Abstract
The total synthesis of hyacinthacine A2 is reported via a novel transannular hydroamination in which planar chirality of a 5-aza-trans-cyclooctene precursor is transferred to point chirality in the product. Key to the success of this strategy was the development of a method for establishing absolute planar chirality via stereocontrolled photoisomerization of a 5-aza-cis-cyclooctene. This was accomplished by constructing a 5-aza-cis-cyclooctene precursor with a trans-fused acetonide. The improved diastereoselectivity observed upon photoisomerization of this derivative is attributed to the conformational strain of the eight-membered ring in the minor diastereomer.
Introduction
The pyrrolizidine framework is common to manifold natural products,1 including the polyhydroxylated natural products hyacinthacine A2 (1), australine, alexine and their epimers (Figure 1). These natural products and analogs have been targeted for synthesis1-5 because of their selective glycosidase inhibition6 and activity against HIV.7 For example, hyacinthacine A2 (8.6 μM),8 australine (5.8 μM),9 and alexine (11 μM)10 and are all selective inhibitors of amyloglucosidase that do not display significant inhibition of β-glucosidases. Because of the diverse biological activities of pyrrolizidine alkaloids,1 the stereocontrolled construction of this privileged scaffold is of considerable interest.
Fig. 1.
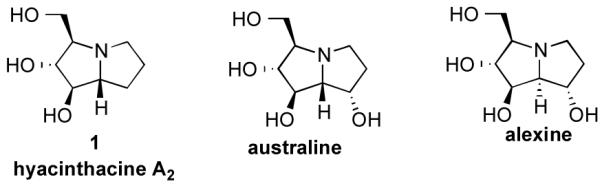
Representative pyrrolizidine alkaloid natural products
A transannular approach to the synthesis of the pyrrolizidine framework was first explored by Wilson, who showed that electrophiles could activate 5-aza-cis-cyclooctenes toward transannular C–N bond construction.11,12 White,4 Lin,13 Madsen5 and Davies14 have elegantly demonstrated the importance of transannular cyclizations in syntheses of pyrrolizidine alkaloids. White first used an epoxidation/transannular cyclization strategy to synthesize australine, and demonstrated that the 5-aza-cis-cyclooctene precursors could be created using ring closing metathesis.4 Madsen later synthesized australine via this approach of epoxidation/transannular cyclization, and demonstrated that the 5-aza-cis-cyclooctene precursors could be prepared very easily using a zinc-mediated fragmentation of iodosugars derived from fructose or sucrose.5 Most recently, Davies described an enantioselective synthesis of 7a-epi-hyacinthacine A1 in which transannular iodoamination was a key step.14
We envisioned that access to the pyrrolizidine alkaloids could be realized through transannular hydroamination15,16 of a 5-aza-cyclooctene (Scheme 1). There are few descriptions of transannular hydroaminations of 5-aza-cis-cyclooctenes. The radical cation of 5-aza-cis-cyclooctene cyclizes in the presence of t-BuSH to pyrrolizidine in 28% yield.12 The two-stage conversion of 3-azabicyclo[3.3.l]non-6-ene to 3-aza-noradamantane proceeds in two steps via the intermediacy of an isolable HCl addition product.16 We envisioned that transannular hydroaminations of 5-aza-trans-cyclooctenes would be facile due to the intrinsic strain of the (E)-cycloalkene. While stereospecific, transannular cyclizations of (E)-cycloalkenes have been studied with larger ring systems,17 relatively few studies have been carried out on trans-cyclooctene derivatives.18-20 Ceré et al. demonstrated that (E)-thiacyclooct-4-ene undergoes acid catalyzed transannular cyclization,21 as does the anion of (E)-4,5-epoxy-1-thiacyclooctane-1,1–dioxide.22 In 2008, we demonstrated that treatment of 4-aza-trans-cyclooctene derivative with bromine provides the bromopyrrolizidine in >90% isomeric purity, and with complementary diastereoselectivity relative to bromoamination of 4-aza-cis-cyclooctene. 23 Previously, the transannular hydroamination of an 5-aza-trans-cyclooctene has not been described, although elegant studies on the intermolecular hydroamination of trans-cycloalkenes have been described by Beauchemin.24
Scheme 1.
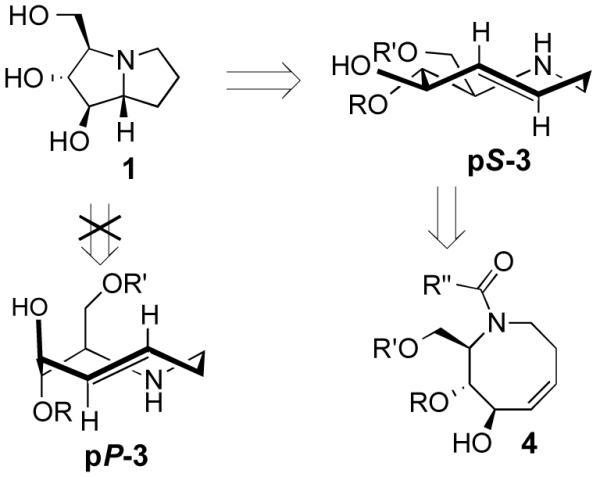
Retrosynthesis of hyacinthacine 1 using transannular hydroamination and diastereoselective photoisomerization as key steps
Retrosynthetic Analysis
A retrosynthetic analysis for the total synthesis of hyacinthacine A2 (1) is displayed in Scheme 1. It was expected that 1 could arise from the hydroamination of a 5-aza-trans-cyclooctene pS-3, which would in turn arise from the photoisomerization of cis-cyclooctene 4.25 cis-Cyclooctene 4 could be prepared via ring closing metathesis.4,5 We planned for the photoisomerization of 4 to be carried out on practical scale using a flow reaction developed by our group, in which trans-isomers selectively complex to AgNO3/silica.23 While our photochemical flow procedure has been applied in bioconjugation chemistry,26,27 it has not been previously applied in a target directed synthesis.
A key consideration for the synthesis of hyacinthacine A2 is stereocontrol in the photoisomerization step, as trans-cyclooctenes possess planar chirality and are configurationally stable. Only the pS isomer of 3 would lead to the natural product; pP-3 would instead lead to 7a epimer of hyacinthacine A2. In our earlier study, poor diastereoselectivity was observed in the photoisomerizations of most cyclooctenes that bear stereogenic centers.23 For example, the photoisomerization of 4,5-dihydroxy-trans-cyclooctene 5 gives 6a and 6b with a dr of only 1.9 : 1 (Scheme 3a). An exceptional case was 7, which gave 8a and 8b in 11 : 1 dr (Scheme 3b). As 7 is simply the acetonide of 5, we hypothesized that the high diastereoselectivity observed in the photoisomerization of 7 was a result of conformational constraints of the ring fusion. The eight-membered rings of both diastereomers of 6 can adopt the most stable crown conformation. By contrast, only the major diastereomer of 8 is able to adopt a crown conformation. The minor diasteromer 8b cannot reasonably support a crown conformation, which would necessitate a trans-diaxial ring fusion. Compound 8b must instead adopt a higher energy conformation. The second lowest energy conformation of trans cyclooctene is the chair conformation, which is calculated to be 5.4–5.6 kcal/mol (22.6–23.4 kJ/mol) higher in energy than the crown conformation. 23,28 This difference in product ground state conformations is apparently mirrored by an energetic difference in the transition states leading to 8a and 8b.
Scheme 3.
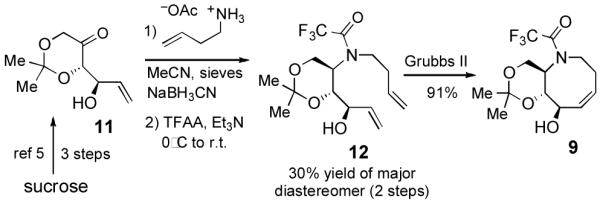
Synthesis of aza-cis-cyclooctene 9
Based on these observations, we predicted that a ring fusion could also impart diastereocontrol in the synthesis of hyacinthacine A2. Thus, acetonide 9 was targeted, and expected to photoisomerize to pS-10, which can adopt the crown conformation (Scheme 2c). The eight-membered ring of minor diastereomer pP-10 would be unable to adopt a crown conformation, and would instead be forced to adopt a higher energy chair conformer.
Scheme 2.
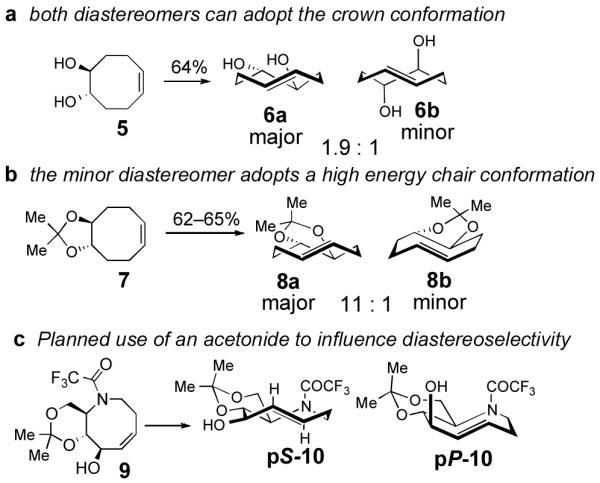
(a) Like most cyclooctenes with stereogenic centers, the photoisomerization of cyclooctene 5 proceeds with poor diastereoselecitvity. (b) The high diastereoselectivity observed in photoisomerization of cyclooctene 7 is attributed to the acetonide ring fusion, which would force the minor diastereomer to adopt a high energy chair conformation. (c) Incorporation of an acetonide in 5-aza-cyclooctene 9 is expected to prefer the formation of pS-10.
Results and Discussion
The synthesis of acetonide 9 was accomplished as illustrated in Scheme 3. The preparation of ketone 11 was carried out using the efficient method previously described by Lauritsen and Madsen.5 Reductive amination of 11 was carried out using a modification of the literature procedure5 to give an inseparable 7:3 mixture of diastereomers. However, the isomers could be readily separated upon trifluoroacetylation, and the major diastereomer 12 was obtained in 30% yield. While our efforts to improve the reductive amination of 11 were unsuccessful, we considered that the brevity of the synthesis of 12 make it an attractive intermediate. Ring closing metathesis using the 2nd Generation Grubbs catalyst gave 9 in 91% yield.
The key photoisomerization of 9 was carried out using our previously described flow system23 in which the very unfavorable cis/trans ratio is driven to completion by active removal of the trans-isomers (Figure 2). Thus, a 4:1 hexanes/ether solution of cis-alkene 9 was irradiated at 254 nm, and continuously cycled through a RediSep™ column that was packed with a bed of AgNO3 impregnated silica gel.29 Unlike cis-cyclooctenes, trans-cyclooctenes form stable coordination complexes with Ag(I), and trans-isomers 10 form stable complexes with AgNO3 that adhere to the column. In contrast, cis-cyclooctene 9 is not retained on the column, and is returned to the reaction flask for isomerization. The aza-trans-cyclooctene complexes were decomplexed from AgNO3 using aqueous ammonia, providing 10 with 8:1 dr, favoring the pS-isomer. The major isomer was separated, and X-ray crystallography confirmed that the eight-membered ring of pS-10 adopts a crown structure in the solid state (Figure 2a).
Figure 2.
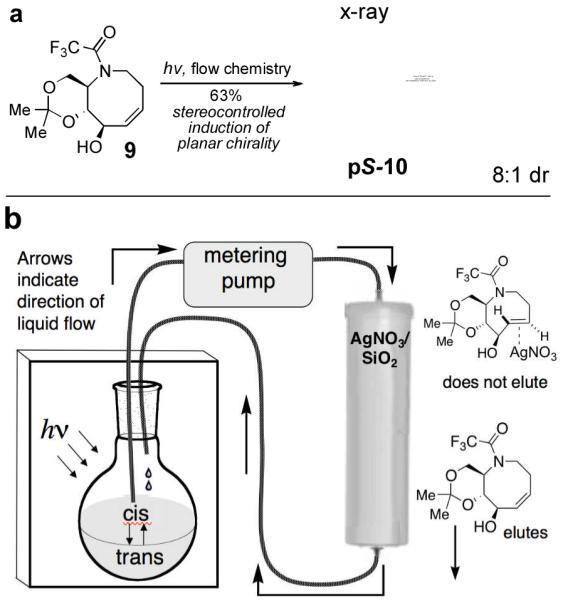
(a) Flow photoisomerization of 9 provides 10 in 8:1 dr, favoring the pS-diastereomer. (b) Schematic description of the flow photochemical apparatus
The total synthesis of hyacinthacine A2 (1) was completed as shown in Scheme 4. The trifluoroacetyl group of pS-10 was cleaved through treatment with MeLi. Without purification, treatment with hydrochloric and acetic acids removed the acetonide to give triol 11 as the ammonium salt. 5-Aza-trans-cyclooctene 11 smoothly underwent transannular hydroamination to give hyacinthacine A2 (1) upon treatment with aqueous ammonium hydroxide and pH adjustment with aqueous hydrochloric acid. In the hydroamination, the absolute planar chirality of 11 is transferred to the C-7a stereocenter of 1 with excellent fidelity: only a single diastereomer of 1 is obtained in the transannular reaction.
Scheme 4.
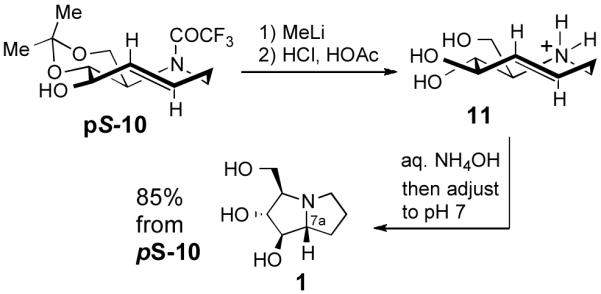
Synthesis of hyacinthacine A2 via transannular hydroamination with planar-to-point chirality transfer
Conclusions
The total synthesis of hyacinthacine A2 is reported using a novel transannular hydroamination of a 5-aza-trans-cyclooctene precursor in which planar chirality is transferred to point chirality in the product. Key to the success of this strategy was the development of a method for establishing absolute planar chirality via stereocontrolled photoisomerization of a 5-aza-cis-cyclooctene.
Supplementary Material
Acknowledgments
We thank Glenn Yap for X-ray crystallography. This work was supported by NIH Grant Number GM068640-01. Spectra were obtained with instrumentation supported by NSF CRIF:MU grants: CHE 0840401 and CHE-0541775.
Footnotes
Electronic Supplementary Information (ESI) available: Provided are experimental and characterization details, 1H and 13C NMR spectra for new compounds, and the CIF file for pS-10. See DOI: 10.1039/b000000x/
References
- 1.Stocker BL, Dangerfield EM, Win-Mason AL, Haslett GW, Timmer MSM. Eur. J. Org. Chem. 2010;2010:1615–1637. [Google Scholar]; López MD, Cobo J, Nogueras M. Curr. Org. Chem. 2008;12:718–750. [Google Scholar]; Pyne SG, Tang M. Curr. Org. Chem. 2005;9:1393–1418. [Google Scholar]; Liddell JR. Nat. Prod. Rep. 2002;19:773–781. doi: 10.1039/b108975g. [DOI] [PubMed] [Google Scholar]
- 2.Syntheses of Hyacinthacine A2: Rambaud L, Compain P, Martin OR. Tetrahedron: Asymmetry. 2001;12:1807–1809. Cardona F, Faggi E, Liguori F, Cacciarini M, Goti A. Tetrahedron Lett. 2003;44:2315–2318. Izquierdo I, Plaza MT, Franco F. Tetrahedron: Asymmetry. 2003;14:3933–3935. Desvergnes S, Py S, Vallée Y. J. Org. Chem. 2005;70:1459–1462. doi: 10.1021/jo048237r. Dewi-Wülfing P, Blechert S. Eur. J. Org. Chem. 2006;2006:1852–1856. Calveras J, Casas J, Parella T, Joglar J, Clapés P. Adv. Syn. Catal. 2007;349:1661–1666. Ribes C, Falomir E, Carda M, Marco JA. Tetrahedron. 2009;65:6965–6971. Delso I, Tejero T, Goti A, Merino P. Tetrahedron. 2010;66:1220–1227. Liu W-J, Ye J-L, Huang P-Q. Org. Biomol. Chem. 2010;8:2085. doi: 10.1039/b926741g.
- 3.Selected syntheses of australine and alexine: Denmark SE, Martinborough EA. J. Am. Chem. Soc. 1999;121:3046–3056. Furneaux RH, Gainsford GJ, Mason JM, Tyler PC. Tetrahedron. 1994;50:2131–2160. Pearson WH, Hines JV. Tetrahedron Lett. 1991;32:5513–5516. Pearson WH, Hines JV. J. Org. Chem. 2000;65:5785–5793. doi: 10.1021/jo000689q. Romero A, Wong CH. J. Org. Chem. 2000;65:8264–8268. doi: 10.1021/jo000933d. Trost BM, Aponick A, Stanzl BN. Chem. Eur. J. 2007;13:9547–9560. doi: 10.1002/chem.200700832. Dressel M, Restorp P, Somfai P. Chem. Eur. J. 2008;14:3072–3077. doi: 10.1002/chem.200701776. Fleet GWJ, Haraldsson M, Nash RJ, Fellows LE. Tetrahedron Lett. 1988;29:5441–5444. Takahashi M, Maehara T, Sengoku T, Fujita N, Takabe K, Yoda H. Tetrahedron. 2008;64:5254–5261. Yoda H, Katoh H, Takabe K. Tetrahedron Lett. 2000;41:7661–7665.
- 4.White JD, Hrnciar P. J. Org. Chem. 2000;65:9129–9142. doi: 10.1021/jo0012748. [DOI] [PubMed] [Google Scholar]; White JD, Hrnciar P, Yokochi AFT. J. Am. Chem. Soc. 1998;120:7359–7360. [Google Scholar]
- 5.Lauritsen A, Madsen R. Org. Biomol. Chem. 2006;4:2898–2905. doi: 10.1039/b605818c. [DOI] [PubMed] [Google Scholar]
- 6.Asano N, Nash RJ, Molyneux RJ, Fleet GWJ. Tetrahedron:Asymmetry. 2000;11:1645–1680. [Google Scholar]; Kato A, Kano E, Adachi I, Molyneux RJ, Watson AA, Nash RJ, Fleet GWJ, Wormald MR, Kizu H, Ikeda K, Asano N. Tetrahedron:Asymmetry. 2003;14:325–331. [Google Scholar]
- 7.Taylor DL, Nash R, Fellows LE, Kang MS, Tyms AS. Antiviral Chemistry & Chemotherapy. 1992;3:273–277. [Google Scholar]
- 8.Asano N, Kuroi H, Ikeda K, Kizu H, Kameda Y, Kato A, Adachi I, Watson AA, Nash RJ, Fleet GWJ. Tetrahedron:Asymmetry. 2000;11:1–8. [Google Scholar]
- 9.Tropea JE, Molyneux RJ, Kaushal GP, Pan YT, Mitchell M, Elbein AD. Biochemistry. 1989;28:2027–2034. doi: 10.1021/bi00431a010. [DOI] [PubMed] [Google Scholar]
- 10.Nash RJ, Fellows LE, Dring JV, Fleet GWJ, Girdhar A, Ramsden NG, Peach JM, Hegarty MP, Scofield AM. Phytochemistry. 1990;29:111–114. [Google Scholar]
- 11.Wilson SR, Sawicki RA, Huffman JC. J. Org. Chem. 1981;46:3887–3891. [Google Scholar]; Wilson SR, Sawicki RA. J. Heterocycl. Chem. 1982;19:81–83. [Google Scholar]
- 12.For transannular cyclizations involving nitrogen-centered radicals: Newcomb M, Marquardt DJ, Deeb TM. Tetrahedron. 1990;46:2329–2344.
- 13.Subramanian T, Lin C-C, Lin C-C. Tetrahedron Lett. 2001;42:4079–4082. [Google Scholar]
- 14.Brock EA, Davies SG, Lee JA, Roberts PM, Thomson JE. Org. Lett. 2011;13:1594–1597. doi: 10.1021/ol103090z. [DOI] [PubMed] [Google Scholar]
- 15.Hydroamination review: Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Chem. Rev. 2008;108:3795–3892. doi: 10.1021/cr0306788. Also see: Cochran BM, Michael FE. J. Am. Chem. Soc. 2008;130:2786–2792. doi: 10.1021/ja0734997. For an transannular, Pd-catalyzed hydroamination to form tropenes: Sakai N, Ridder A, Hartwig JF. J. Am. Chem. Soc. 2006;128:8134–8135. doi: 10.1021/ja061349a.
- 16.Udding JH, Papin N, Hiemstra H, Speckamp WN. Tetrahedron. 1994;50:8853. [Google Scholar]
- 17.Nubbemeyer U. Eur. J. Org. Chem. 2001;2001:1801–1816. [Google Scholar]; Edstrom ED. J. Am. Chem. Soc. 1991;113:6690–6692. [Google Scholar]; Sudau A, Münch W, Nubbemeyer U, Bats JW. J. Org. Chem. 2000;65:1710–1720. doi: 10.1021/jo991484o. [DOI] [PubMed] [Google Scholar]; Surprenant S, Lubell WD. Org. Lett. 2006;8:2851–2854. doi: 10.1021/ol0609863. [DOI] [PubMed] [Google Scholar]
- 18.Recent studies on E-silacycloalkenes: Prévost M, Woerpel KA. J. Am. Chem. Soc. 2009;131:14182–14183. doi: 10.1021/ja906204a. Prévost M, Ziller JW, Woerpel KA. Dalton Transactions. 2010;39:9275–9275. doi: 10.1039/c003227a. Ventocilla CC, Woerpel KA. J. Am. Chem. Soc. 2011;133:406–408. doi: 10.1021/ja109631z.
- 19.Recent studies on formation and conformational control in E-cyclononenes: Tomooka K, Komine N, Fujiki D, Nakai T, Yanagitsuru S.-i. J. Am. Chem. Soc. 2005;127:12182–12183. doi: 10.1021/ja053347g. Tomooka K, Suzuki M, Shimada M, Yanagitsuru S.-i., Uehara K. Org. Lett. 2006;8:963–965. doi: 10.1021/ol053141k. Tomooka K, Akiyama T, Man P, Suzuki M. Tetrahedron Lett. 2008;49:6327–6329. Tomooka K, Uehara K, Nishikawa R, Suzuki M, Igawa K. J. Am. Chem. Soc. 2010;132:9232–9233. doi: 10.1021/ja1024657.
- 20.Recent total synthesis of rhazinilam with transfer of axial-to-point chirality: Gu Z, Zakarian A. Org. Lett. 2010;12:4224–4227. doi: 10.1021/ol101523z.
- 21.Cerè V, Peri F, Pollicino S, Antonio A. J. Chem. Soc, Perkin Trans. II. 1998:977–980. [Google Scholar]
- 22.Cerè V, Paolucci C, Pollicino S, Sandri E, Fava A. J. Org. Chem. 1991;56:4513–4520. [Google Scholar]; Cerè V, Paolucci C, Pollicino S, Sandri E, Fava A. J. Org. Chem. 1992;57:1457–1461. [Google Scholar]
- 23.Royzen M, Yap GPA, Fox JM. J. Am. Chem. Soc. 2008;130:3760–3761. doi: 10.1021/ja8001919. [DOI] [PubMed] [Google Scholar]
- 24.Moran J, Cebrowski PH, Beauchemin AM. J. Org. Chem. 2008;73:1004–1007. doi: 10.1021/jo701985w. [DOI] [PubMed] [Google Scholar]
- 25.Maeda R, Wada T, Mori T, Kono S, Kanomata N, Inoue Y. J. Am. Chem. Soc. 2011;133:10379–10381. doi: 10.1021/ja203781f. Inoue Y, Yokoyama T, Yamasaki N, Tai A. J. Am. Chem. Soc. 1989;111:6480–6482. Yamasaki N, Inoue Y, Yokoyama T, Tai A. J. Photochem. Photobiol. A-Chemistry. 1989;48:465–465. Inoue Y, Yamasaki N, Yokoyama T, Tai A. J. Org. Chem. 1993;58:1011–1018. Inoue Y, Matsushima E, Wada T. J. Am. Chem. Soc. 1998;120:10687–10687. Inoue T, Matsuyama K, Inoue Y. J. Am. Chem. Soc. 1999;121:9877–9877. For a recent review on the use of photochemistry in natural product synthesis, see Bach T, Hehn JP. Angew. Chem. Int. Ed. 2011;50:1000–1045. doi: 10.1002/anie.201002845. 2011.
- 26.Taylor MT, Blackman ML, Dmitrenko O, Fox JM. J. Am. Chem. Soc. 2011;133:9646–9649. doi: 10.1021/ja201844c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvaraj R, Liu S, Hassink M, Huang C.-w., Yap L.-p., Park R, Fox JM, Li Z, Conti PS. Bioorg. Med. Chem. Lett. 2011 doi: 10.1016/j.bmcl.2011.04.116. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li Z, Cai H, Hassink M, Blackman ML, Brown RCD, Conti PS, Fox JM. Chem. Commun. 2010;46:8043–8043. doi: 10.1039/c0cc03078c. [DOI] [PMC free article] [PubMed] [Google Scholar]; Blackman ML, Royzen M, Fox JM. J. Am. Chem. Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bach RD. J. Am. Chem. Soc. 2009;131:5233–5243. doi: 10.1021/ja8094137. [DOI] [PubMed] [Google Scholar]
- 29.Williams CM, Mander LN. Tetrahedron. 2001;57:425–447. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


