Abstract
Rationale
The default mode network (DMN), one of several resting-state networks (RSN) in the brain, is thought to be involved in self-referential thought, awareness, and episodic memories. Nicotine improves cognitive performance, in part by improving attention. Nicotinic agonists have been shown to decrease activity in regions within DMN and increase activity in regions involved in visual attention during effortful processing of external stimuli. It is unknown if these pharmacological effects also occur in the absence of effortful processing.
Objectives
This study aims to determine if nicotine suppresses activity in default mode and enhances activity in extra-striate RSNs in the absence of an external visual task.
Methods
Within-subject, single-blinded, counterbalanced study of 19 non-smoking subjects who had resting functional MRI scans after 7 mg nicotine or placebo patch. Group independent component analysis was performed. The DMN component was identified by spatial correlation with a reference DMN mask. A visual attention component was identified by spatial correlation with an extra-striate mask. Analyses were conducted using statistical parametric mapping.
Results
Nicotine was associated with decreased activity in regions within the DMN and increased activity in extra-striate regions.
Conclusions
Suppression of DMN and enhancement of extra-striate resting-state activity in the absence of visual stimuli or effortful processing suggest that nicotine’s cognitive effects may involve a shift in activity from networks that process internal to those that process external information. This is a potential mechanism by which cholinergic agonists may have a beneficial effect in diseases associated with altered resting-state activity.
Keywords: Resting-state networks, Default mode network, Nicotine, Attention, Posterior cingulate, Extra-striate cortex
Introduction
Acute administration of nicotine can enhance performance across many cognitive domains, especially those involving attention, vigilance, and working memory (Foulds et al. 1996; Rezvani and Levin 2001; Swan and Lessov-Schlaggar 2007). Improvements in performance have been consistently observed in nicotine-dependent individuals and also reported in minimally deprived smokers and non-smokers, suggesting an effect beyond that of withdrawal alleviation alone. The mechanisms of cognitive enhancement are unknown but likely involve neuronal activation directly through nicotinic cholinergic receptors (Poorthuis et al. 2009), and indirectly through modulation of glutamate, GABA, or dopamine neurotransmission, or MAO inhibitors (Brody et al. 2004; Swan and Lessov-Schlaggar 2007).
To understand nicotine’s effects on cognition at the circuit level, numerous studies have examined the blood-oxygen-level-dependent (BOLD) functional MR imaging (fMRI) response to nicotine or nicotinic cholinergic agonists during cognitive tasks, most often involving memory (Kumari et al. 2003) or attention (Thiel et al. 2005; Lawrence et al. 2002; Hahn et al. 2007). Several studies have reported nicotine-associated reduction in the BOLD response in parietal regions during target-detection tasks (Thiel et al. 2005; Giessing et al. 2006; Thiel and Fink 2008; Hahn et al. 2007). Reduced medial parietal activity has also been reported with the nicotinic agonist physostigmine, a cholinesterase inhibitor, in subjects performing a sustained attention task (Bentley et al. 2004). The overlap between nicotine-associated decreased activity in medial parietal and posterior cingulate cortex and the “default network” supports the hypothesis that nicotine enhances cognitive performance, in part, by suppressing default mode network (DMN) activity (Hahn et al. 2007).
The DMN is one of the several brain networks that show spontaneous, synchronous low-frequency fluctuations at rest. The DMN has been identified as deactivating during effortful tasks and is comprised by the posterior cingulate cortex, medial prefrontal cortex, medial and inferior-lateral parietal and medial temporal lobes. The DMN concept arose from the observation of deactivation in these regions during cognitive tasks, or conversely, activation during baseline states (Raichle et al. 2001). The degree of deactivation or suppression is proportional to the demands of the task (McKiernan et al. 2003) suggesting a reallocation of resources away from DMN toward regions involved in task performance. Resting brain “activity” has been conceptualized as an antagonism or “toggle” between an introspective, task-negative default network and an extrospective, task-positive network (Broyd et al. 2009). Nicotine may improve cognitive performance by enhancing extrospective task-positive networks such as those that involve visuospatial attention, while suppressing introspective, task-negative networks. Previous studies have focused on nicotine’s effects on brain activity with cognitively demanding tasks involving attention, information processing, and memory. It has not been shown whether nicotine’s effect on DMN or other resting-state networks (RSNs) occurs in the absence of external tasks. Such a finding would support a sensory-driven pharmacological mechanism of nicotine’s effect on cognition. The purpose of this study was to test the hypothesis that nicotine, but not placebo, would be associated with decreased activity in DMN regions in the absence of an external task.
While the DMN has received much attention in the literature, it is only one of several RSNs characterized by spontaneous, synchronous, low-frequency fluctuations. Other major RSNs include sensorimotor, visual, auditory, dorsal attention, and executive control networks (Biswal et al. 1995; Zhang and Raichle 2010). Among the RSNs, a high degree of inter-subject consistency has been shown for the peri-striate visual association network (Damoiseaux et al. 2006). Because nicotine is known to improve visual attention, our second hypothesis was that nicotine, but not placebo, would be associated with increased resting-state activity in the extra-striate cortex, consistent with enhancement in visual attention areas, in the absence of visual stimuli.
Methods
Subjects
Twenty-five healthy adults participated in the study. After a nicotine tolerance session, described below, four did not return for imaging sessions due to side effects from the nicotine (two experienced significant nausea and two experienced both nausea and emesis) and one subject moved out of state. One subject did not complete all scanning sessions. We report on the remaining 19 (11 male and 8 female) subjects (average age of 30 years, SD 9). Three of 19 were considered former smokers (one was abstinent for 3 years and had a lifetime use of 100 cigarettes; one was abstinent for 22 years and smoked 2–3 months; one was abstinent for 3 years and had a lifetime use of 20 cigarettes). Three subjects had smoked fewer than five cigarettes in their lifetime. All subjects were nicotine-free for at least 3 years prior to the study. Subjects underwent Structured Clinical Interview (SCID) and were excluded for axis I disorders including schizophrenia, bipolar disorder, depression, anxiety, and lifetime substance dependence. Subjects were not excluded for any lifetime conditions. One subject had a single episode of depression 9 years prior to study entry. No others reported a past diagnosis. Additional exclusions were neurological illness, prior significant head trauma, and major medical illness. Subjects provided written informed consent as approved by the Colorado Multiple Institutional Review Board.
Experimental design and drug administration
This was a single-blinded, placebo-controlled, cross-over study comparing the effects of nicotine vs. placebo patch on the DMN and extra-striate network during rest with eyes closed. The physician who was aware of the drug condition (L.F.M.) was not involved with the MRI data analysis and blinded the data so that the researchers involved with data analysis were blinded to drug condition. Subjects participated in three visits separated by approximately 1 week. During the first visit, medical, psychiatric, and smoking history were obtained. Subjects underwent a nicotine tolerance study. A 7 mg transdermal nicotine (Nicoderm CQ) clear patch (Alza Corp, subsidiary of Johnson & Johnson, New Brunswick, NJ, USA) was applied for 90 min. Tape was placed over the patch to maintain the single-blind status. During this time, blood pressure, heart rate, and subjective reporting of side effects were recorded at baseline, 30, 60, and 90 min.
During the second and third visits, subjects underwent fMRI of the brain before and after receiving nicotine patch (7 mg Nicoderm CQ clear patch) covered with tape during one of these visits and a placebo patch (two pieces of medical tape cut to the size of the nicotine patch) during the other session. Nine subjects received nicotine first and ten received placebo first. Resting fMRI scans were acquired 90 min after application of the patch, corresponding to previous work showing near peak levels for the Alza transdermal system (Fant et al. 2000). For purposes of blinding, the patch and tape overlay were applied and removed with the subjects’ eyes closed. After removal of the patches, bandage tape was applied to the site to conceal clues about the patch based on erythematous skin appearance.
MRI parameters
Images were acquired on a 3T whole body MR scanner (General Electric, Milwaukee, WI, USA) using a standard quadrature head coil. A high-resolution 3D T1-weighted anatomic scan was collected. Functional scans were acquired with the following parameters: TR 2,000 ms, TE 32 ms, FOV 240 mm2, matrix 64×64, voxel size 3.75× 3.75 mm2, slice thickness 3 mm, gap 0.5 mm, interleaved, flip angle 70°. Resting fMRI scan duration was 10 min. Subjects were instructed to rest with eyes closed. The study was performed along with a parametric finger tapping fMRI study that will be reported separately. An MR-compatible photoplethysmograph was used to record heartbeat and respiratory fluctuations during the MR scan. The signal was sampled every 25 msec.
MRI data analysis
fMRI data were pre-processed using SPM8 (Wellcome Dept. of Imaging Neuroscience, London, UK) running on Matlab R2009b. The first four images were excluded for saturation effects. Images were realigned to the first volume, normalized to the Montreal Neurological Institute (MNI) space, and spatially smoothed with an 8-mm FWHM Gaussian kernel.
Spatial independent component analysis (ICA) was performed using GIFT software v1.3g (http://icatb.sourceforge.net) (Calhoun et al. 2001). Group ICA was conducted separately for four conditions: post-placebo, pre-placebo, post-nicotine, pre-nicotine. For each condition, the dimensionality of the data from each subject was reduced using principle component analysis and concatenated into an aggregate data set. Twenty independent sources were estimated with an ICA using the infomax algorithm (Bell and Sejnowski 1995). The rationale for selecting 20 components was (1) it was the default parameter in GIFT and (2) previous work comparing across ICA methods, components, and RSNs showed that 20 components was associated with overall highest consistency, based on temporal and spatial correlations (Schopf et al. 2010).
Component selection by template matching
Default mode network (DMN)
The DMN component was identified by selecting the component with the highest spatial correlation to the DMN template supplied by the GIFT software. This method of component selection has been described previously (Calhoun et al. 2008; Correa et al. 2007). The template is a weighted atlas-based mask consisting of posterior cingulate cortex, posterior parietal cortex, precuneus, frontal pole, and occipitotemporal junction. Weighting of posterior and anterior cingulate nodes has been shown to improve reliability in identifying resting-state DMN(Franco et al. 2009).
Visuospatial attention
To determine if nicotine increased resting-state activity in regions involved in visuospatial attention, an extra-striate template was created using the WFU Pickatlas (http://www.fmri/wfubmc.edu). The template was defined by combining Brodmann Area (BA) 18 and 19, and smoothing the result with an 8-mm FWHM Gaussian kernel to match the functional data, similar to other atlas-based approaches for defining other RSNs (Calhoun et al. 2008).
Removal of physiological component
The analysis above was repeated after removing the component corresponding to aliased heartbeat. This was performed by down-sampling the heartbeat to 2 s (equal to TR) for each subject, followed by identification and removal of the component with the highest temporal correlation to the down-sampled heartbeat.
Statistical comparison of images
Component maps were analyzed using a 2×2, repeated measures ANOVA (rmANOVA), with drug and session as within-subject factors. To test the hypothesis that nicotine would decrease activity in DMN, directionally specific, planned t-contrasts of post-nicotine<pre-nicotine and post-placebo<pre-placebo were performed. To test the hypothesis that nicotine would increase activity in extra-striate visual regions, directionally specific, planned t-contrasts of post-nicotine>pre-nicotine and post-placebo>pre-placebo were performed. Contrast maps were set at threshold of p<0.001 and masked with the effect of condition, set at p<10−6. The t-statistic for these contrasts control for within-subject effects.
We next tested for a drug (nicotine vs. placebo) by session (pre vs. post-patch) interaction. Because we did not hypothesize an effect of placebo or session, these analyses were considered exploratory, and therefore, contrast maps of the interaction were set at a threshold of p<0.05 and masked with the effect of condition, set at p<10−6.
Spectral analysis group comparison
The statistical image analyses above test for differences in signal amplitude or signal strength for a given component. Those differences may arise from any number of frequency signatures, and are not limited to the low frequencies characteristic of RSNs, in the range of 0.01 to 0.12 Hz (Cordes et al. 2001). To assess the effect of nicotine on this low-frequency range, we compared the spectral power before and after nicotine for the DMN and extra-striate networks separately. Low-frequency signals were arbitrarily assigned to bins of 0.02–0.07 Hz and 0.08–0.12 Hz using the spectral comparison utility in GIFT.
Physiological data
Heart rate measured outside of and in the scanner and mean arterial blood pressure (MABP) measured outside the scanner were analyzed with rmANOVA with time and drug as within-subject factors (SPSS).
Results
Physiological measures
Outside the scanner
Heart rate
There was no main effect of time, drug, or drug by time interaction across the four time periods (baseline, 30, 60, and 90 min after patch) on heart rate. Blood pressure: There was no main effect of drug or interaction of drug by time on MABP. There was a main effect of time on MABP (F(3,48)=6.26, p=0.001) with a quadratic increase over time (Table 1).
Table 1.
Effects of nicotine and placebo on heart rate and mean arterial blood pressure (MABP) over time as measured outside the scanner
| Baseline | Patch on
|
|||
|---|---|---|---|---|
| 30 min | 60 min | 90 min | ||
| Heart rate (bpm) | ||||
| Placebo | 70.8 (12.7) | 65.2 (12.1) | 62.4 (12.3) | 64.1 (9.3) |
| Nicotine | 68.0 (15.8) | 65.8 (12.1) | 67.7 (11.8) | 68.0 (11.4) |
| MABP (mm Hg) | ||||
| Placebo | 90.5 (10.6) | 86.4 (12.1) | 86.7 (9.1) | 89.4 (9.7) |
| Nicotine | 89.2 (8.7) | 87.4 (9.4) | 89.6 (9.6) | 92.4 (8.1) |
Values are mean (SD)
Heart rate during scanning
Thirteen of the 76 heartbeat tracings acquired during MR scans were excluded due to poor quality. Of the remaining 63 data points, there was no main effect of drug on heart rate during MR scanning. There was an effect of time (F(1, 9)=9.12, p=0.014), with heart rate lower in the second compared to the first session (69.30 (2.84) vs. 66.1 (3.14), pre-patch vs. post-patch). There was a drug by time interaction (F(1,9)=8.7, p=0.016) with placebo associated with a lowering heart rate (65.88 (10.31) vs. 59.75 (10.74), pre-placebo vs. post-placebo), whereas nicotine was not associated with a change in heart rate (64.13 (10.97) vs. 66.53 (10.74), pre-nicotine vs. post-nicotine).
Effect of nicotine on default mode network (DMN)
Compared to baseline, nicotine was associated with a decrease in activity in the default network in the posterior cingulate cortex, precuneus, paracentral lobule, and medial orbitofrontal cortex (Fig. 1; Table 2). Compared to baseline, placebo was associated with a smaller extent of decreased activity in default network regions: precuneus, paracentral lobule, and angular gyrus (Fig. 1; Table 2).
Fig. 1.
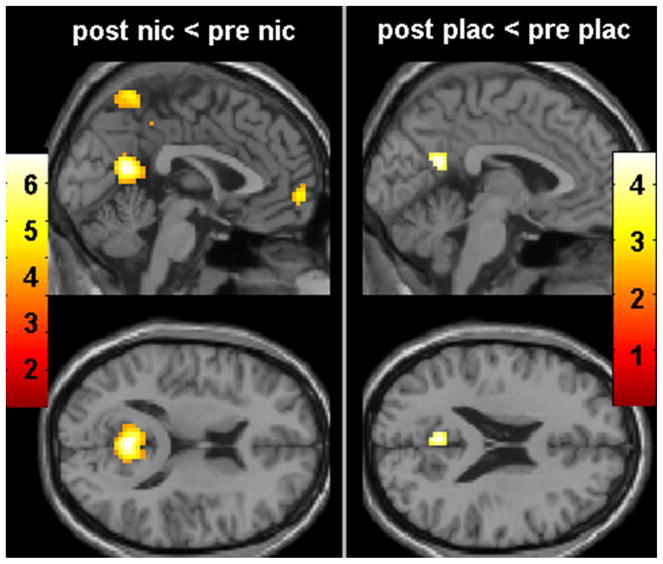
Effect of drug on DMN. Compared to baseline, nicotine was associated with decreased activity in precuneus, posterior cingulate, and medial frontal cortex (left). Placebo was associated with a smaller region of decreased activity in posterior cingulate (right). Maps set at threshold p<0.001, masked with effect of condition set at p<10–6. Colored bars represent weighted contrast t-scores for within-subject rmANOVA
Table 2.
Areas within the default network, where nicotine and placebo decreased resting-state activity (Fig. 1) and where significant interaction between drug and session were observed (Fig. 2). Coordinates are given in MNI space
| Post-nic<pre-nic | BA | x | y | z | t | Extent |
|---|---|---|---|---|---|---|
| Posterior cingulate | 30 | 0 | −55 | 16 | 6.78 | 183 |
| Precuneus | 7/23 | 6 | −55 | 64 | 4.72 | 93 |
| Paracentral lobule | 4/31 | 9 | −31 | 49 | 5.74 | 42 |
| −9 | −37 | 41 | 3.86 | 29 | ||
| Medial orbitofrontal gyrus | 10 | 12 | 59 | −2 | 4.71 | 51 |
| Post-plac<pre-plac | BA | x | y | z | t | Extent |
| Angular gyrus | 19 | −45 | −73 | 43 | 4.56 | 10 |
| Posterior cingulate | 23/30 | −3 | −55 | 22 | 4.44 | 31 |
| Paracentral lobule | 4/31 | 6 | −28 | 49 | 4.04 | 10 |
| Interaction | BA | X | Y | Z | T | Extent |
| Precuneus | 7/23 | 6 | −61 | 64 | 6.65 | 945 |
| −6 | −55 | 58 | 5.68 | |||
| Posterior cingulate | 30 | 0 | −58 | 16 | 2.78 | 98 |
A drug by session interaction was observed in the precuneus and posterior cingulate. Nicotine was associated with a decrease and placebo associated with an increase in activity in precuneus (Fig. 2, right; Table 2). Nicotine was associated with greater reduction in DMN in posterior cingulate region compared to placebo (Fig. 2, left; Table 2).
Fig. 2.
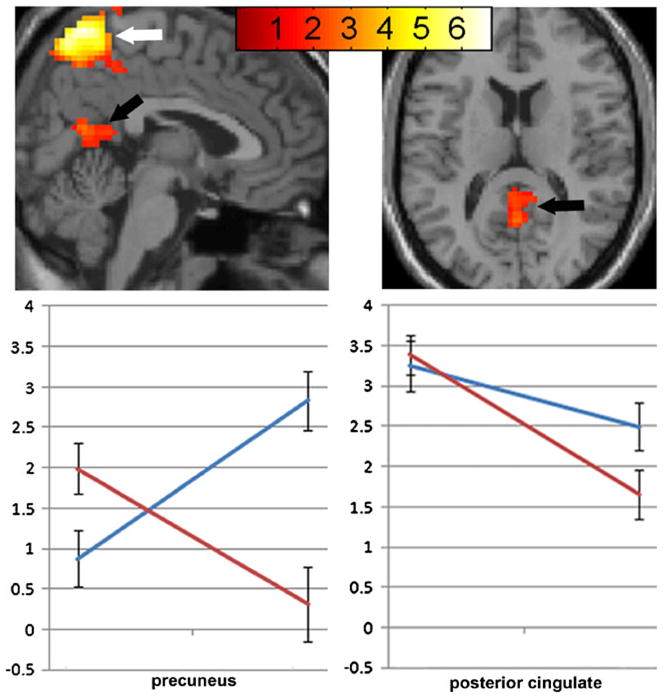
Drug by session interaction on DMN. Significant interaction of drug (nicotine vs. placebo) and session (pre- vs. post-patch) in precuneus (white arrow) and posterior cingulate (black arrow). Nicotine: red line, placebo: blue line. Data are mean (SEM) blood-oxygen-level dependent (BOLD) signal. Map set at threshold p<0.05, masked with effect of condition set at p<10–6. Colored bars represent weighted contrast t-scores for within-subject rmANOVA
Effect of nicotine on default mode network activity after physiological correction
Thirteen of the 76 heartbeat tracings acquired during MR scans were excluded due to poor quality. Excluded data were distributed similarly over the four conditions. For the remaining 63 data sets, the aliased heartbeat was removed using the GIFT utility for component removal. There was no significant change in the statistical contrast maps after removing this component.
Effect of nicotine on extra-striate cortex
Compared to baseline, nicotine was associated with an increase in resting-state activity in bilateral extra-striate cortex. Compared to baseline, placebo was associated with an increase in resting-state activity in the cuneus (Table 3). There was an interaction between session and drug in bilateral extra-striate regions (BA 18, 19, and 37), with nicotine associated with an increase in activity and placebo not significantly changed (Fig. 3; Table 3).
Table 3.
Areas where nicotine and placebo increased activity for the component corresponding to extrastriate cortex resting fluctuations
| Post-nic>pre-nic | BA | x | y | z | t | Extent |
|---|---|---|---|---|---|---|
| Left mid temp/occip | 37 | −54 | −64 | 4 | 8.40 | 259 |
| 37 | −51 | −71 | 10 | 8.27 | ||
| 19 | −45 | −73 | 4 | 7.91 | ||
| Right mid temp/occip | 37 | 48 | −67 | 16 | 6.65 | 180 |
| 37 | 51 | −58 | 10 | 5.50 | ||
| Post-plac>pre-plac | BA | x | y | z | t | Extent |
| Left cuneus | 18 | −6 | −85 | 31 | 14.8 | 432 |
| Right cuneus | 18 | 12 | −85 | 28 | 13.18 | |
| 18 | 3 | −91 | 22 | 9.79 | ||
| Interaction | BA | X | Y | Z | T | Extent |
| Left mid temp/occip | 37 | −54 | −67 | 1 | 6.30 | 541 |
| 37 | −54 | −73 | 10 | 6.05 | ||
| 19 | −33 | −91 | −8 | 5.96 | ||
| Right mid temp/occip | 18 | 36 | −91 | −8 | 5.37 | 297 |
| 19 | 42 | −85 | −2 | 4.55 | ||
| 37 | 57 | −54 | 13 | 4.20 |
Fig. 3.
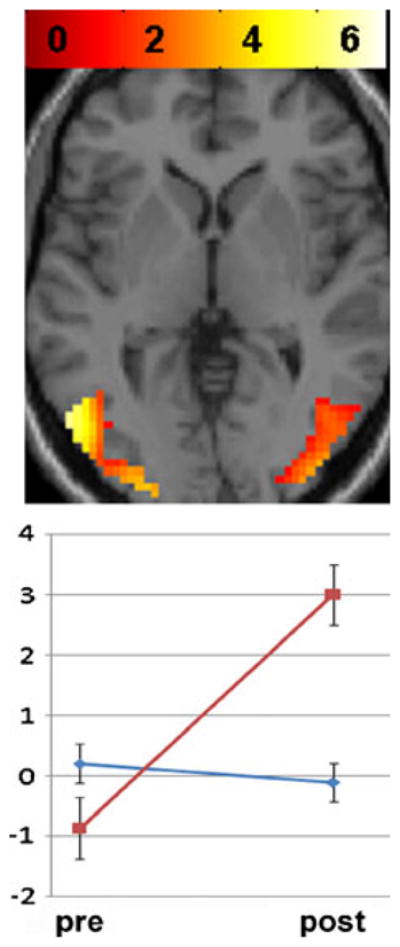
Drug by session interaction on extra-striate resting network. Significant interaction of drug (nicotine vs. placebo) and session (pre- vs. post-patch) in bilateral extra-striate cortex. Nicotine: red line, placebo: blue line. Data are mean (SEM) BOLD signal. Map set at threshold p<0.05, masked with effect of condition set at p<10–6. Colored bars represent weighted contrast t-scores for within-subject rmANOVA
Spectral power comparison
For the DMN component, nicotine was associated with a decrease in power, consistent with lower amplitude resting-state signal fluctuations. For the extra-striate component, nicotine was associated with a slight decrease in power in the very low (0.02–0.07 Hz), but much larger increase in power in the low (0.08–0.12 Hz) frequency range, suggesting an overall increase in amplitude of resting-state signal fluctuations (Fig. 4).
Fig. 4.
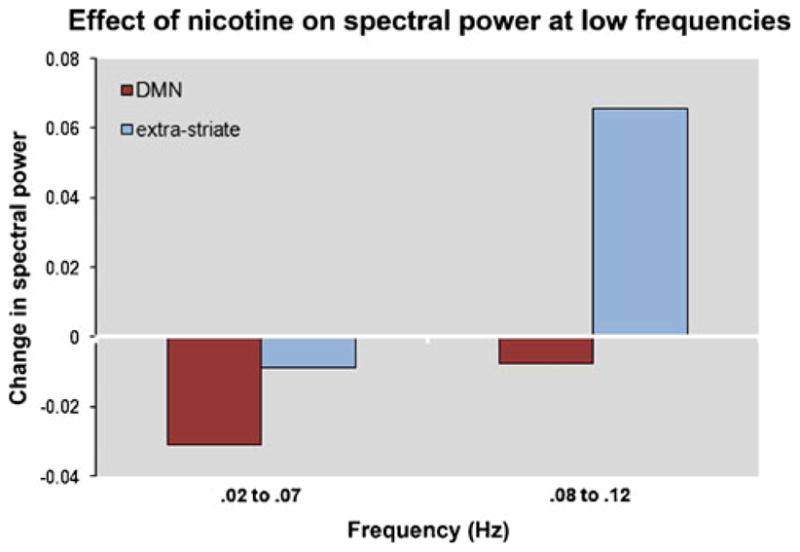
Effect of nicotine on spectral power at low frequencies (0.02–0.07 Hz and 0.08–0.12 Hz) for default mode and extra-striate RSNs
Discussion
The main finding of this study is that nicotine was associated with reduced default network activity in non-smokers during true rest; that is, in the absence of an externally cued cognitively demanding task. This is consistent with previous studies demonstrating reduced BOLD-related activity in regions within the DMN after acute administration of nicotine or nicotinic cholinergic agonists (Bentley et al. 2004; Hahn et al. 2007; Thiel et al. 2005; Giessing et al. 2006; Thiel and Fink 2008). Those studies did not focus on resting-state activity, but rather on task-specific aspects of attention and the modulating effects of cholinergic agonists. Our results extend these findings by demonstrating that effortful processing is not required to detect similar effects of nicotine. The emphasis in the literature has been on knowledge-driven or top-down mechanisms of cholinergic modulation of sustained attention processes (Sarter et al. 2001; Yu and Dayan 2005). Our results suggest that sensory-driven or bottom-up processes also play a potentially important role in nicotine’s effect on cognition. A previous study similar to ours evaluated the effects of transdermal nicotine on both top-down and bottom-up responses during a visually cued target attention task in non-smokers. Nicotine was associated with less BOLD activity than placebo within cuneus, precuneus, and posterior cingulate cortex for both top-down and bottom-up contrasts (Hahn et al. 2007). Another study in non-smokers reported nicotine-associated decreases in BOLD signal in precuneus during reorienting of attention (Thiel et al. 2005). Bentley et al. reported decreased activity associated with physostigmine during an attention task in a region nearly overlapping our results within the superior medial parietal cortex (Bentley et al. 2004). Taken together, these studies suggest that nicotine may downregulate DMN processing independent of cognitive effort.
The functional correlates of the DMN are of considerable interest given a growing number of studies suggesting its importance for understanding information processing and potential utility as a marker of diseases (Broyd et al. 2009), although it is noted that the significance has been somewhat controversial (Morcom and Fletcher 2007). One interpretation of our results is that by suppressing internally directed processes, nicotine allows for greater receptiveness to external sensory cues. This could have therapeutic implications in mental disorders where abnormal increased resting-state activity has been observed such as schizophrenia (Zhou et al. 2007; Garrity et al. 2007), depression (Greicius et al. 2007), or attention deficit hyperactivity disorder (Tian et al. 2006).
We use the term “activity” here to reflect the signal strength or amplitude of the component of interest. It is important to keep in mind that the difference in components does not measure a difference in signal coherence or correlation. Rather, it measures differences in the amplitude of the given component. It is possible, for example, for two signals to have perfect coherence and correlation but different amplitudes. The finding that nicotine was associated with decreased DMN “activity” does not indicate which frequencies may have contributed to the decreased signal amplitude. Since RSNs are characterized by frequencies less than 0.12 Hz (Cordes et al. 2001), we conducted a spectral analysis of the low-frequency signals. Nicotine was associated with less power at low frequencies for the DMN component, consistent with suppression of DMN signal. In contrast, nicotine was associated with increased power for the extra-striate component, consistent with increased resting-state signal amplitude (Fig. 4). It remains uncertain if nicotine suppression of DMN reflects a general change in arousal state or a specific pharmacological effect of nicotine. Other drugs, such as Midazolam, a short acting benzodiazepine, given at doses sufficient to cause conscious sedation, is also associated with decreased DMN activity (Greicius et al. 2008). Further studies are needed to determine if such changes in the magnitude of RSNs represent state effects (Harrison et al. 2008) or specific pharmacological effects. We note that some studies have reported nicotine-associated increases, not decreases, in activity within regions overlapping with DMN (Lawrence et al. 2002). Our study differs significantly from the previous paper in that the Lawrence study was performed in the context of a visual attention task and the increased activity reported in inferior parietal and supra-gyral regions is lateral to the decreased activity reported here.
The second finding of this paper is that nicotine was associated with increased resting-state “activity” in extra-striate cortex in the absence of external visual stimuli. The extra-striate cortex, comprised of BA 18 and 19, carries out higher level visual processing. Animal and human neuroimaging studies have shown that selective attention biases neuronal responses in extra-striate regions (Kastner and Ungerleider 2000). Among several functional RSNs, the visual network is one of the more robust in terms of its extent of signal change and consistency across individuals (Damoiseaux et al. 2006). Our findings are consistent with increased BOLD activity associated with nicotinic agonists in the lateral occipital cortex during attentional tasks (Bentley et al. 2004; Thiel et al. 2005) and rapid visual information processing (Lawrence et al. 2002). One interpretation is that while nicotine suppresses internally generated processes, it also prepares or alerts areas important for processing external visual stimuli. Preparatory BOLD signal changes in extra-striate areas preceding visual stimuli have been observed in other studies of spatial attention (Sapir et al. 2005). Our results are somewhat inconsistent with Thiel and Fink who reported no difference in extra-striate cortex activity associated with nicotine compared to placebo (Thiel and Fink 2008) in a visual reorienting task. Their study differed significantly from ours, however, as it was designed to measure nicotine’s effect on top-down modulation of visual cues, while our study focused on bottom-up mechanisms.
An outstanding question regarding the DMN is the extent to which these synchronous, low-frequency signal fluctuations represent non-neuronal physiological signals (Bandettini and Bullmore 2008). It is unlikely that cardiorespiratory noise related to nicotine would explain our results. First, nicotine had no significant effect on heart rate as measured outside the scanner (Table 1). Second, there is no evidence that nicotine alters respiration which, compared to aliased cardiac signal, would be more likely to confound DMN fluctuations. Third, an advantage of data-driven independent component analysis methods is that components related to structured physiological noise should be separable from DMN component. This is consistent with a recent study showing that physiological corrections account for only a minor amount of variance in group ICA resting-state studies (Starck et al. 2010). Nonetheless, because there was an interaction of drug and session on heart rate during scanning, we repeated the group ICA analysis after removing the cardiac noise (i.e., that component with the highest temporal correlation to aliased heartbeat) and this did not significantly change the results.
There are limitations of this study. First, although the study controlled for placebo, session, and order, these results must be considered preliminary given the sample size. While RSNs are robust, quantitative test–retest stability has not been established and state effects, which may include placebo effects, are unknown. The inclusion of former smokers is a limitation. The findings did not change after removing the three subjects conservatively defined as former smokers. We believe this potential confound is relatively minor, although future studies comparing current to former smokers would be useful given expected differences in response to nicotine. Third, the single dose of nicotine limits the interpretations about behavioral and pharmacological effects. Although nicotine is known to increase visual attention, we lack independent confirmation that 7 mg nicotine administered as patch improves cognition.
In conclusion, this study found that nicotine has differential effects on different RSNs in a pattern consistent with sensory-driven modulation of brain regions involved in functionally differential aspects of attention. Reduced DMN and increased extra-striate cortex activity may reflect a shift away from introspective towards preparatory extrospective information processing. This alteration could have therapeutic implications for disorders associated with altered resting-state signals.
Acknowledgments
This publication was made possible by NIH Grant Numbers R21DA024104, R01DA027748 and the VA Biomedical Laboratory and Clinical Science Research and Development Service. We thank Dr. Robert Freedman for his helpful discussion.
Footnotes
Conflicts of interest
Dr. Laura Martin has received compensation from Pfizer as a sub-investigator for a trial using Chantix. The remaining authors have no financial disclosures or other conflicts of interest.
Contributor Information
Jody Tanabe, Email: jody.tanabe@ucdenver.edu, Department of Radiology, University of Colorado Denver School of Medicine, Denver, CO, USA. Department of Psychiatry, University of Colorado Denver School of Medicine, Denver, CO, USA.
Eric Nyberg, Department of Radiology, University of Colorado Denver School of Medicine, Denver, CO, USA.
Laura F. Martin, Department of Psychiatry, University of Colorado Denver School of Medicine, Denver, CO, USA. Research Service, Denver VA Medical Center, Denver, CO, USA
Jesse Martin, Department of Psychiatry, University of Colorado Denver School of Medicine, Denver, CO, USA.
Dietmar Cordes, Department of Radiology, University of Colorado Denver School of Medicine, Denver, CO, USA.
Eugene Kronberg, Department of Psychiatry, University of Colorado Denver School of Medicine, Denver, CO, USA.
Jason R. Tregellas, Department of Psychiatry, University of Colorado Denver School of Medicine, Denver, CO, USA. Research Service, Denver VA Medical Center, Denver, CO, USA. 12700 E. 19th Ave, Mailstop C278, Aurora, CO 80045, USA
References
- Bandettini PA, Bullmore E. Endogenous oscillations and networks in functional magnetic resonance imaging. Hum Brain Mapp. 2008;29:737–739. doi: 10.1002/hbm.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bentley P, Husain M, Dolan RJ. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron. 2004;41:969–982. doi: 10.1016/s0896-6273(04)00145-x. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, et al. Smoking-induced ventral striatum dopamine release. Am J Psychiatr. 2004;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2008;29:1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Correa N, Adali T, Calhoun VD. Performance of blind source separation algorithms for fMRI analysis using a group ICA method. Magn Reson Imag. 2007;25:684–694. doi: 10.1016/j.mri.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant RV, Henningfield JE, Shiffman S, Strahs KR, Reitberg DP. A pharmacokinetic crossover study to compare the absorption characteristics of three transdermal nicotine patches. Pharmacol Biochem Behav. 2000;67:479–482. doi: 10.1016/s0091-3057(00)00399-3. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology (Berl) 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Franco AR, Pritchard A, Calhoun VD, Mayer AR. Interrater and intermethod reliability of default mode network selection. Hum Brain Mapp. 2009;30:2293–2303. doi: 10.1002/hbm.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatr. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Giessing C, Thiel CM, Rosler F, Fink GR. The modulatory effects of nicotine on parietal cortex activity in a cued target detection task depend on cue reliability. Neuroscience. 2006;137:853–864. doi: 10.1016/j.neuroscience.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatr. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yucel M. Modulation of brain resting-state networks by sad mood induction. PLoS ONE. 2008;3:e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, et al. Cognitive effects of nicotine in humans: an fMRI study. NeuroImage. 2003;19:1002–1013. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cognit Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. NeuroImage. 2007;37:1073–1082. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Goriounova NA, Couey JJ, Mansvelder HD. Nicotinic actions on neuronal networks for cognition: general principles and long-term consequences. Biochem Pharmacol. 2009;78:668–676. doi: 10.1016/j.bcp.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatr. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Sapir A, d’Avossa G, McAvoy M, Shulman GL, Corbetta M. Brain signals for spatial attention predict performance in a motion discrimination task. Proc Natl Acad Sci USA. 2005;102:17810–17815. doi: 10.1073/pnas.0504678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Schopf V, Windischberger C, Kasess CH, Lanzenberger R, Moser E. Group ICA of resting-state data: a comparison. MAGMA. 2010;23(5–6):317–325. doi: 10.1007/s10334-010-0212-0. [DOI] [PubMed] [Google Scholar]
- Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V. Correction of low-frequency physiological noise from the resting state BOLD fMRI–Effect on ICA default mode analysis at 1.5T. J Neurosci Meth. 2010;186:179–185. doi: 10.1016/j.jneumeth.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Fink GR. Effects of the cholinergic agonist nicotine on reorienting of visual spatial attention and top-down attentional control. Neuroscience. 2008;152:381–390. doi: 10.1016/j.neuroscience.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology. 2005;30:810–820. doi: 10.1038/sj.npp.1300633. [DOI] [PubMed] [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, et al. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2006;400:39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]


