacts associated with storage, receptacles (50–100) were preincubated in 2-L flasks containing 1 L of SW for ≥6 h in the light (150–200 μmol photons m−2 s−1) with water motion provided by an orbital shaker (150 rpm, Lab-Line Instruments, Inc., Melrose Park, IL) prior to experiments, unless otherwise stated. Slightly different periods of potentiation were used in different experiments as a result of small seasonal effects in responses of tissue. Gamete release in experiments was quantified by counting the number of eggs present in the medium following a 30-min transfer to darkness (unless otherwise stated) with a dissecting microscope, and is expressed as a function of the fresh weight of receptacle tissue after release.
Time Course of Gamete Expulsion in Darkness
The time course of gamete expulsion in darkness (two receptacles per replicate in 15 mL of ASW, n = 5) was determined following potentiation under calm conditions in the light for 6 h. Following potentiation, receptacles were placed in darkness for 30 s, 1 min, 5 min, or 30 min, and then irradiated for an additional period of 30 min (to allow completion of any gamete expulsion under way) before quantitation of release.
Time Course of Stimulation and Inhibition of Potentiation Related to Hydrodynamic Conditions
To study the time course of potentiation in the light, which is known to be inhibited by high water motion in this and other species of fucoid algae (Serrão et al., 1996; Pearson et al., 1998), two to three receptacles (approximately 0.5–1.0 g fresh weight) per replicate (n = 5) were incubated in 25 mL of filtered SW in 125-mL flasks. Receptacles in flasks were incubated at 15°C ± 1°C in the light (250 μmol photons m−2 s−1) under either agitated (150 rpm, Lab-Line Instruments, orbital shaker) or calm conditions for 8 or 13 h. Other treatments (n = 5) included agitation first for 8 h and then 1, 2, 3, 4, or 5 h of light under calm conditions. A second experiment was performed under the same culture conditions as described above, except that receptacles were placed under calm conditions for 6 h in the light and then agitated for 1, 2, 3, 4, or 5 h in the light. Positive and negative controls were incubated under calm or agitated conditions for 6 or 11 h in the light. In both experiments, receptacles were used directly from storage in darkness at 5°C without pretreatment (see above), and gamete release was determined under a dissecting microscope by counting the number of eggs released after transfer to darkness (30 min).
Inhibition of Photosynthetic Electron Transport
The effects of inhibiting photosynthesis in the light on gamete expulsion following potentiation were investigated by adding an inhibitor of PSII electron transport, DCMU, to receptacles after 7 h in light (two receptacles per replicate in 4 mL of ASW, n = 5). DCMU was added from a 100 mm stock in 95% ethanol to a final concentration of 10 μm. Control treatments were incubated in ASW or ASW plus 0.01% ethanol. Gamete release was determined after 30 min in the light with no dark transfer.
Malate Sensitivity
The effect of malate on gamete release in P. compressa was studied by addition of l-(−)malate and d-(+)malate (as sodium salts, Sigma) during potentiation. Malate (up to 50 mm) was added to receptacles (two per replicate in 15 mL of ASW, n = 5) at the beginning of a 6-h potentiation period, and gamete release was quantified following a 30-min dark transfer.
Effects of Light Quality on Potentiation and Gamete Release
To investigate the effects of light quality on gamete release independently of photosynthetic rate, the photosynthesis versus irradiance responses of receptacles to white, red, and blue light were determined in preliminary experiments using oxygen electrodes (data not shown). Red and blue wavelengths were provided using 50-mm-diameter dichroic color separation glass filters (Edmund Scientific Co., Barrington, NJ). In subsequent experiments to investigate the effects of light quality on potentiation and gamete expulsion, photon flux densities were selected that gave equal rates of O2 production in different treatments. Relatively low fluences of blue light were obtainable with the fluorescent light source used, and photon flux densities were lower than those used in other experiments (white light = 50 μmol photons m−2 s−1; red light = 35 μmol photons m−2 s−1; and blue light = 70 μmol photons m−2 s−1). Receptacles (three per replicate) were potentiated for 8 h in 60-mm Petri dishes wrapped in aluminum foil containing 15 mL of ASW (n = 6). The transparent lids of the dishes were either unaltered (white light) or replaced with the appropriate filter (red or blue light). Following potentiation, receptacles were incubated for an additional 30 min in darkness or in white light, red light, or blue light. Gamete release was assayed as described previously.
Inhibitors of Ion Channels
An inhibitor of K+ channels (Taylor and Brownlee, 1993), TEA-chloride (100 mm), was added either at the start of the potentiation period (i.e. for 5 h) or by transfer of receptacles to ASW plus TEA+ for 60 min or for 5 min prior to dark treatment. The appropriate controls for both osmotic effects (100 mm NaCl) and for transfers of receptacles between different solutions were used to allow the comparison of inhibitor and noninhibitor treatment effects.
Several compounds reported to block S-type anion channels in both animals and plants were tested for their effects on gamete release. 9-AC was prepared as a 0.5 n stock solution in ethanol:DMSO (95:5 [v/v]) and used at a final concentration of 1 mm in ASW. Probenicid was used at a final concentration of 2 mm, from a 1 n stock in 1 n NaOH. Niflumic acid was prepared as a 200 mm stock in ethanol and used at a final concentration of 1 mm. Control treatments were done in ASW and in ASW with the appropriate solvent concentration (9-AC control, 0.02% [v/v] 95:5 ethanol:DMSO; probenicid control, 0.02% [v/v] 1 n NaOH; and niflumic acid control, 0.5% [v/v] ethanol). Experiments were carried out in 35- × 10-mm Petri dishes containing 4 mL of Tris-buffered ASW (pH 7.8). Each dish (n = 5) contained two receptacles. Anion-channel blockers were added at the beginning of the 7-h potentiation period, after which time receptacles were removed to new dishes containing appropriate inhibitor or control solutions for a 30-min dark period; the number of gametes expelled was thus quantified independently for both the light (potentiation) and the dark phases. The results were analyzed by a two-factor analysis of variance (SYSTAT, version 5.2.1, SPSS, Inc., Chicago, IL) and significant differences between means (α = 0.05) were identified using Tukey's test.
In similar experiments, the anion-exchange inhibitor 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid was added at concentrations of 200 μm and 1 mm (two receptacles per treatment in 4 mL of ASW, n = 5). After 5 h of potentiation, gamete release was quantified following a 30-min transfer to darkness. Gamete release in the light was not observed in these experiments and was not quantified separately.
Inhibitors of Protein Phosphorylation
The involvement of protein phosphorylation in gamete release was investigated in several experiments with the use of drugs known to be active against protein kinases in other systems. Staurosporine, a broad-range inhibitor of Ser/Thr protein kinases, was added to a concentration of 10 μg mL−1, from a 10 mg mL−1 stock solution in DMSO. Tyrphostins A25 and A63, specific inhibitors of protein Tyr kinases (Yaish et al., 1988), were added at 50 to 200 μm from stock solutions in DMSO, either at the beginning of the potentiation period or for 30 min prior to the dark transfer period. Controls for the effects of solvents (0.1% DMSO), in addition to ASW controls, were used in these experiments.
RESULTS
Time Course of Gamete Expulsion and of Stimulation and Inhibition of Potentiation by Hydrodynamic Conditions
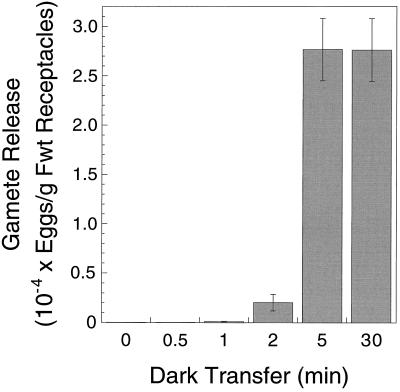
A quantitative analysis of the temporal effect of darkness on potentiated receptacles under calm conditions (Jaffe, 1954) showed that a sustained period of darkness was required to stimulate gamete expulsion (Fig. 1). Receptacles returned to light after periods of ≤2 min of darkness released few gametangia. A period of darkness between 2 and 5 min was sufficient to saturate gamete expulsion (Fig. 1). The response of potentiated receptacles to darkness was gradual, as demonstrated by the data for 1, 2, and 5 min of darkness (Fig. 1).
Figure 1.
Gamete expulsion from potentiated receptacles following transfer to darkness for periods between 0 and 30 min. Potentiation was for 6 h in the light. Following dark transfers, receptacles were returned to the light for an additional 30 min to allow for the completion of gamete release. Results are means ± se (n = 5). Fwt, Fresh weight.
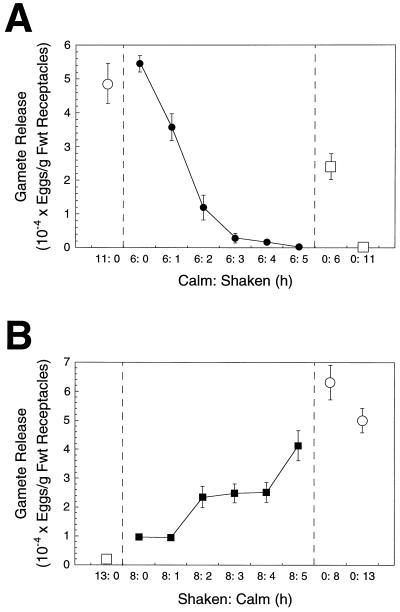
The potentiation of gamete release in P. compressa was inhibited by high water motion (agitation) relative to calm conditions in laboratory experiments (Fig. 2). Gamete release from receptacles was maximized by incubation for 6 h under calm conditions; no further increase was observed by extending the potentiation period to 11 h (Fig. 2A). However, incremental increases in the duration of agitation (1–5 h) following a 6-h period of calm resulted in an exponential decline in gamete release. Following an 11-h potentiation period, 48,720 ± 5,937 eggs g−1 fresh weight were released under calm conditions, but only 306 ± 161 eggs g−1 fresh weight were released when 6 h of calm was followed by 5 h of agitation (Fig. 2A). Agitated controls in both experiments (Fig. 2) indicated that gamete release was reduced progressively by periods of agitation for 6 to 13 h. Several properties are evident using receptacles in calm:shaken and shaken:calm treatments: (a) an extended period of agitation is required to fully reverse potentiation, (b) potentiated (calm) receptacles are very sensitive; only 1 to 2 h of agitation greatly reduced the number of gametes released (Fig. 2), and (c) the rapid, exponential kinetics of the reversal of potentiation by agitation (Fig. 2A) compared with the slower increases in potentiation after agitation (Fig. 2B) suggest that these two processes might not be simply the inverse of one another. Following 8 h of agitation, >1 h of calm incubation was necessary to observe increases in gamete release (Fig. 2B), which then increased with the duration of the calm interval.
Figure 2.
The effects on potentiation of shaking receptacles following calm conditions (A, •) and calm following shaking conditions (B, ▪). The numbers of gametes released were determined following a 30-min dark transfer after completion of each experimental treatment. Results are shown relative to calm controls (○) and shaken controls (□). Values are means ± se (n = 5). Fwt, Fresh weight.
Inhibition of Photosynthetic Electron Transport
When added to potentiated receptacles in the light, DCMU caused a massive expulsion of gametes, mimicking a transfer to darkness (Table I). The time course of the response (about 30 min for completion following addition of the inhibitor) was slower than that seen in response to darkness (Fig. 1). But given the time necessary for DCMU to reach its site of action in the chloroplasts, these results are consistent with a role for photosynthesis in signaling the rapid response of gamete expulsion in P. compressa, in addition to the requirement for photosynthesis during potentiation shown in a previous report (Serrão et al., 1996).
Table I.
DCMU triggers gamete expulsion in the light from potentiated receptacles
| Treatment | Gamete Release |
|---|---|
| eggs/g fresh wt receptacles | |
| ASW | 589.1 ± 377.6 |
| ASW + ethanol | 632.8 ± 322.2 |
| ASW + DCMU | 28,759.0 ± 2,327.5 |
DCMU (10 μm) or ethanol (0.01%) was added following 7 h of potentiation of receptacles in the light, and the number of gametes released was determined 30 min after addition. Control (ASW) and treated receptacles remained in the light for a total of 7.5 h. Values are means ± se (n = 5).
Effects of Light Quality
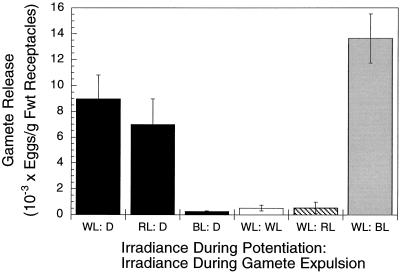
Receptacles potentiated in white light expelled gametes normally when incubated in darkness for 30 min (Fig. 3). As expected, gamete expulsion was very low when white-light-potentiated receptacles were transferred to white or red light, but a transfer from white to blue light triggered gamete expulsion that exceeded that induced by darkness (although in another experiment, it was of a similar magnitude). Moreover, although potentiation in red light was effective (similar to white light) and resulted in a large expulsion of gametes after transfer to darkness, very few gametes were expelled when potentiation was carried out in blue light (Fig. 3). Thus, blue light was insufficient to potentiate gamete release, despite providing photosynthetically active irradiance, and had a similar effect to darkness in triggering gamete expulsion following normal potentiation in white light.
Figure 3.
The effects of light quality on potentiation and gamete expulsion. Receptacles were potentiated for 8 h in red light (RL), blue light (BL), or white light (WL). Receptacles were then transferred to darkness (D) for 30 min and gamete release was assayed. For white-light-potentiated receptacles additional treatments were given by transferring to red or blue light for 30 min, while controls were kept in white light, and gamete release was assayed. Results are means ± se (n = 6). Fwt, Fresh weight.
Effects of Malate and Inhibitors of Ion Channels
When l-(−)malate (50 mm) was present in ASW during potentiation, gamete release was reduced significantly relative to controls in ASW or to treatments with the biologically inactive stereoisomer (Table II, F2,12 = 4.56; P = 0.034). We found that the effective concentration of l-malate necessary to inhibit potentiation varied from experiment to experiment; 50 mm was always effective, but in some experiments a significant response was observed with concentrations as low as 5 mm.
Table II.
The effects of d- and l-malate on potentiation
| Treatment | Gamete Release |
|---|---|
| eggs/g fresh wt receptacles | |
| Control (ASW) | 30,534 ± 3,414 |
| d-(+)Malate | 31,942 ± 2,733 |
| l-(−)Malate | 21,227 ± 1,786 |
Malate (50 mm) was added at the start of potentiation in ASW (6 h), and gamete release from receptacles was assayed following 30 min of darkness. Values are means ± se (n = 5).
The K+-channel blocker TEA+ (100 mm) inhibited potentiation, thereby reducing gamete release by approximately 50% (Table III), suggesting that K+ movements are involved in achieving potentiation in receptacles. In contrast, gamete expulsion was unaffected when TEA+ was added to fully potentiated receptacles for periods of up to 1 h before placing these receptacles (still in TEA+) in darkness (data not shown).
Table III.
Inhibition of potentiation by the K+-channel blocker TEA+
| Treatment | Cl− Osmolality | Gamete Release |
|---|---|---|
| mmol/kg | eggs/g fresh wt receptacles | |
| ASW | 518.00 | 43,424 ± 5,889 |
| ASW + NaCl | 618.00 | 40,409 ± 4,776 |
| TEA+ | 618.00 | 16,456 ± 2,596 |
Receptacles were potentiated for 6 h in ASW (518 mmol/kg Cl−), ASW plus NaCl (618 mmol/kg Cl−), or ASW plus 100 mm TEA+. Gamete release was assayed following 30 min of darkness. Values are means ± se (n = 5).
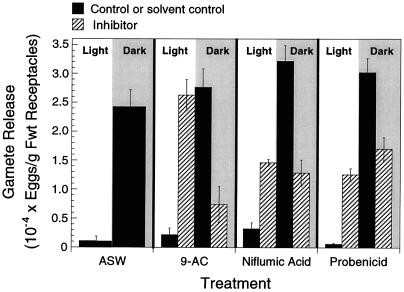
Gamete release was significantly greater in the light relative to controls in the presence of all three anion-channel inhibitors tested (Fig. 4; two-factor analysis of variance, F6,56 = 39.12; P <0.0001). The most effective inhibitor at the concentrations used was 9-AC, which resulted in levels of gamete expulsion in the light that were similar to levels of dark-induced expulsion in controls (Fig. 4). The presence of solvents (DMSO or ethanol) or carrier (NaOH) at concentrations equal to those used in inhibitor treatments had no significant effects on gamete release relative to controls; in every case very few gametes were expelled in the light, almost all expulsion occurring rapidly following the transfer of receptacles to darkness. Cumulative gamete release (light plus dark periods) did not differ significantly between treatments (analysis of variance, F6,28 = 1.58; P = 0.190). Therefore, the biological significance of the observed reduction in gamete expulsion during the dark phase in the presence of inhibitors might simply have been a consequence of depletion of the gamete pool due to release in the light (i.e. the magnitude of release in the dark was not independent of release in the light in these treatments). These results suggest that anion channels play a central role in the normal functioning and regulation of gamete expulsion in P. compressa.
Figure 4.
The effects of anion-channel blockers on gamete release. Receptacles were incubated for 7 h in the light in 9-AC (1 mm), niflumic acid (1 mm), or probenicid (2 mm). Gametes released were counted at the end of the light period and after an additional 30 min of darkness. Hatched bars represent treatments in which inhibitor was present, and black bars represent ASW controls or ASW plus solvent controls. Values are means ± se (n = 5). Fwt, Fresh weight.
Effects of Inhibitors of Protein Phosphorylation
Gamete release was almost completely blocked when specific inhibitors of Tyr kinases were added to ASW at the beginning of potentiation (Table IV), whereas staurosporine (10 μg/mL), a Ser/Thr kinase inhibitor, had no effect (Table IV). Tyrphostin A63 (200 μm) reduced gamete release from 19,107 ± 2,200 eggs g−1 fresh weight in controls (ASW) to 733 ± 409 eggs g−1 fresh weight (n = 5). A second tyrphostin, A25 (200 μm), had no significant effect on gamete release, indicating that inhibition was not a consequence of any general cytotoxicity of these compounds or of the concentration used. Neither tyrphostin had any effect on gamete expulsion when added after potentiation, for up to 30 min before receptacles were transferred to darkness (data not shown). Thus, we conclude that protein Tyr phosphorylation is essential for potentiation but appears not to be involved in the rapid response to darkness that triggers gamete expulsion.
Table IV.
Effects of protein kinase inhibitors on potentiation
| Treatment | Gamete Release following Dark Transfer |
|---|---|
| eggs/g fresh wt receptacles | |
| ASW | 19,107 (2,200) |
| ASW + DMSO | 22,038 (3,420) |
| Tyrphostin A25 | 21,027 (3,340) |
| Tyrphostin A63 | 733 (409) |
| ASW | 11,846 (1,169) |
| ASW + DMSO | 19,307 (3,842) |
| Staurosporine | 19,166 (1,844) |
Inhibitors of Tyr kinases, tyrphostin A25 and A63 (200 μm), and, in a separate experiment, the Ser/Thr kinase inhibitor staurosporine (10 μg/mL) were added at the beginning of potentiation (6 h), and gamete release was determined following a 30-min transfer to darkness. Values are means ± se (n = 5).
DISCUSSION
A model that summarizes our present understanding of potentiation and gamete expulsion is presented in Figure 5. Our data indicate that photoreception and subsequent signal transduction occur via two pathways. One of these requires photosynthesis (Serrão et al., 1996; see Results), and to become fully potentiated, receptacles must experience inorganic carbon limitation during at least a portion of the potentiation period (see figure 9 in Pearson et al., 1998). Fucoid algae have a carbon-concentrating mechanism that provides relatively high [CO2] to the chloroplasts (Surif and Raven, 1989; Raven and Osmond, 1992), and they continue to evolve O2 in inorganic carbon-free SW (Surif and Raven, 1989). This suggests that internal stores of carbon might be utilized under carbon-limiting conditions (e.g. via the decarboxylation of organic acids), which could drive the uptake of K+ as a counterion. Gamete expulsion in the dark from receptacles treated with TEA+ during potentiation was only one-half that of controls; thus, part of the requirement for potentiation is K+ uptake.
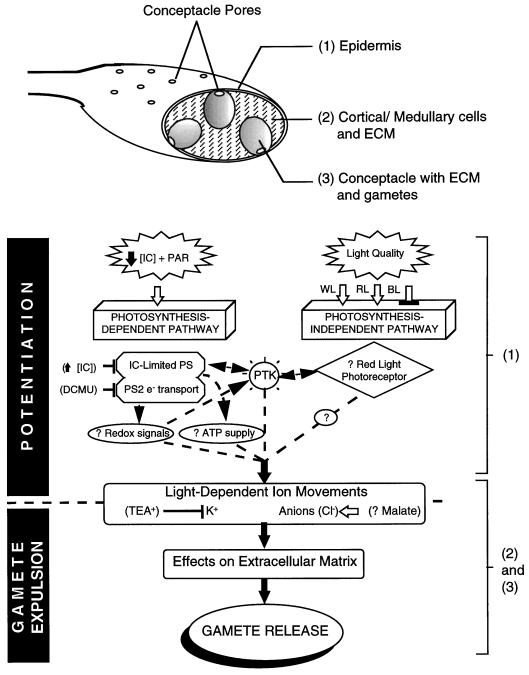
Figure 5.
A model for the signaling pathways controlling light-dependent potentiation and gamete expulsion in fucoid algae. The top part of the figure shows a diagrammatic section through a receptacle illustrating the general organization of tissues and extracellular matrix (ECM) in receptacles of P. compressa. The black blocks on the left of the model indicate those parts of the pathway involved in potentiation and gamete expulsion, respectively. The brackets on the right indicate the probable sites within the receptacle for the events indicated. Stimulation of pathway components is shown by open arrows, and inhibitors by blocked lines. The photosynthesis-dependent pathway activates under low inorganic carbon (IC) conditions. Potentiation is prevented by increasing [IC] and also by blocking PSII electron transport with DCMU (Serrão et al., 1996). Potentiation under red light (RL) or white light (WL) is blocked in blue light (BL), independently of photosynthetic rate. Protein Tyr kinase (PTK) activity is required during potentiation. Unconfirmed components of the pathway are shown by a “?,” and possible pathways are shown by broken arrows. Potentiation ultimately results in movements of K+ and anions, and the extracellular matrix provides the osmomechanical forces necessary to cause gamete release from conceptacles. PS, Photosynthesis.
Light Regulation of Gamete Release
The epidermal cells of the receptacle are likely to be key sites for potentiation (Fig. 5) because chlorophyll autofluorescence is found here primarily (S.H. Brawley, unpublished data), and the plasma membrane of the epidermal cells is deeply invaginated, suggesting the importance of these cells in secretion and/or absorption (McCully, 1968a). Ions taken up during potentiation, however, are likely to be distributed throughout the receptacle during potentiation because the cells are coupled by distinctive cytoplasmic junctions (Fritsch, 1945; McCully, 1968b; Moss, 1983; G.A. Pearson and S.H. Brawley, unpublished data). Taken together, these observations suggest that the receptacle is a reproductive organ that functions as an ionic syncytium.
Gamete expulsion is complete within about 30 min after the application of DCMU to receptacles in the light (see Results). This is dramatic, although somewhat slower than the time course demonstrated here for darkness. Hypotheses (Fig. 5) arising from the observed effects of darkness/DCMU include: (a) photosynthetically supplied ATP may be required by ion pumps involved in maintenance of potentiation (Fig. 5) and/or (b) redox signaling (Campbell et al., 1993; Danon and Mayfield, 1994; Escoubas et al., 1995) may be important in the transition from potentiation to gamete release. Other signals could arise under high light or carbon limitation as a result of oxidative stress and/or the thioredoxin pathway (Danon and Mayfield, 1994; Allen, 1995; Bohnert et al., 1995; Ingram and Bartels, 1996).
A second photosynthesis-independent pathway must have been present, because potentiation occurred in red or white light, but not in blue light, independently of photosynthetic rate. Since potentiation occurs normally in white light, it appears that blue light is not inhibitory. Blue light was as effective as darkness in triggering gamete expulsion from potentiated receptacles. Investigations of the action spectra for the response will reveal whether this was simply due to removal of red light or to a blue-light effect. Blue light does have substantial effects on some processes in fucoids, including photopolarization of zygotes (Hurd, 1920; Robinson and Miller, 1997), which may occur via rhodopsin, since retinal has been identified in P. compressa zygotes (Robinson et al., 1998). The ubiquitous red-far-red photoreceptors of higher plants, the phytochromes (Quail et al., 1995; Chamovitz and Deng, 1996), are candidates for the red-light photoreceptor of fucoid receptacles. Phytochrome has not been identified in fucoids, but genes with homology to phytochrome are widely distributed (prokaryotes: Schneider-Poetsch et al., 1991; Kehoe and Grossman, 1996; Hughes et al., 1997; green algae: Kidd and Lagarias, 1990; Winlands and Wagner, 1996; lower plants: Thümmler et al., 1992; Pratt, 1995; Ermolayeva et al., 1996; ascomycete fungi and slime moulds: Griffith et al., 1994; Starostzik and Marwan, 1995). Given the identification of retinal in P. compressa, it is also of interest that animals obtain selective spectral information, including from red light, with retinal in combination with different opsin proteins (Bowmaker, 1991).
Role of Protein Phosphorylation
Successful potentiation requires Tyr kinase activity. Gamete release was reduced to 13% of controls when tyrphostin A63 was present during the potentiation period. Gamete release was unaffected, however, when the drug was added 30 min prior to dark transfer, leading to the (tentative) conclusion that a protein Tyr kinase is not involved in the direct triggering of gamete expulsion in darkness. Tyr phosphorylation has been found as part of a number of processes that may be relevant to our model (e.g. in stress-response pathways involving mitogen-activated protein kinase [Shinozaki and Yamaguchi-Shinozaki, 1997]; modulation of K+ channels [Holmes et al., 1996]; regulation of some Rubisco [Aggarwal et al., 1993]; phytochrome-mediated signaling [Bowler et al., 1994; Sommer et al., 1996]). It should be noted, however, that translation from 70S and 80S ribosomes was not required for potentiation in this study (data not shown); thus, the functional consequences of light signaling and/or protein phosphorylation are probably not expressed through changes in gene regulation and transcription. Inward-rectifying K+ channels in guard cells are sensitive to TEA+ (Blatt, 1992), with regulation through calmodulin-dependent protein phosphatase 2B (calcineurin); however, we have not detected any significant change in gamete release when inhibitors of Ser/Thr kinases (staurosporine, H-7, ML-7) or the phosphatase inhibitor okadaic acid were present during potentiation, and inconsistent results have been obtained in experiments with the calmodulin inhibitors W7 and W5 (data not shown).
Anion-Channel Regulation and Gamete Expulsion
Experiments using anion-channel blockers suggest that S-type anion channels play a role in controlling gamete expulsion. Several anion-channel antagonists caused gametes to be released prematurely in the light during the normal potentiation phase. However, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid, a potent inhibitor of rapid-type anion channels (Marten et al., 1993), had no effect on gamete release under the same conditions. The control of anion efflux through S-type anion channels in guard cells is central to the process of turgor regulation and thus stomatal opening (Schroeder and Keller, 1992; Schroeder et al., 1993; Schroeder, 1995; Schwartz et al., 1995; Pei et al., 1997). These channels can remain open over a wide range of membrane potentials and allow for sustained membrane depolarization, upon which turgor loss and stomatal closing depend. The activation state of these channels is also important in the control of stomatal opening, which is enhanced in the presence of anion-channel blockers (Schwartz et al., 1995). We propose that anion-channel activity is necessary to maintain the potentiated state, because of the effects of inhibitors in causing gamete expulsion in the light; however, further studies are needed to clarify their regulation and role (e.g. in the maintenance of turgor). The inhibitory effect of malate on potentiation that we observed might be due to disruption of turgor by an activation of anion efflux channels (Hedrich and Marten, 1993; Hedrich et al., 1994).
A Model for Signal Transduction Leading to Gamete Release
Our view of the potentiated receptacle is one in which light and inorganic carbon-limited uptake of K+ has occurred. Underlying the epidermal and cortical cells of the receptacles is a thick layer consisting of medullary filaments in a copious extracellular matrix of alginic acid and fucoidan (McCully, 1968b). We hypothesize that a rapid release of ions into the extracellular matrix occurs when potentiated receptacles are transferred to darkness, such that this extracellular gel swells rapidly to provide the physical force required for gamete expulsion (Fig. 5). As shown above, TEA+ inhibits gamete release by partially blocking potentiation. The failure of TEA+ to inhibit gamete expulsion following potentiation in the absence of the inhibitor might seem contrary to our model, but the inhibitor may not penetrate to sites deep within the receptacle and, or the channels through which K+ efflux occurs may be TEA+ insensitive (for reviews, see Cook, 1990; Hedrich and Dietrich, 1996). Preliminary studies of the spatial distribution of ions within receptacles during potentiation and during gamete expulsion with x-ray microanalysis are supportive of the proposed K+ flux into the extracellular matrix (Speransky et al., 1998).
The exquisite control mechanisms of potentiation and gamete expulsion in P. compressa in response to water motion ([inorganic carbon]) and light signals may have arisen as adaptations allowing increased fertilization success in intertidal habitats. Potentiation builds gradually under calm conditions but declines exponentially if water motion increases (see Results), conditions under which gamete dilution would be expected to decrease fertilization success. Rapid light-to-dark transitions, which trigger gamete expulsion in the laboratory, are unlikely to happen in nature; however, sharp reductions in light intensity and changes in quality (reduction in the relative amount of red light) may occur when algae are immersed by the incoming tide. Questions concerning the potentiation status of receptacles of P. compressa in natural populations, and thus the timing of natural gamete release (Johnson and Brawley, 1998), might be best addressed using a physiological marker, e.g. the spatial distribution of important ions at different phases of the tidal cycle (Speransky et al., 1998). Our model for signal transduction during gamete release suggests the presence of elements common to other osmoregulatory model systems (guard cells and sensitive plants) and offers a basis for further investigation.
ACKNOWLEDGMENTS
The authors would like to thank Jon Ashen for providing regular shipments of P. compressa. We appreciate the help and support of Dr. Ester Serrão during both the experimental work and the preparation of the manuscript. Comments by two anonymous reviewers improved an earlier version of the manuscript.
Abbreviations:
- 9-AC
anthracene-9-carboxylic acid
- ASW
artificial seawater
- S-type
slow-type
- SW
seawater
- TEA+
tetraethylammonium ion
Footnotes
This work was supported by National Science Foundation award OCE 92 16981 to S.H.B.
LITERATURE CITED
- Aggarwal KK, Saluja D, Sachar RC. Phosphorylation of Rubisco in Cicer arietinum: non-phosphoprotein nature of Rubisco in Nicotiana tabacum. Phytochemistry. 1993;34:329–335. [Google Scholar]
- Ahmad M, Cashmore AR. Seeing blue: the discovery of cryptochrome. Plant Mol Biol. 1996;30:851–861. doi: 10.1007/BF00020798. [DOI] [PubMed] [Google Scholar]
- Allen RD. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995;107:1049–1054. doi: 10.1104/pp.107.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antkowiak B, Engelmann W. Oscillations of apoplasmic K+ and H+ activities in Desmodium motorium (Houtt.) Merril. pulvini in relation to the membrane potential of motor cells and leaflet movements. Planta. 1995;196:350–356. [Google Scholar]
- Assmann SM. Signal transduction in guard cells. Annu Rev Cell Biol. 1993;9:345–375. doi: 10.1146/annurev.cb.09.110193.002021. [DOI] [PubMed] [Google Scholar]
- Blatt MR. K+ channels of stomatal guard cells. J Gen Physiol. 1992;99:615–644. doi: 10.1085/jgp.99.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Nelson DE, Jensen RG. Adaptations to environmental stresses. Plant Cell. 1995;7:1099–1111. doi: 10.1105/tpc.7.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Yamagata H, Neuhaus G, Chua N-H. Phytochrome signal transduction pathways are regulated by reciprocal control mechanisms. Genes Dev. 1994;8:2188–2202. doi: 10.1101/gad.8.18.2188. [DOI] [PubMed] [Google Scholar]
- Bowmaker JK. The evolution of vertebrate visual pigments and photoreceptors. In: Cronly-Dillon JR, Gregory RL, editors. Vision and Visual Dysfunction, Vol 2: Evolution of the Eye and Visual Systems. London: Macmillan; 1991. pp. 63–65. [Google Scholar]
- Campbell D, Houmard J, Tandeau de Marsac N. Electron transport regulates cellular differentiation in the filamentous cyanobacterium Calothrix. Plant Cell. 1993;5:451–463. doi: 10.1105/tpc.5.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamovitz DA, Deng X-W. Light signaling in plants. Crit Rev Plant Sci. 1996;15:455–478. [Google Scholar]
- Cook NS (1990) Potassium Channels: Structure, Classification, Function and Therapeutic Potential. E Horwood, Chichester, UK
- Danon A, Mayfield SP. Light-regulated translation of chloroplast messenger RNAs through redox potential. Science. 1994;266:1717–1719. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- Durnford DG, Falkowski PG. Chloroplast redox regulation of nuclear gene transcription during photoacclimation. Photosynth Res. 1997;53:229–241. [Google Scholar]
- Ermolayeva E, Hohmeyer H, Johannes E, Sanders D. Calcium-dependent membrane depolarization activated by phytochrome in the moss Physcomitrella patens. Planta. 1996;199:352–358. [Google Scholar]
- Escoubas J-M, Lomas M, LaRoche J, Falkowski PG. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA. 1995;92:10237–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch FE (1945) The Structure and Reproduction of the Algae, Vol 2. Cambridge University Press, Cambridge, UK, pp 366–368
- Griffith GW, Jenkins GI, Milner-White EJ, Clutterbuck AJ. Homology at the amino acid level between plant phytochromes and a regulator of asexual sporulation in Emericella (=Aspergillus) nidulans. Photochem Photobiol. 1994;59:252–256. doi: 10.1111/j.1751-1097.1994.tb05030.x. [DOI] [PubMed] [Google Scholar]
- Hedrich R, Dietrich P. Plant K+ channels: similarity and diversity. Bot Acta. 1996;109:94–101. [Google Scholar]
- Hedrich R, Marten I. Malate-induced feedback regulation of plasma membrane anion channels could provide a CO2 sensor to guard cells. EMBO J. 1993;12:897–901. doi: 10.1002/j.1460-2075.1993.tb05730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Marten I, Lohse G, Dietrich P, Winter H, Lohaus G, Heldt H-W. Malate-sensitive anion channels enable guard cells to sense changes in the ambient CO2 concentration. Plant J. 1994;6:741–748. [Google Scholar]
- Holmes TC, Fadool DA, Ren R, Levitan IB. Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science. 1996;274:2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- Hughes J, Lamparter T, Mittman F, Hartmann E, Gartner W, Wilde A, Borner T. A prokaryotic phytochrome. Nature. 1997;386:663. doi: 10.1038/386663a0. [DOI] [PubMed] [Google Scholar]
- Hurd AM. Effect of unilateral monochromatic light and group orientation on the polarity of germinating Fucus spores. Bot Gaz. 1920;70:25–50. [Google Scholar]
- Ingram J, Bartels D. The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:377–403. doi: 10.1146/annurev.arplant.47.1.377. [DOI] [PubMed] [Google Scholar]
- Jaffe LF. Stimulation of the discharge of gametangia from a brown alga by a change from light to darkness. Nature. 1954;174:743. [Google Scholar]
- Johnson LE, Brawley SH (1998) Dispersal and recruitment of a canopy-forming intertidal alga, Pelvetia compressa (Phaeophyceae). Oecologia (in press) [DOI] [PubMed]
- Kehoe DM, Grossman AR. Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science. 1996;273:1409–1412. doi: 10.1126/science.273.5280.1409. [DOI] [PubMed] [Google Scholar]
- Kidd DG, Lagarias JC. Phytochrome from the green alga Mesotaenium caldariorum: purification and preliminary characterization. J Biol Chem. 1990;265:7029–7035. [PubMed] [Google Scholar]
- Lee Y (1990) Ion movements that control pulvinar curvature in nyctinastic legumes. In RL Satter, HL Gorton, TC Vogelmann, eds, The Pulvinus: Motor Organ of Leaf Movement. American Society of Plant Physiologists, Rockville, MD, pp 130–141
- Li J, Lee Y-RJ, Assmann SM. Guard cells possess a calcium-dependent protein kinase that phosphorylates the KAT1 potassium channel. Plant Physiol. 1998;116:785–795. doi: 10.1104/pp.116.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S, Li W, Rusnak F, Assmann SM, Schreiber SL. Immunosuppressants implicate protein phosphatase regulation of K+ channels in guard cells. Proc Natl Acad Sci USA. 1993;90:2202–2206. doi: 10.1073/pnas.90.6.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten I, Busch H, Raschke K, Hedrich R. Modulation and block of the plasma membrane anion channel of guard cells by stilbene derivatives. Eur Biophys J. 1993;21:403–408. [Google Scholar]
- McCully ME. Histological studies on the genus Fucus. III. Fine structure and possible functions of the epidermal cells of the vegetative thallus. J Cell Sci. 1968a;3:1–16. doi: 10.1242/jcs.3.1.1. [DOI] [PubMed] [Google Scholar]
- McCully ME. Histological studies on the genus Fucus. II. Histology of the reproductive tissues. Protoplasma. 1968b;66:205–230. [Google Scholar]
- Moss BL. Sieve elements in the Fucales. New Phytol. 1983;93:433–437. [Google Scholar]
- Pearson GA, Brawley SH. Reproductive ecology of Fucus distichus (Phaeophyceae): an intertidal alga with successful external fertilization. Mar Ecol Prog Ser. 1996;143:211–223. [Google Scholar]
- Pearson GA, Serrão EA, Brawley SH. Control of gamete release in fucoid algae: sensing hydrodynamic conditions via carbon acquisition. Ecology. 1998;79:1725–1739. [Google Scholar]
- Pei Z-M, Kuchitzu K, Ward JM, Schwartz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LH. Phytochromes—differential properties, expression patterns and molecular evolution. Photochem Photobiol. 1995;61:10–21. [Google Scholar]
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. Phytochromes: Photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Raven JA, Osmond CB. Inorganic C acquisition processes and their ecological significance in inter- and sub-tidal macroalgae of North Carolina. Funct Ecol. 1992;6:41–47. [Google Scholar]
- Robinson KR, Lorenzi R, Ceccarelli N, Gualtieri P. Retinal identification in Pelvetia fastigiata. Biochem Biophys Res Commun. 1998;243:776–778. doi: 10.1006/bbrc.1998.8176. [DOI] [PubMed] [Google Scholar]
- Robinson KR, Miller BJ. The coupling of cyclic GMP and photopolarization of Pelvetia zygotes. Dev Biol. 1997;187:125–130. doi: 10.1006/dbio.1997.8600. [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Prins HBA. The membrane potential of Arabidopsis thaliana guard cells; depolarizations induced by apoplastic acidification. Planta. 1998;205:100–112. doi: 10.1007/s004250050301. [DOI] [PubMed] [Google Scholar]
- Satter RL, Morse MJ, Lee Y, Crain RC, Coté GG, Moran N. Light- and clock-controlled leaflet movements in Samanea saman: a physiological, biophysical and biochemical analysis. Bot Acta. 1988;101:205–213. [Google Scholar]
- Schmidt C, Schelle I, Liao Y-J, Schroeder JI. Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc Natl Acad Sci USA. 1995;92:9535–9539. doi: 10.1073/pnas.92.21.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Poetsch HAW, Braun B, Marx S, Schaumburg A. Phytochromes and bacterial sensor proteins are related by structural and functional homologies. Hypothesis on phytochrome-mediated signal-transduction. FEBS Lett. 1991;281:245–249. doi: 10.1016/0014-5793(91)80403-p. [DOI] [PubMed] [Google Scholar]
- Schroeder JI. Anion channels as central mechanisms for signal transduction in guard cells and putative functions in roots for plant-soil interactions. Plant Mol Biol. 1995;28:353–361. doi: 10.1007/BF00020385. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Keller BU. Two types of anion channel currents in guard cells with distinct voltage regulation. Proc Natl Acad Sci USA. 1992;89:5025–5029. doi: 10.1073/pnas.89.11.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Schmidt C, Sheaffer J. Identification of high-affinity slow anion channel blockers and evidence for stomatal regulation by slow anion channels in guard cells. Plant Cell. 1993;5:1831–1841. doi: 10.1105/tpc.5.12.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Ilan N, Schwarz M, Scheaffer J, Assmann SM, Schroeder JI. Anion-channel blockers inhibit S-type anion channels and abscisic acid responses in guard cells. Plant Physiol. 1995;109:651–658. doi: 10.1104/pp.109.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrão EA, Pearson GA, Kautsky L, Brawley SH. Successful external fertilization in turbulent environments. Proc Natl Acad Sci USA. 1996;93:5286–5290. doi: 10.1073/pnas.93.11.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva PC. California seaweeds collected by the Malaspina expedition, especially Pelvetia (Fucales, Phaeophyceae) Madroño. 1996;43:345–354. [Google Scholar]
- Sommer D, Wells TA, Song P-S. A possible tyrosine phosphorylation of phytochrome. FEBS Lett. 1996;393:161–166. doi: 10.1016/0014-5793(96)00876-9. [DOI] [PubMed] [Google Scholar]
- Speransky VV, Brawley SH, McCully ME (1998) Ion fluxes during potentiation and gamete release in the fucoid alga Pelvetia compressa (J. Agardh) De Toni (abstract). J Phycol 34(suppl): 56
- Starostzik C, Marwan W. A photoreceptor with characteristics of phytochrome triggers sporulation in the true slime mould Physarum polycephalum. FEBS Lett. 1995;370:146–148. doi: 10.1016/0014-5793(95)00820-y. [DOI] [PubMed] [Google Scholar]
- Surif MB, Raven JA. Exogenous inorganic carbon source for photosynthesis in seawater by members of the Fucales and Laminariales (Phaeophyta): ecological and taxonomic implications. Oecologia. 1989;78:97–105. doi: 10.1007/BF00377203. [DOI] [PubMed] [Google Scholar]
- Taylor A, Brownlee C. Calcium and potassium currents in the Fucus egg. Planta. 1993;139:109–119. [Google Scholar]
- Thiel G, Blatt MR. Phosphatase antagonist okadaic acid inhibits steady-state K+ currents in guard cells of Vicia faba. Plant J. 1994;5:727–733. [Google Scholar]
- Thümmler F, Algarra P, Fobo GM. Molecular cloning of a novel phytochrome gene of the moss Ceratodon purpureus which encodes a putative light-regulated protein kinase. Plant Mol Biol. 1992;20:1003–1017. doi: 10.1007/BF00028888. [DOI] [PubMed] [Google Scholar]
- Winlands A, Wagner G. Phytochrome of the green alga Mougeotia: cDNA sequence, autoregulation and phylogenetic position. Plant Mol Biol. 1996;32:589–597. doi: 10.1007/BF00020200. [DOI] [PubMed] [Google Scholar]
- Yaish P, Gazit A, Gilon C, Levitski A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988;242:933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]