Abstract
Hypersecretion of mucin plays an important role in the pathophysiology of many inflammatory airway diseases, including asthma, chronic bronchitis, and cystic fibrosis. Myristoylated alanine-rich C-kinase substrate (MARCKS) protein has been shown to play an important role in regulation of airway mucin secretion, as peptides analogous to the amino (N)-terminus of MARCKS attenuate mucin secretion by airway epithelium in vitro and in vivo. Here, we investigated a potential role for the protease Calpain, a calcium-dependent cysteine protease that can cleave MARCKS, in the MARCKS-related secretory mechanism. We theorized that Calpain might cleave MARCKS near the N-terminus, thereby attenuating the ability of MARCKS to bind to membranes and/or creating a small N-terminal peptide that could act as a competitive intracellular inhibitor to remaining endogenous full-length MARCKS molecules. Primary normal human bronchial epithelial (NHBE) cells and the virally-transformed human bronchial epithelial HBE1 cell line were exposed to phorbol-12-myristate-13-acetate (PMA) to stimulate the Protein Kinase C (PKC) pathway, leading to enhanced mucin secretion, and Calpain activity within the cells was measured with a fluorescent cleavage assay. Calpain activity was increased by PMA, and pretreatment of the cells with Calpain inhibitors reduced both Calpain activity and mucin secretion in a concentration-dependent manner. Thus, as opposed to the original hypothesis, inactivating Calpain caused a decrease rather than an increase in secretion. HBE1 cells transfected with DNA constructs encoding a MARCKS-YFP fusion protein showed cleavage at a putative site near the N-terminus in response to PMA. Cleavage of MARCKS by Calpain may have an important role in regulation of the PKC/MARCKS pathway regulating airway mucin secretion.
Keywords: Airway, Mucin, MARCKS, Calpain, Secretion
1. Introduction
Hypersecretion of mucus plays an important role in the pathophysiology of many inflammatory airway diseases, as excessive mucus can obstruct airways, leading to inhibited respiration, enhanced susceptibility to infection, and even mortality. Mucus is a gel made up of water and mucins, which are complex glycoproteins 20 to 200kDa in size. They can be extensively post-translationally modified by myristoylation, glycosylation and/or phosphorylation, and their carbohydrate content may account for between 50-90% of their total mass. It is mucins that give mucus its viscosity and elasticity [1]. At least 20 different human mucins have been identified, and they are present in the respiratory, gastrointestinal, ocular and reproductive systems as either membrane-tethered or secreted. In the respiratory system, 11 different mucin genes have been identified at the protein or mRNA level, and the mucins MUC5AC, MUC5B, MUC19 and MUC2 are secreted by both goblet cells and submucosal glands, with MUC5AC and MUC5B predominating [2].
Myristoylated alanine-rich C-kinase substrate (MARCKS) protein, specifically the evolutionarily-conserved N-terminal region, has been shown to have an important role in airway mucin secretion, as peptides analogous to the N - terminus of MARCKS attenuate mucin secretion by airway epithelial cells both in vitro [3-5] and in vivo [6-9]. MARCKS can be activated by PKC, especially the delta isoform, in airway epithelium [10,11]
Calpain is a calcium-dependent cysteine protease lacking a specific primary sequence for cleavage; it is thought to have site recognition based on tertiary structure. Interestingly, Calpain has been shown to cleave MARCKS protein, although the location(s) of the cleavage site(s) are still uncertain [12,13]. Reducing the expression of Calpain genes leads to accumulation of MARCKS in cultured myogenic cells [14]. Calpain cleavage may increase accessibility of the phosphorylation site domain (PSD) on MARCKS, thereby increasing its ability to bind actin [15]. This cleavage site is possibly the same site identified between asparagine 147 and glutamate 148 in the bovine sequence of MARCKS, only three amino acids away from the amino-terminal side of the PSD [13]. It also has been speculated that MARCKS may be cleaved between the 6th and 7th amino acid from the N-terminus [12].
A convergent point between MARCKS and Calpain activation in these cells could be phospholipase C, a common cellular signaling pathway. Activated phospholipase C will cleave phosphatidylinositol into diacylglycerol (DAG) and inositol triphosphate (IP3). DAG can then go on to activate PKC, which phosphorylates MARCKS, while IP3 binds to specific receptors on endoplasmic reticular membranes, resulting in release of intracellular stores of calcium into the cytoplasm, which may activate Calpains (along with other calcium-sensitive molecules) [16].
In the studies described here, a possible role for Calpain in modulating the MARCKS-related mechanism of airway mucin secretion was investigated, utilizing cultured primary human airway epithelial cells and a virally-transformed human airway epithelial cell line. The results suggest that inhibition of Calpain in these cells decreases mucin secretion in response to PKC activation, and that MARCKS might be cleaved near its N-terminus by Calpain during the secretory event. The exact role of Calpain in the MARCKS-related secretion mechanism, however, remains speculative.
2. Materials & Methods
2.1. Cell Culture
Primary normal human bronchial epithelial (NHBE) cells, purchased from Lonza (Walkersville, MD), and human papilloma virus-transformed bronchial epithelial cells (HBE1 cells; a generous gift from Dr. Reen Wu, University of California, Davis, CA) [17] were seeded on Corning® Transwell® collagen-coated membrane inserts and maintained in a humidified air/5% CO2 incubator for 14 days in air-liquid interface culture, as described previously [3,18]. Two different cell types were used in these studies:HBE1 cells were used when experiments called for molecular manipulations (eg transfection) as the transfection efficiency is low (~ 5%) for primary NHBE cells and over 50% for HBE1 cells. When studies were complementary to these molecular manipulations, HBE1 cells were used for consistency. Studies on mucin secretion were performed using primary NHBE cells because these cells produce more mucin than the HBE1 cell line.
2.2. Treatments
NHBE or HBE1 cells were exposed to 250nM PMA (EMD Biosciences, La Jolla, CA) for 3 min to provoke mucin secretion. Two separate inhibitors of Calpain, Z-LLY-FMK (MBL International Corporation, Woburn, MA) or Z-LLY-CHO (Enzo Life Sciences, Farmingdale, NY) were added at 20 μM to cells for 15 min prior to addition of PMA (or medium control) and Calpain activity or mucin secretion after exposure to PMA for the indicated time periods measured as described below. All reagents were applied both apically and basolaterally.
2.3. Calpain Activity Assay
After exposure of cells to PMA (or control) for 30 min, cells were assayed for Calpain activity using a Calpain Activity Assay Kit (Abcam, Cambridge, MA) according to the manufacturer’s suggestions. Cell lysates were exposed to substrate bound to AFC fluorophore (7-Amino-4-trifluoromethylcoumarin). Calpain cleaves the substrate, releasing the AFC fluorophore and allowing it to fluoresce. Relative activity of Calpain vs. a standard was then determined using a fluorescent plate reader.
2.4. Measurement of Mucin Secretion
Mucin was collected and assayed as described previously [3]. Briefly, after “baseline” mucin samples were collected, cells were rested overnight and exposed to test reagents the next day for indicated times. After each treatment period, secreted mucin was collected as the treatment sample and quantified by sandwich enzyme-linked immunosorbent assay using the 17Q2 antibody (Covance Research Products, Berkeley, CA), a monoclonal antibody that reacts specifically with a carbohydrate epitope on human airway mucins [19]. The 17Q2 antibody was purified using an ImmunoPure(G) IgG purification kit (Pierce Biotechnology, Rockford, IL) following the manufacturer’s protocol and then conjugated with alkaline phosphatase (EMD Biosciences). To account for variability between cultures and experiments, levels of mucin secretion were reported relative to non-treated controls, as described previously [3].
2.5. Transfections
HBE1 cells were grown to approximately 50% confluence and transfected with plasmids containing yellow fluorescent protein (YFP) fused to the N-terminus of full length human MARCKS. FuGENE® 6 (Roche, Indianapolis, IN) was used as the transfection reagent according to the manufacturer’s protocol. Forty-eight hrs later, cells were exposed to either 100 uM ATP (a potent activator of Calpain), or control media, for 3 min. Cells were lysed and protein was extracted in buffer containing Complete Mini Protease inhibitor (Roche).
2.6. Western Blot Analysis
Briefly, cells were washed with cold PBS and scraped into lysis buffer [50 mmol/L Tris-Cl (pH 7.6), 1mmol/L ethylenediamine tetraacetic acid, 100 mmol/L NaCl, 100 mmol/L MgCl,1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L dithiothreitol, 1% (v/v) protease inhibitor cocktail, and phosphatase inhibitor cocktail (Sigma, St. Louis, MO)]. Western blots used a primary antibody against GFP (which cross reacts with YFP in the constructs) followed by biotinylated goat anti-mouse secondary (both from Abcam) and then Streptavidin HRP (Santa Cruz, Santa Cruz, CA) After these blots were performed, they were normalized to actin using an antibody from Santa Cruz,
2.7. Statistical Analysis
Graphpad Prism® version 5 was used for the statistical analysis. A one way analysis of variation (ANOVA) was used in the time course study of PMA treated HBE1 cells. Student’s t-test was used for the remainder of the experiments. A p value < 0.05 for the two-tailed t test was considered statistically significant.
3. RESULTS
3.1. Effects of PMA on Calpain Activity
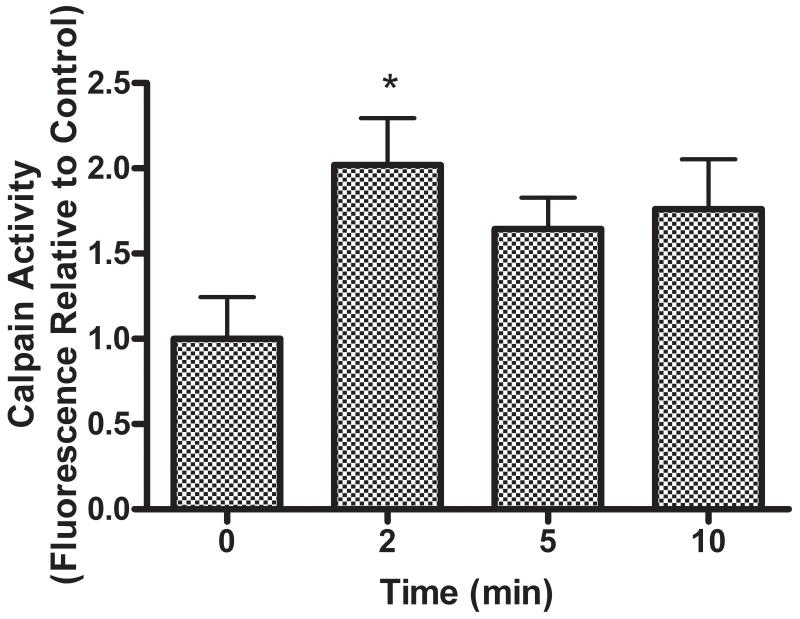
An approximate twofold increase in Calpain activity compared to untreated cells was observed in HBE1 cells exposed to 250nM PMA within a two min period post exposure. This activity appeared to plateau and remained elevated for at least 10 min (Figure 1).
Figure 1.
PMA exposure increases Calpain activity in airway epithelial cells. Relative levels of Calpain activity in HBE1 cells after stimulation with 250nM PMA are shown. Label on X axis (time) refers to time post PMA addition. Calpain activity is significantly increased within 2 min of exposure to PMA. (* p<0.05 relative to 0 time control, all values expressed as mean ± SEM, n=3).
3.2. Effects of Calpain Inhibitors on Calpain Activity
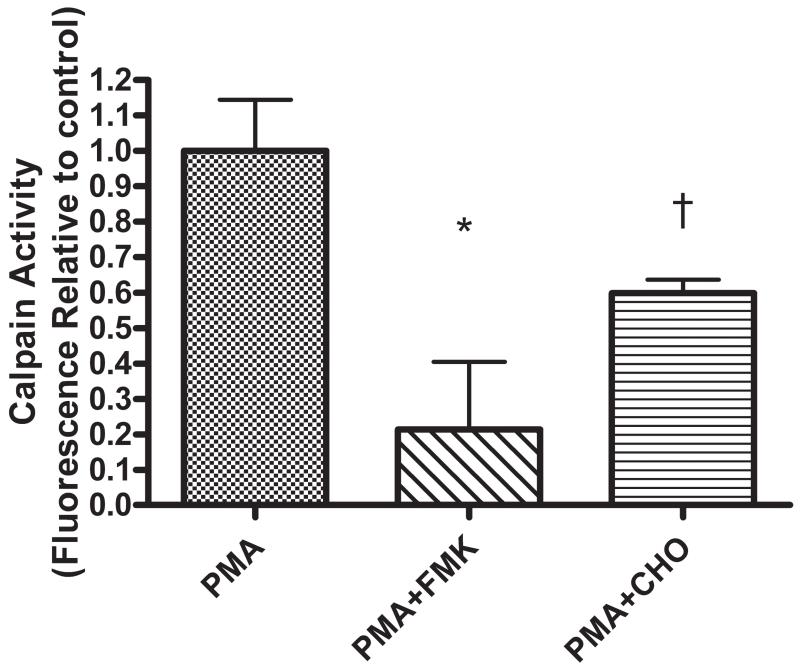
Pretreatment with 20 μM of the commercially available Calpain inhibitors Z-LLY-FMK or Z-LLY-CHO for 15 minutes significantly decreased the PMA-induced enhancement of Calpain activity in HBE1 cells (Fig 2).
Figure 2.
Calpain inhibitors decrease Calpain activity in airway epithelium. HBE1 cells were exposed to 250nM PMA for 3 min after 15 min pretreatment with 20 μM of the Calpain inhibitors Z-LLY-FMK or Z-LL-CHO (* p<0.05 † p<0.005 relative to PMA - stimulated, all values expressed as mean ± SEM, n=3).
3.3. Effects of Calpain Inhibitors on Mucin Secretion
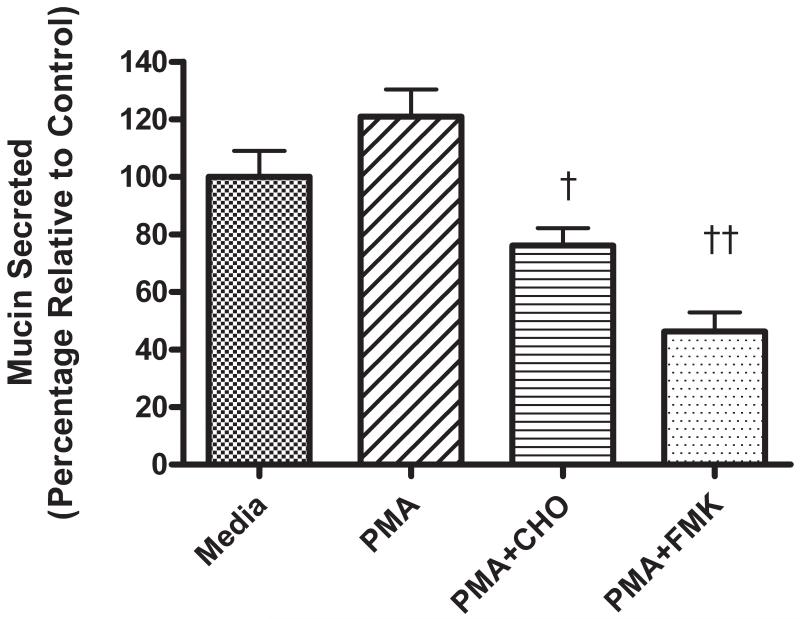
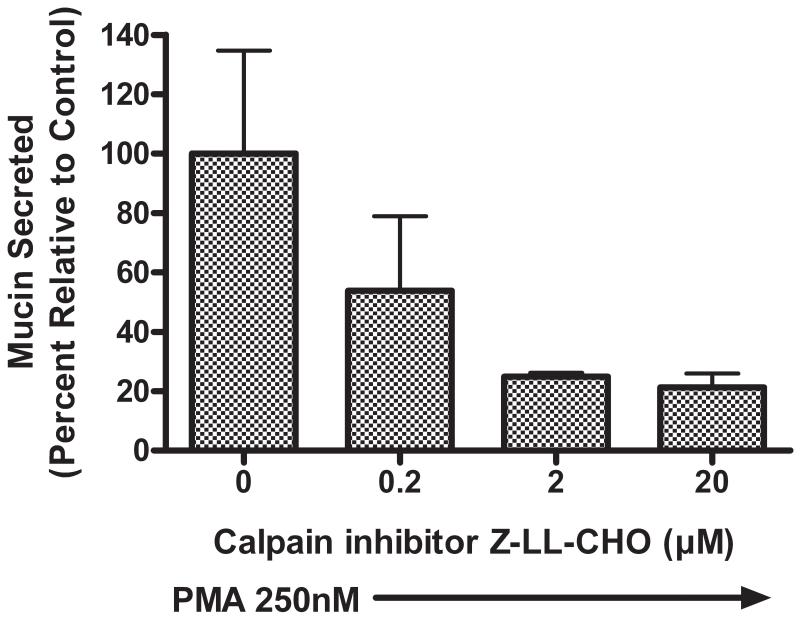
As illustrated in Figure 3, pretreatment of NHBE cells for 15 min with 20 μM of either Z-LLY-FMK (FMK) or Z-LLY-CHO (CHO) significantly attenuated mucin secretion in response to exposure of the cells to 250nM PMA. When NHBE cells were pretreated for 15 min with CHO over a range of concentrations (0.2 - 20 μM), there was a concentration-dependent attenuation of mucin secretion in response to PMA measured 3 min after PMA exposure (Figure 4).
Figure 3.
Pretreatment of airway epithelial cells with Calpain inhibitors attenuates mucin secretion. Relative levels of mucin secretion in NHBE cells after treatment with 250nM PMA with or without pre-treatment with Calpain inhibitors: 20μM Z-LLY-CHO (CHO) or 20μM Z-LLY-FMK (FMK) for 15 min. Secretion was measured 3 min after PMA addition. († p<0.01, †† p<0.001 relative to PMA-stimulated, all values expressed as mean ± SEM, n=4).
Figure 4.
Pretreatment of NHBE cells for 15 min with the Calpain inhibitor Z-LL-CHO (CHO) attenuates mucin secretion in response to 250nM PMA in a concentration-dependant manner. Secretion was measured 3 minutes after PMA addition and is normalized to media control. (* P < 0.05 relative to PMA-stimulated, all values expressed as mean ± SEM, n=3).
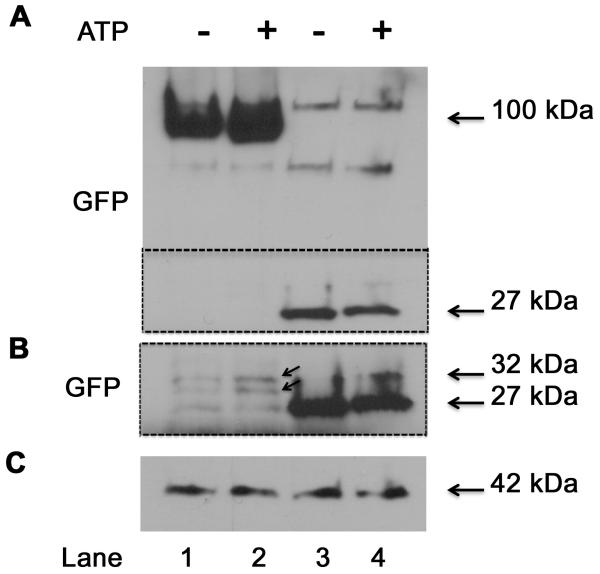
3.4. Western Blot Analysis of Cells Transfected with MARCKS Constructs
HBE 1 cells transfected with the YFP-labeled MARCKS showed, upon Western blot of lysed cells, enhanced induction of potential cleavage products after ATP exposure and thus presumptive Calpain activity. As shown in Figure 5, these cleavage products were observed at a molecular weight of approximately 27-32 kDa, suggesting that Calpain cleaves MARCKS near the YFP-tagged amino terminus.
Figure 5.
Western Blot probing for YFP (using antibody against GFP) in HBE1 cells transfected with YFP fused to the N-terminus of MARCKS. A) the entire blot; B) the area of the blot within the dotted line in “A”; this region was exposed longer to bring out blot details; C) actin used for normalization. Lanes 1,2: MARCKS constructs; Lanes 3,4: empty vector. Treatment of cells for 3 min with 100 μM ATP (B; lane 2) shows apparent induction of MARCKS cleavage products of molecular weight 27 - 32 kDa (indicated by arrows).
4. Discussion
In this study, Calpain was shown to be activated in response to PMA in human airway epithelial cells in vitro. In addition to its previously-described secretagogue effect on airway mucin secretion [3,5], PMA also activates a number of PKC (typical and atypical) channels, which could then open calcium channels in the plasma membrane, with the resultant increase in intracellular calcium potentially activating Calpain proteases. The increased activity of Calpains in response to PMA in airway epithelial cells was effectively blocked by pretreatment of cells with Calpain inhibitors, and, interestingly, pretreatment with these Calpain inhibitors caused a decrease in PMA-provoked secretion of mucin. It is interesting to note that mucin secretion in cells treated with FMK (see Figure 3) was attenuated to a level below that seen in control cells. This is a phenomenon that occurs often in cells exposed to strong inhibitors of various molecules involved in the secretory process, as was seen with MARCKS-inhibitory peptides both in vitro and in vivo in previous studies [3,6]. This would indicate that Calpain could be involved in both stimulated and constitutive mucin secretion by human airway epithelial cells. Of the two commercially available inhibitors, FMK appears to be the more potent in attenuating both Calpain activity mucin secretion, as equimolar concentrations appeared to have a greater inhibitory effect on both parameters (see Figures 3 and 4). In extracts of HBE1 cells, which were transfected with a fusion protein of MARCKS with YFP joined to the N-terminus, a band possibly corresponding to a protein fragment where MARCKS has been cleaved near the N-terminus was present in Western Blots probed for GFP/YFP. This fragment could be the result of Calpain cleaving MARCKS near the N-terminus, possibly at a putative cleavage site between Lysine 6 and Threonine 7 [12].
MARCKS is a highly conserved and ubiquitously expressed protein. It has been shown to have major roles in, for example: secretion, including airway mucin secretion [3-11], cellular migration [20-24] brain development [25], cell adhesion [14], phagocytosis [26], and cell cycle regulation [27]. While precise mechanisms for these diverse functions remain unkown, MARCKS is known to interact with cytoskeletal components and can bind and crosslink actin filaments within cells [28]. Structurally, MARCKS contains three evolutionarily conserved sites: a multiple homology (MH2) region of unknown function, an effector or phosphorylation site domain (PSD) that serves as the site for PKC phosphorylation as well as binding of actin and calmodulin, and a myristoylated, hydrophobic N-terminal domain associated with binding of MARCKS to the plasmalemma and other intracellular membranes.
In elucidating the function of MARCKS, the N-terminus recently has received much interest. This highly conserved domain has been shown to have an important role in airway mucin secretion based on inhibition of secretion, both in vitro and in vivo, by a peptide, the MANS peptide, which corresponds to the conserved myristic acid + 24 amino acid - containing N-terminal region of MARCKS [3]. Although the exact nature of the inhibition of MARCKS function in the secretory process by the MANS peptide is uncertain, it may act as a competitive inhibitor for endogenous, full length MARCKS, preventing MARCKS from interacting with membranes of secretory granules as part of the mucin secretion process [6]. Calpains are calcium-activated cysteine proteases. Calpains 1 and 2 (known as μ- and m-calpain, respectively, from the relative molarity of calcium needed to activate them) are ubiquitously expressed. Calpains do not cleave based upon primary sequence of their substrates. Rather, they are thought to recognize their cleavage sites based upon the higher order structure of the substrate. Calpain 1 has been demonstrated to cleave MARCKS on the N-terminal side of the PSD in a manner dependent on the activity of PKC phosphorylation of MARCKS. MARCKS cleaved at this site appears only in cytoplasm, as MARCKS bound to the cell membrane does not appear susceptible to Calpain cleavage [12,13]. MARCKS and Calpain 2 have been shown to co-localize in caveolae, and Calpain can indirectly affect MARCKS phosphorylation by modulating PKC activity and thereby affecting MARCKS binding to membranes and translocation to the cytosol after it is phosphorylated [29].
The original hypothesis behind these studies was that Calpain-induced cleavage of MARCKS protein would inactivate MARCKS, therefore increased Calpain activity would increase secretion of mucin in airway epithelial cells. However, the results appeared to be opposite of what was hypothesized; inactivating Calpain caused a decrease in secretion. If, as it seems, cleavage of MARCKS by Calpain occurs at the predicted site between the 6th and 7th amino acid of the N-terminus, as has been suggested [2], this cleavage would separate the bulk of the molecule from its intensely hydrophobic end. Alternately, if the relevant site of MARCKS cleavage is the other known site, close to the PSD, this could allow for more favorable access of the site to allow MARCKS to bind to actin, leading to increased actin crosslinking. How these factors affect the MARCKS-related mechanism of mucin secretion is not known, and clearly additional studies to determine the mechanism of Calpain-induced modulation of mucin secretion in these cells clearly are needed. For example, the exact site of Calpain cleavage of MARCKS needs to be determined, as does the role of cellular compartmentalization and intracellular localization of Calpain where it can affect MARCKS function in secretion. Other important information may relate to, for example, determining the specific Calpain gene(s) that are important in this function and/or determining other potential cellular targets of Calpain whose cleavage may be relevant to the secretory process. Clearly, there is a connection between Calpain, MARCKS and mucin secretion, but at this point this connection has not been fully elucidated.
It also should be pointed out that these studies were done with both a cell line (HBE1 cells) and primary human bronchial epithelial cells (NHBE) maintained in air/liquid interface culture to maintain their differentiated characteristics. In order to elucidate more about the role of Calpain in airway mucin secretion, it is clear that in vivo studies using physiologically relevant stimuli for mucin secretion in animal models would be an appropriate next step. In fact, most of our work in the literature related to mechanisms of MARCKS function in secretion were first reported using these in vitro approaches [3-5, 10, 11] and later expanded to animal studies [6-9].
In conclusion, the results of this study show that Calpain is activated in human airway epithelial cells by exposure to PMA, suggesting that PKC increases Calpain activity by raising intracellular Ca++; treatment of airway epithelial cells with commercially available Calpain inhibitors reduces Calpain activity in these cells; inhibiting Calpain activity appears to attenuate mucin secretion, and a MARCKS YFP fusion protein transfected into airway epithelial cells appears to be cleaved at a site near the N-terminus, possibly at a suspected Calpain cleavage site.
Acknowledgements
Funded by grant # R37 HL36982 from NIH. The authors thank of Dr. Reen Wu (University of California, Davis) for his generous gift of HBE1 cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–78. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 2.Thai P, Loukoianov A, Wachi S, Wu R. Regulation of airway mucin gene expression. Annu Rev Physiol. 2008;70:405–29. doi: 10.1146/annurev.physiol.70.113006.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Martin LD, Spizz G, Adler KB. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem. 2001;276:40982–90. doi: 10.1074/jbc.M105614200. [DOI] [PubMed] [Google Scholar]
- 4.Lin K-W, Park j, Fang S, Crews AL, Adler KB. MARCKS and related chaperones bind to unconventional myosin V isoforms in airway epithelial cells. Am J Resp Cell Molec Biol. 2010;43:131–36. doi: 10.1165/rcmb.2010-0016RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JJ, Fang S, Crews AL, Lin K-W, Adler KB. MARCKS regulation of mucin secretion by airway epithelial cells in vitro: interactions with chaperone proteins. Am J Resp Cell Mol Biol. 2008;102:949–55. doi: 10.1165/rcmb.2007-0139OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–6. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 7.Foster WM, Adler KB, Crews AL, Potts EN, Fischer BM, Voynow JA. MARCKS-related peptide modulates in vivo secretion of airway Muc5ac. Am J Physiol:Lung Cell Mol Physiol. 2010;299:L345–52. doi: 10.1152/ajplung.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Agrawal A, Murphy EC, III, Park J, Adler KB, Parikh I. Aerosolized BIO-11006, a novel MARCKS-related peptide, improves airway obstruction in a mouse model of mucus hypersecretion. J Epith Biol Pharmacol. 2011;3:13–20. [Google Scholar]
- 9.Agrawal A, Rengarajan S, Adler KB, Ram A, Ghosh B, Fahim M, Dickey BF. Inhibition of mucin secretion with MARCKS-related peptide improves airflow obstruction in a mouse model of asthma. J Appl Physio. 2007;102:399–405. doi: 10.1152/japplphysiol.00630.2006. [DOI] [PubMed] [Google Scholar]
- 10.Park J-A, Park JJ, Fang S, Crews AL, Lampe WR, Adler KB. Protein Kinase Cδ regulates mucin secretion via phosphorylation of MARCKS protein in human airway epithelial cells in vitro. Am J Pathol. 2007;171:1822–1830. doi: 10.2353/ajpath.2007.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J-A, He F, Martin LD, Li Y, Adler KB. Human neutrophil elastase induces hypersecretion of mucin from human bronchial epithelial cells in vitro via a PKCδ - mediated mechanism. Am J Pathol. 2005;167:651–661. doi: 10.1016/s0002-9440(10)62040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun T, McIlhinney RA, Vergeres G. Myristoylation-dependent N-terminal cleavage of the myristoylated alanine-rich C kinase substrate (MARCKS) by cellular extracts. Biochimie. 2000;82:705–715. doi: 10.1016/s0300-9084(00)01154-8. [DOI] [PubMed] [Google Scholar]
- 13.Manenti S, Taniguchi H, Sorokine O, Van Dorsselaer A, Darbon JM. Specific proteolytic cleavage of the myristoylated alanine-rich C kinase substrate between Asn 147 and Glu 148 also occurs in brain. J Neurosci Res. 1997;48:259–63. doi: 10.1002/(sici)1097-4547(19970501)48:3<259::aid-jnr8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Dedieu S, Poussard S, Mazeres G, Grise F, Dargelos E, Cottin P, et al. Myoblast migration is regulated by calpain through its involvement in cell attachment and cytoskeletal organization. Exp Cell Res. 2004;292:187–200. doi: 10.1016/j.yexcr.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Tapp H, Al-Naggar IM, Yarmola EG, Harrison A, Shaw G, Edison AS, et al. MARCKS is a natively unfolded protein with an inaccessible actin-binding site. J Biol Chem. 2005;280:9946–56. doi: 10.1074/jbc.M414614200. [DOI] [PubMed] [Google Scholar]
- 16.Fukami K, Inanobe S, Kanemaru K, Nakamura Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog Lipid Res. 2010;49:429–37. doi: 10.1016/j.plipres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Yankaskas JR, Haizlip JE, Conrad M, Koval D, Lazarowski E, Paradiso AM, et al. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am J Physiol. 1993;264:C1219–30. doi: 10.1152/ajpcell.1993.264.5.C1219. [DOI] [PubMed] [Google Scholar]
- 18.Krunkosky TM, Martin LD, Fischer BM, Voynow JA, Adler KB. Effects of TNFα on expression of ICAM-1 in human airway epithelial cells in vitro. Oxidant mediated pathways of transcription factor activation. Free Rad Biol Med. 2003;35:1158–67. doi: 10.1016/s0891-5849(03)00498-2. [DOI] [PubMed] [Google Scholar]
- 19.Lin H, Carlson DM, St George JA, Plopper CG, Wu R. An ELISA method for the quantitation of tracheal mucins from human and nonhuman primates. Am J Respir Cell Mol Biol. 1989;1:41–8. doi: 10.1165/ajrcmb/1.1.41. [DOI] [PubMed] [Google Scholar]
- 20.Dedieu S, Mazeres G, Poussard S, Brustis JJ, Cottin P. Myoblast migration is prevented by a calpain-dependent accumulation of MARCKS. Biol Cell. 2003;95:615–23. doi: 10.1016/j.biolcel.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Dulong S, Goudenege S, Vuillier-Devillers K, Manenti S, Poussard S, Cottin P. Myristoylated alanine-rich C kinase substrate (MARCKS) is involved in myoblast fusion through its regulation by protein kinase Calpha and calpain proteolytic cleavage. Biochem J. 2004;382:1015–23. doi: 10.1042/BJ20040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis M, Zanou N, Van Schoor M, Gailly P. TRPC1 regulates skeletal myoblast migration and differentiation. J Cell Sci. 2008;121:3951–3959. doi: 10.1242/jcs.037218. [DOI] [PubMed] [Google Scholar]
- 23.Eckert BS, Sharief Y, Crews AL, Adler KB, Jones SL. Myristoylated Alanine-Rich C-kinase Substrate (MARCKS) Protein regulation of human neutrophil migration in vitro. Am J Resp Cell Molec Biol. 2010;42:586–94. doi: 10.1165/rcmb.2008-0394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JF, Lankford SM, Adler KB, Brody AR. Mesenchymal stem cells require MARCKS protein for directed chemotaxis in vitro. Am J Resp Cell Mol Biol. 2010;43:253–58. doi: 10.1165/rcmb.2010-0015RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamada H, Zhang YL, Kawai A, Li F, Hibino Y, Hirashima Y, et al. Development-associated myristoylated alanine-rich C kinase substrate phosphorylation in rat brain. Childs Nerv Syst. 2003;19:152–8. doi: 10.1007/s00381-002-0713-x. [DOI] [PubMed] [Google Scholar]
- 26.Carballo E, Pitterle DM, Stumpo DJ, Sperling RT, Blackshear PJ. Phagocytic and macropinocytic activity in MARCKS-deficient macrophages and fibroblasts. Am J Physiol. 1999;277:C163–73. doi: 10.1152/ajpcell.1999.277.1.C163. [DOI] [PubMed] [Google Scholar]
- 27.Los AP, de Widt J, Topham MK, van Blitterswijk WJ, Divecha N. Protein kinase C inhibits binding of diacylglycerol kinase-zeta to the retinoblastoma protein. Biochim Biophys Acta. 2007;1773:352–57. doi: 10.1016/j.bbamcr.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Yarmola EG, Edison AS, Lenox RH, Bubb MR. Actin filament cross-linking by MARCKS: characterization of two actin-binding sites within the phosphorylation site domain. J Biol Chem. 2001;276:22351–58. doi: 10.1074/jbc.M101457200. [DOI] [PubMed] [Google Scholar]
- 29.Goudenege S, Poussard S, Dulong S, Cottin P. Biologically active milli-calpain associated with caveolae is involved in a spatially compartmentalised signalling involving protein kinase C alpha and myristoylated alanine-rich C-kinase substrate (MARCKS) Int J Biochem Cell Biol. 2005;37:1900–10. doi: 10.1016/j.biocel.2005.04.010. [DOI] [PubMed] [Google Scholar]