Abstract
Emerging evidence indicates that many aspects of alcohol and drug dependence involve changes in glutamate transmission. A number of studies have reported that drugs of abuse, including alcohol and cocaine, alter glutamate transport. Extracellular glutamate is regulated by a number of glutamate transporters in various brain regions. Of these transporters, glutamate transporter (GLT1) is a key player in the removal of most of the extracellular glutamate. Similar to neurodegenerative disease models, in which there is dysfunction of the glutamatergic excitatory system, the role of GLT1 has been tested in drug dependence models that show dysfunction of glutamate transmission. We and others have recently found that ceftriaxone, an FDA-approved drug known to elevate GLT1 expression, attenuates cue-induced cocaine relapse. Moreover, we recently found that alcohol-preferring rats treated with ceftriaxone showed a significant dose-dependent reduction in alcohol consumption. We also demonstrated that ceftriaxone-induced upregulation of GLT1 expression was associated with increases in glutamate uptake in Huntington’s disease mouse model. Importantly, ceftriaxone is currently in clinical trials for the treatment of amyotrophic lateral sclerosis. This review provides information about the potential therapeutic role of GLT1 for the treatment of alcohol abuse and dependence.
Keywords: GLT1, EAAT2, glutamate, alcohol dependence, alcohol addiction, cocaine, GLAST, EAAT1, glutamate transporters, alcohol-preferring rats, glutamate uptake, cystine-glutamate exchanger, basal extracellular glutamate, nucleus accumbens, prefrontal cortex
I. INTRODUCTION
Alcohol dependence has been and continues to be a widespread phenomenon affecting the lives of many people worldwide. A national epidemiological survey reported that nearly 14 million people in the Unites States meet diagnostic criteria for alcohol use disorders [1]. The National Institute on Alcohol Abuse and Alcoholism defines alcohol dependence as a disease characterized by four symptoms – craving, loss of control, physical dependence and tolerance. Nearly 79,000 annual deaths are caused, directly or indirectly, by excessive alcohol consumption (Center for Disease Control and Prevention, 2008).
The acute reinforcing effects associated with alcohol are due to its pharmacological interaction with various neurotransmitter systems in the brain’s reward and stress circuits. Chronic exposure to alcohol causes changes in neuronal function, thereby precipitating associated symptoms, namely sensitization, tolerance, withdrawal, and dependence [2]. Alcohol tolerance, a common symptom of chronic alcohol consumption, is defined as the reduced response to a given dose of alcohol or the need for greater dose of ethanol to produce a desired level of response. Although the exact mechanism of development of alcohol tolerance remains unknown, studies have indicated the role of the endocannabinoid system in the development of alcohol tolerance [3]. Importantly, administration of N-methyl D-aspartate (NMDA) receptor antagonists induced inhibition of the development of tolerance to alcohol [4]. This demonstrates the implication of glutamatergic system in alcohol tolerance. Alternatively, behavioral sensitization is defined as the gradual enhancement in behavioral response following repeated administration of ethanol. Sensitization is characterized by various neuroadaptations of neurotransmitter systems which promote addictive behavior and hence considered a classified model for drug addiction. For example, studies have shown that dopamine 1 (D1) receptors and mu-opioid receptors play an important role in the development of sensitization to alcohol [5, 6]. In regards to the implication of glutamatergic system in sensitization to alcohol, studies demonstrated that metabotropic glutamate 1 receptor (mGluR1) and NMDA receptor are implicated in the expression of alcohol-induced sensitization [7]. In addition, other studies revealed the existence of links between the alterations of extracellular glutamate levels and alcohol-induced behavioral sensitization [8].
Our focus, in this review, is on the glutamatergic system as a target system for the treatment of alcohol dependence. Glutamate is the major excitatory neurotransmitter in the brain and acts via interaction with various glutamatergic receptors, including NMDA receptors. The glutamatergic system has been implicated in the development of acute reinforcing effects of alcohol. Alcohol interferes with the glutamatergic signal transmission by altering the functions of NMDA receptor as well as metabotropic glutamate receptor subtype 5 (mGluR5) [9, 10]. In addition, alcohol is known to inhibit glutamatergic transmission by blocking NMDA receptors [11, 12]. As a result of a compensatory mechanism, chronic alcohol intake has been shown to be associated with upregulation of NMDA receptors [13, 14]. Moreover, alcohol withdrawal increased the extracellular glutamate levels in the striatum along with heighted sensitivity of NMDA receptors in the nucleus accumbens (NAc) [15, 16].
Furthermore, acute alcohol exposure leads to decreased extracellular glutamate levels and reduced glutamatergic transmission in central reward brain regions, including NAc and amygdala [17, 18]. However, following chronic alcohol exposure, glutamate signal transmission was found to be elevated in the amygdala. Although there are no existing compounds targeting glutamatergic system for the treatment of alcoholism, acamprosate, a GABA agonist, has been suggested to act as non-selective antagonist for NMDA receptors and mGluRs, and thus consequently may block excessive alcohol consumption by reducing the excessive glutamate activity [19]. Importantly, we have recently identified in our laboratory that ceftriaxone, a β-lactam antibiotic known to upregulate glutamate transporter 1 (GLT1) termed also as excitatory amino acid transporter 2 (EAAT2) in human [20–23], reduced alcohol intake in alcohol-preferring (P) rat model [24]. The reduction in alcohol intake was associated with upregulation or activation of GLT1. Thus, GLT1 is considered a target for the treatment of alcohol dependence and addiction. In this review article, the neurocircuitry involved in alcohol-drinking behavior implicating glutamatergic system, the role of GLT1 and other glutamate transporters in development of alcohol dependence, and studies evaluating the effects of GLT1 by ceftriaxone on other drugs of abuse are described in detail.
II. NEUROCIRCUITRY INVOLVING GLUTAMATERGIC SYSTEM IN DRUGS OF ABUSE, INCLUDING ALCOHOL
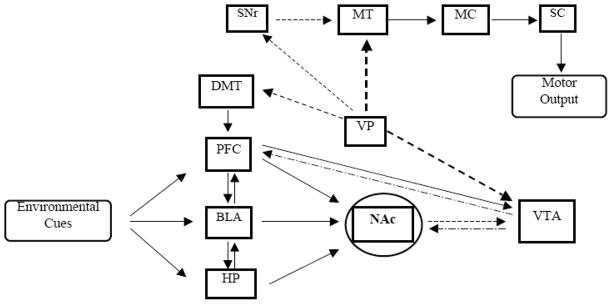
The NAc, located in the ventral striatum (VS), has been well studied for its role in reward mechanism associated with drugs of abuse. It is currently believed that NAc acts as a gateway for limbic structures to reach the motor system [25, 26]. When a novel stimulus, capable of motivating a behavioral response, is encountered, the limbic system is engaged to process new and previously learned information about the stimulus, whereas the prefrontal cortex (PFC) is involved in producing goal oriented behavior [27]. The limbic structures, the basal lateral amygdala (BLA) and the hippocampus, are responsible for emotional processing and making contextual associations, respectively [28, 29]. Moreover, the mesocorticolimbic structures responsible for sensory information processing and action determination relay through the NAc to produce motor actions necessary to execute intended goals. When the NAc is activated through inputs from the PFC and limbic areas, the substantia nigra reticulata and motor thalamus become disinhibited and activate the motor cortex which further projects to the spinal cord to produce movement [30, 31]. Thus, the NAc serves to integrate information contained in the mesocorticolimbic circuit and projects that information to the motor system to produce an appropriate behavioral response as shown in Fig. (1). This behavioral response is a key factor in drugs of abuse-seeking behavior.
Fig. (1).
Simplified neurocircuitry involving glutamatergic, GABAergic and dopaminergic pathways in drugs of abuse, including alcohol. The nucleus accumbens serves as an integrator of environmental stimuli and as a gateway between the mesocorticolimbic circuit and the motor output pathway. Abbreviations: NAc, nucleus accumbens; PFC, prefrontal cortex; BLA, basal lateral amygdala; HP, hippocampus; DMT, dorsomedial thalamus; VTA, ventral tegmental area; VP, ventral pallidum; SNr, substantial nigra; MT, motor thalamus; MC, motor cortex; SC, spinal cord.
Both projections from amygdala and PFC to the NAc and the connections between BLA and PFC are glutamatergic [32]. Glutamatergic neurons arising from PFC also project towards dopaminergic neurons in the VTA. Alternatively, activation of glutamatergic neurons arising from the PFC and amygdala has been directly linked to the development of addiction [32]. The blood flow, as revealed with neuroimaging studies, is increased in brain regions associated with reward mechanism, including PFC and amygdala, with craving to addictive drugs such as alcohol [33, 34]. Moreover, employing the reinstatement model, BLA has been proven to be critical for reinstatement produced by drug-associated cue [35–37]. In addition, NAc receives glutamatergic inputs from amygdala and PFC, which suggests that NAc has a critical role in drug-seeking behavior [38, 39].
It is noteworthy that studies tested compounds targeting NMDA receptor and mGluR5 have revealed the importance of glutamatergic system in alcohol dependence [40, 41]. Furthermore, chronic exposure to alcohol has been associated with a marked increase in extracellular glutamate levels in the NAc [42, 43]. Given the role of glutamatergic system in the development of alcohol dependence, various approaches have been focused on finding compounds to attenuate the elevated level of extracellular glutamate and over activation of glutamatergic system. In this review, we focus on glutamate transporters, in particular GLT1, as target for the treatment of alcohol dependence. It is well known that GLT1 is responsible for the uptake of the majority of extracellular glutamate, which is critical in the induction of alcohol dependence. Thus, manipulating GLT1 level or activity is a key player in determining propensity for alcoholism. Prior to discuss the role of GLT1 in alcohol dependence, it is important to review and discuss the interactive role of glutamatergic system with key neurotransmitter systems in the regulation of alcohol intake.
III. INTERACTION OF GLUTAMATERGIC SYSTEM WITH OTHER NEUROTRANSMITTER SYSTEMS IN THE MODULATION OF ALCOHOL-DRINKING BEHAVIOR
Acute alcohol exposure was first identified to increase the binding capacity of low affinity GABA receptor binding sites in a rat model [44]. This, combined with the dampened glutamatergic transmission, leads to a net sedative effect. It was further suggested that alcohol acts by facilitating GABA action through GABAA receptors and increases the chloride influx, thus depressing neuronal excitability [45, 46]. Therefore, alcohol acts to suppress neurotransmission by potentiating GABA receptors and inhibiting NMDA receptors. The effects of chronic alcohol use, however, are associated with a multitude of compensatory actions.
Seizures related to excessive excitation have been observed in subjects going through periods of alcohol withdrawal [47]. Chronic alcohol use has been shown to decrease the cell surface expression of GABAA receptors and decrease their sensitivity [48, 49]. Repeated alcohol exposure at pharmacologically relevant levels increased NMDA receptor subunits expression, synaptic clustering of these receptors, and receptor functionality [50]. Although acute alcohol exposure seems to suppress neurotransmission, chronic use has been linked to over excitation, possibly due to the compensatory mechanisms associated with repeated administration. Studies have shown that Sprague-Dawley rats withdrawn from chronic alcohol exposure show a significant increase in glutamate output relative to controls when administered with NMDA [51]. These studies demonstrated that administration of NMDA directly into the striatum increased extracellular glutamate in alcohol withdrawn rats as opposed to sucrose controls. These results suggest that the compensatory mechanisms during alcohol withdrawal can lead to an increase in synaptic glutamate release. Moreover, it was found that chronic alcohol exposure increases the density of NMDA receptors in the hippocampus [12]. The hippocampus has been shown to be associated with alcohol withdrawal seizures, and the use of NMDA antagonist, MK801, led to decreased number and intensity of seizures associated with alcohol withdrawal [11–14].
The importance of NAc activation in drug and alcohol dependence has been well studied due to its integrative role in mediating rewarding behavior. The majority of excitatory transmission to the NAc stems from glutamatergic efferents located in the PFC [52], which is composed primarily of glutamatergic pyramidal neurons. Evidence has also shown that dopamine type II (D2) receptors are downregulated in PFC following chronic alcohol consumption [52]. D2 receptor binding sites on pyramidal neurons within the PFC has been shown to have an inhibitory influence, whereas D2 receptors binding sites on GABAergic interneurons have an excitatory influence [31]. D2 receptors are located presynaptically on glutamate efferents projecting from the PFC to other brain regions [31]. Studies have shown that infusion of selective D2 antagonist, sulpiride, into the NAc dose-dependently increased alcohol consumption in P rats [53]. The results obtained from these studies could be attributed to increased glutamate transmission in the NAc by the inability to inhibit pyramidal neuron efferents. The PFC also receives glutamatergic afferents from the dorsomedial thalamus, an area involved in the neurocircuitry of drugs of abuse. Together, these results suggest that during alcohol withdrawal, there is an excessive amount of glutamate within the PFC, causing hyperactivation of pyramidal neurons that further project to the NAc and promote alcohol-seeking behavior. Excessive extracellular glutamate levels are regulated by several glutamate transporters, among them GLT1 is key player in regulating the majority of glutamate uptake [54].
IV. GLUTAMATE TRANSPORTERS
Glutamate is a major excitatory neurotransmitter of the central nervous system (CNS). Excess extracellular glutamate can lead to increased production of reactive oxygen and nitrogen species. Subsequently, this causes an increase in oxidative stress, leading to neuronal death [55]. Therefore, extracellular glutamate levels are tightly controlled via series of homeostatic pathways. The extracellular glutamate levels are regulated by a number of glutamate transporters (for review see ref. [54]). Glutamate transporters are classified under two categories namely, the Excitatory Amino Acid Transporters (EAATs) and the Vesicular Glutamate Transporters (vGLUTs). The EAATs depend on electrochemical gradient of sodium and potassium ions for their actions. EAATs are membrane bound pumps, which are responsible for maintaining a low physiological extracellular glutamate levels [56]. Five subtypes have been identified in humans as well as rodents [57, 58]. Three of these transporters were first identified in rodents as glutamate/aspartate transporter (GLAST), GLT1 and excitatory amino acid carrier type 1 (EAAC1); the human homologues were later identified as EAAT1, EAAT2, and EAAT3, respectively. The other two excitatory amino acid transporters have been classified as EAAT4 and EAAT5. These two transporters have been identified in both rodents and humans. Immunostaining and protein expression studies have demonstrated that EAAT1 is most prominently distributed in the cerebellum and moderately expressed in other brain regions such as the hippocampus and the forebrain. GLT1 distribution, on the other hand, is mostly limited to forebrain, with little expression in the cerebellum.
As opposed to the EAATs, the vesicular glutamate transporters (vGLUTs) do not involve Na+ electrochemical gradient to accumulate glutamate [59]. Unlike the EAATs that recognize and transport both glutamate and aspartate, the vGLUTs are selective for transporting glutamate [60]. Three types of vesicular glutamate transporters have been identified, namely vGLUT1, vGLUT2 and vGLUT3 [61]. The three isoforms are highly homologous, with up to 90% homology for the membrane spanning region. Alternatively, the N- and C- terminal regions have little homology and give rise to functional differences [62].
Since increased in extracellular glutamate levels has been shown to be closely associated with development of alcohol dependence, the role of glutamate transporters has drawn substantial attention to date. With the inherent potential to regulate the synaptic levels of glutamate, the glutamate transporters, in particular GLT1, represent potential targets for the treatment of alcohol dependence.
V. GLT1 AND ALCOHOL DEPENDENCE
Among various glutamate transporters, GLT1 is expressed in the brain and spinal cord. GLT1 is responsible for the removal of about 90% of extracellular glutamate [54, 63–65]. Dysfunction or reduced expression of GLT1 level results in impaired glutamate reuptake from the extracellular space, which has been implicated in the pathogenesis of various in vitro and in vivo disease models [66–70]. The mRNA coding for GLT1 has been found as multiple splice variants throughout the brain resulting in variant protein forms [71–73]. Splice variant forms of GLT1 have been found altered in neurodegenerative diseases and neurological disorders [74–77]. Furthermore, study has shown that reduction in extracellular glutamate was a result of increased expression of the glutamate transporters, such as GLT1 and EAAT1 [78].
The increased in extracellular glutamate levels has been observed in postmortem tissues of amyotrophic lateral sclerosis (ALS) patients [79–81]. This increase in extracellular levels of glutamate in ALS patients was accompanied by selective loss of GLT1 [64]. Drugs upregulating the expression of GLT1 therefore represent a viable solution and may help restore normal glutamatergic transmission. For example, Harmine, a naturally found beta-carboline alkaloid, is one of the lead compounds identified during high-throughput screening efforts and has been found to upregulate the GLT1 expression in both in vitro and in vivo models [82]. Cell based enzyme-linked immunosorbent assay was used in a high-throughput screen of about 140,000 compounds [83]. Few lead compounds were identified from these studies and served a starting point for the development of GLT1 upregulators/activators. In addition, Thiopyridazine containing compound was shown to increase the levels of GLT1 protein in a dose-dependent manner. Following promising results, a structure-activity relationships study has focused on further improving the scaffold to identify a potent GLT1 activator [84].
Moreover, pharmaceutical modulation of GLT1 has long been studied in several types of neurodegenerative disorders and drug addiction models. Rothstein and colleagues have tested 1,040 FDA-approved drugs and identified that β-lactam antibiotics were some of the most potent stimulators of GLT1 protein expression [21]. These studies revealed that β-lactam antibiotic, ceftriaxone, increased the expression of GLT1 and its functional activity in both the hippocampus and spinal cord treated at a dose of 200 mg/kg administered i.p. once a day for five days. Since these findings, ceftriaxone’s positive effects on GLT1 have been well documented in other experiments both in vitro [85, 86] and in vivo [23, 87, 88]. Recent studies have also shown that ceftriaxone causes an increased expression of GLT1 in the spinal cord [89]. In Huntington’s disease mouse model, it was demonstrated that single daily i.p. injections of 200 mg/kg ceftriaxone for five days increased glutamate uptake in striatum, a primary target of cortical glutamatergic inputs [20]. Also, ceftriaxone is neuroprotective in models of ischemic injury and motor neuron degeneration in which glutamate reaches neurotoxic levels [90]. Thus, the ceftriaxone-induced increase in GLT1 expression has a direct effect on glutamate homeostasis.
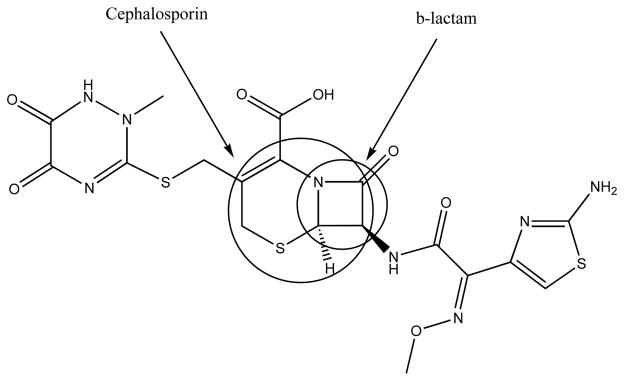
Although other β-lactam antibiotics and cephalosporins appeared promising in the initial high-throughput screen, only ceftriaxone has been selected to be further studied [21]. This was partly due to the low bioavailability observed with other compounds as well as the lower EC50 values as compared to ceftriaxone. This observation puts emphasis on the key substitutions on the compound contributing to its activity. Apart from the pharmacophore, the β-lactam ring (Fig. 2), ceftriaxone differs from the other promising leads in the side chain substitution alpha to the amide bond of the β-lactam ring. Following the initial high-throughput screening, the lead compounds (including the β-lactam antibiotics) were tested in cell lines for their ability to activate the GLT1 promoter fragment. Promising results were obtained with ceftriaxone and amoxicillin while vancomycin did not produce any change in the basal level. Penicillin, although considered active in the initial screen, was unable to elicit a response in in vivo models due to the lack of ability of this compound to cross the blood-brain barrier. However, ceftriaxone has been well studied and was found to cross the blood-brain barrier [91–94] and elevate GLT1 expression in the CNS [20, 21, 23, 24]. Thus, ceftriaxone is currently considered a compound that has the potential to regulate GLT1 expression in the CNS.
Fig. (2).
The role of GLT1 has been tested in drugs of abuse models that show dysfunction of glutamate neurotransmission as a result of chronic exposure to these drugs. For example, activation of GLT1 by an activator drug, MS-153, was effective in a drug abuse mouse model [95]. Thus, administration of MS-153 attenuated conditioned place preference in mice that have been conditioned to morphine, methamphetamine and cocaine [95]. Also, treatment with ceftriaxone attenuated abstinence-induced withdrawal from cocaine in planarians [96]. Importantly, we have found recently that ceftriaxone attenuates cue-induced cocaine relapse in a dose-dependent manner [23]. Rats were trained to self-administer cocaine (0.125 mg per i.v. infusion) in a lever-pressing task in a daily two-hour session for 10–14 days, followed by five days of extinction training. Immediately after each extinction session, rats received ceftriaxone (i.p.) or saline vehicle. On the following day, presentation of the cue (light and tone) previously associated with cocaine self-administration reinstated lever-pressing in rats treated with vehicle, whereas 100 or 200, but not 50 mg/kg ceftriaxone blocked this response [23]. Immunoblotting confirmed that the ceftriaxone-induced blockade to cue-induced relapse to cocaine-seeking behavior to cocaine relapse was associated with an increase in GLT1 expression in both the PFC and NAc, two forebrain regions in which elevated glutamate transmission appears to drive drug craving. Thus, accelerated removal of extracellular glutamate appears to be a key factor in the ability of ceftriaxone to dampen cocaine craving. In accordance, Kalivas and colleagues found similar effects regarding cocaine relapse [87]. This effect is correlated with an increase in GLT1 expression in PFC and NAc [23, 87].
Related to cocaine-seeking behavior, if an increase in glutamate transmission plays a critical role in alcohol consumption, then up-regulation or activation of GLT1 should attenuate this behavior. We recently tested the hypothesis that GLT1 plays a role in alcohol consumption in male P rats. P rats were selectively bred to determine the neurobiology of chronic alcohol-drinking behavior and the consequences of excessive alcohol intake behaviorally, neurochemically and physiologically. P rats are an established animal model of alcoholism; these rats can consume intoxicating levels of alcohol [97–99]. P rats have been characterized and demonstrate all of the criteria for animal model of alcoholism [100, 101]. P rats are considered a model of alcoholism; these rats drink greater than 4 g of ethanol/kg body weight/day, whereas alcohol non-preferring (NP) rats drink less than 1g/kg/day under similar conditions [102]. When P rats are on an alcohol drinking schedule, their blood alcohol concentrations (BACs) may reach between 50–200 mg% (which corresponds to 0.05–0.20 in clinical terminology) under 24-hour and limited-access conditions [103–105]. It has been shown that P rats develop behavioral tolerance in as little as five weeks of free-choice alcohol consumption [106]. Thus, recent studies from our laboratory used P rats that were given 24-hour concurrent access to 15% and 30% ethanol, water, and food for five weeks [24]. On Week 6, P rats received 25, 50, 100, or 200 mg/kg ceftriaxone (i.p.) or a saline vehicle for five consecutive days. Alcohol consumption was measured daily for 15 days, starting on Day 1 of injections, in order to test both the immediate and long-term effects of ceftriaxone. We revealed a significant reduction in daily alcohol consumption for 15 consecutive days [24]. At higher doses (100 and 200 mg/kg), ceftriaxone-mediated reductions in alcohol intake were correlated with an upregulation of GLT1 expression in the PFC and NAc on Day 8 [24]. The lower doses (25 and 50 mg/kg), did not cause the same increase in GLT1 expression, but did temporarily reduce alcohol consumption as compared to the highest doses. Our findings from this study [24] demonstrated that rats that were administered ceftriaxone showed a reduction in alcohol intake as compared to those that received saline vehicle. The long-lasting effects of ceftriaxone in alcohol intake were found correlated with the upregulation of GLT1 expression in PFC and NAc brain regions. We suggest that upregulation of GLT1 can counteract the increase in basal extracellular glutamate levels that might be caused by chronic alcohol consumption.
Basal extracellular glutamate levels differ between some of drugs of abuse, including cocaine and alcohol. In contrast to animal model with chronic alcohol intake, basal extracellular glutamate levels are decreased in NAc in animals exposed to cocaine [39, 107–109], yet ceftriaxone has similar effects in both models [23, 24, 87]. As reviewed in details by Kalivas that decreased basal extracellular glutamate levels after chronic cocaine self-administration is associated with reduction in cystine-glutamate exchanger (xCT) and reduction in GLT1 levels, but elevation of synaptic glutamate release in NAc occurred during cue-induced relapse to cocaine-seeking behavior [87, 110]. During cue-induced relapse to cocaine-seeking behavior, increased in glutamate release in NAc was found associated with activation of the PFC targeting this brain region [107]. In contrast to chronic alcohol consumption where basal extracellular glutamate is increased, cocaine exposure can lead to decrease in basal extracellular glutamate level. But during reinstatement to cocaine seeking-behavior there is increase in synaptic glutamate release in NAc and decrease in the glutamate uptake [for review see ref. [110]. Although basal extracellular glutamate level is different between cocaine and alcohol models, ceftriaxone was found to restore dysfunction of the glutamate homeostasis in both models, and consequently attenuates alcohol intake and cue-induced relapse to cocaine-seeking behavior [23, 24, 87]. This was due to the fact that ceftriaxone restored GLT1 and xCT levels in NAc and PFC that were found reduced after cocaine exposure [87] and perhaps in alcohol intake model, in particular with GLT1 [24]. Alternatively, it is unclear whether ceftriaxone can induce upregulation of GLT1 expression in other non-reward brain regions in alcohol and cocaine drug abuse models. Studies are warranted to investigate the effects of ceftriaxone in these other non-reward brain regions and to determine whether this effect may affect the overall brain glutamate homeostasis. It is unclear whether this potential overall effect of ceftriaxone in all brain regions may or may not have negative effects in the neurochemistry of the brain.
The precise cellular mechanism by which ceftriaxone enhances the level of GLT1 remains unknown. It is suggested that there is at least one pathway (which might have direct or indirect interaction) involved in GLT1 expression. Lee and colleagues demonstrated that the canonical NF-κB signaling pathway is necessary for the ceftriaxone-induced increase in GLT1 in human primary fetal astrocytes [85]. Other known GLT1 upregulators, like the epidermal growth factor receptor agonists, have also been shown to be mediated via pathway involving the transcription factor NF-κB [111]. Amitriptyline, an antidepressant, has also been found to upregulate the expression of GLT1 and GLAST through NF-κB dependent route in chronic morphine-induced rats [112]. Also, in other in vitro studies, the pathway involving mammalian target for rapamycin (mTOR) has also been shown to regulate GLT1 expression and glutamate uptake [113]. In this pathway, mTOR was phosphorylated by Akt, which in turn was found to be responsible for GLT1 expression.
Additionally, ceftriaxone was found to inhibit alcohol intake with lower doses without any change in GLT1 expression thereby suggesting possible additional pharmacological effects of the drug [24]. Other studies have demonstrated that ceftriaxone increased the activity of GLT1, without affecting its expression, in brain regions including hippocampus, striatum, and frontal cortex of an animal stroke model [88]. Furthermore, studies have shown that ceftriaxone increased glutamate uptake in hippocampus region of rats without upregulation of GLT1 expression [114]. A functional increase could involve changes in several mechanisms, involving for example protein phosphorylation such as protein kinase C (PKC) that was found to interfere with cell surface expression of GLT1 [115]. Studies are warranted to investigate other potential mechanisms of action of ceftriaxone in GLT1 function.
It is noteworthy that higher doses of ceftriaxone might cause diarrhea, as reported in humans [116]. However, in rats, ceftriaxone at higher doses (100 and 200 mg/kg) was found to be very tolerable without the occurrence of diarrhea [24]. The dose of 100 mg/kg has also been tested in Huntington’s disease mouse model and no severe side effects were observed in this study as well [20]. Moreover, ceftriaxone is currently being evaluated for ALS treatment and no major side effects have been reported from phase I and phase II clinical trials. Phase III of the clinical study is currently underway.
VI. ROLE OF OTHER GLUTAMATE TRANSPORTERS IN ALCOHOL INTAKE
Evidence suggests that chronic alcohol exposure not only leads to functional increases in glutamate output but also to an impaired ability in glutamate transport. Unlike other neurotransmitters, glutamate is not metabolized in the synaptic cleft [117]. The primary means by which it is removed from the synapse is by glutamate transporters, including GLAST and GLT1 (EAAT2) [118]. Studies have shown that alcohol-preferring cAA rats chronically exposed to ethanol (10%, v/v) for 20 months exhibited a reduction in glutamate transport in the cerebral cortex [119]. Recent studies examined the expression levels of GLAST and GLT1 in post-mortem human alcoholics. A significant decrease in levels of GLAST and GLT1 in the BLA was found in post-mortem alcoholics as compared to non-alcoholic controls [120]. Glutamate uptake via these transporters is also altered via chronic alcohol exposure, although with varying results in vitro [121–123] and in vivo [124, 125]. In experiments involving Xenopus oocytes, chronic exposure to high doses of alcohol has been shown to reduce the activity of both EAAT3 and EAAT4 [126, 127]. Also, the expression of EAAT1 and EAAT3 in the parietal cortex was unaltered following chronic alcohol administration in rats, as opposed to EAAT2 (GLT1), which was downregulated in high-dose alcohol fed rats [128] (Table 1).
Table 1.
Different Types of Glutamate Transporters, their Distribution, and the Effects of Alcohol in their Levels of Expression or Activity
| Glutamate transporter | Neuronal/Glial | Effects of alcohol on expression/activity | References |
|---|---|---|---|
|
| |||
| 1. EAATs | |||
| EAAT1 | Glial/low expression on neurons | ⇧ | [119] |
| EAAT2 (GLT1) | Glial/low expression in neurons | ⇩ | [110, 118] |
| EAAT3 (EAAC1) | Neuronal | Acute ⇧; Chronic ⇩ | [117, 120] |
| EAAT4 | Neuronal | ⇩ | [116–117] |
| EAAT5 | Retina | NA | - |
|
| |||
| 2. vGLUTs | |||
| vGLUT1 | Neuronal | Unchanged | [121] |
| vGLUT2 | Neuronal | ⇧ | [121] |
| vGLUT3 | Neuronal | NA | - |
NA: Information not available.
In postmortem human PFC samples, a marked increase in the expression of EAAT1 was observed in case of alcoholics suggesting a neuroadaptation may occur to reverse the increased extracellular glutamate levels [129] (Table 1). The increased expression of EAAT1 found throughout the PFC of chronic alcoholics indicates that all layers of PFC are affected by increased glutamate levels. Moreover, acute alcohol exposure leads to an increased activity of EAAT3, and this has been linked to some of the alcohol-induced features like sedation, impaired cognition and general anesthesia [130]. Alternatively, chronic exposure has been shown to decrease the activity of EAAT3 via a PKC-dependent mechanism. It has been hypothesized that this may be a compensatory mechanism to overcome the inhibitory effects of alcohol on the excitatory glutamatergic transmission in the CNS [127] (Table 1).
Alcohol abuse also changes the expression of vGLUTs in the central reward brain regions, particularly the NAc. We have previously reported that repeated deprivation of alcohol in P rats led to an increased vGLUT2 expression in the NAc shell [131]. However, the expression of vGLUT1 levels, which are part of the cognitive circuit of the brain, remained unchanged. The vGLUT2 expressing glutamate neurons are associated with the motor circuit and this might explain the effects of alcohol intake on motor activity. It is suggested that the increase in number of vGLUT2 carrying glutamate neurons following repeated deprivation of alcohol is associated with a significant change in the ratio of dopamine to glutamate in the NAc shell, which is a key region of the reward circuitry [131] (Table 1).
VII. ROLE OF GLT1 ACTIVATION ON OTHER DRUGS OF ABUSE
Increased glutamate activity produced in response to repeated exposure of morphine has been implicated in the development of physical dependence associated with morphine [132]. Moreover, studies have demonstrated that chronic exposure to morphine is associated with reduced mRNA GLT1 levels in the CNS [133, 134]. Rawls and colleagues have tested the hypothesis that decreased extracellular glutamate levels following administration of β-lactam antibiotic, ceftriaxone, can lead to decreased antinociceptive tolerance caused by chronic exposure to morphine [135, 136]. This study showed that ceftriaxone inhibited the tolerance developed for morphine, after repeated exposure, in a dose-dependent manner. Moreover, inhibition of GLT1 by dihydrokainate (DHK) abolished the effect of ceftriaxone on morphine tolerance demonstrating the key role played by GLT1 in the development of morphine tolerance.
The effect of ceftriaxone was also studied on morphine-induced hyperthermia rat model [137]. Ceftriaxone was shown to inhibit the hyperthermia observed following morphine administration in rats. Upon pretreatment with DL-threo-β-Benzyloxyaspartic acid (TBOA), a non-specific inhibitor of glutamate transporters, the effect of ceftriaxone on morphine-induced hyperthermia was not observed further; this indicates the relationship between glutamate uptake and morphine-induced hyperthermia. Furthermore, the effect of ceftriaxone on kappa-opioid receptor agonist induced hypothermia has also been investigated [138]. This study demonstrated that subcutaneous administration of U50,488H produced hypothermia in rats. While a single dose of ceftriaxone was found to have no effect on hypothermia observed following acute administration of U50,488H, however, repeated exposure to ceftriaxone was shown to block the tolerance for U50,488H-induced hypothermia.
Repeated administration of ceftriaxone was also found to inhibit the hyperactivity associated with amphetamine administration [139]. Also, ceftriaxone was found to inhibit the amphetamine-induced behavior sensitization in rats. The results were attributed to the fact that ceftriaxone induced increase in GLT1 level leads to increased glutamate uptake thereby preventing the increase in glutamatergic activity at NMDA and AMPA receptors typically observed following amphetamine administration. On the other hand, coadministration of ceftriaxone with nicotine induced inhibition of the development of antinociceptive tolerance [140]. These results have been attributed to the fact that increased GLT1 expression following ceftriaxone administration results in increased glutamate uptake.
VIII. CONCLUSION
Despite of the involvement of multiple neurotransmitters, glutamatergic system seems to play a critical role in the development of alcohol dependence. Alcohol consumption affects the extracellular glutamate level and expression of various glutamate transporters in a complex manner. While acute alcohol exposure may lead to an overall suppression of extracellular glutamate levels, chronic exposure of alcohol has been shown to increase the extracellular glutamate level. The overall effect is the contribution of glutamatergic system towards development of alcohol dependence. Alcohol dependence is associated with a significant decrease in expression of glutamate transporters. Of these transporters, GLT1 is responsible for the uptake of the majority of extracellular glutamate. We suggested that upregulation of GLT1 level in the central reward brain regions would decrease extracellular glutamate and consequently reduce alcohol consumption. We have tested ceftriaxone, a β-lactam antibiotic known to upregulate GLT1, and found that ceftriaxone-induced upregulation of GLT1 in PFC and NAc was associated with reduction in alcohol intake in P rats. It is noteworthy that ceftriaxone-induced upregulation of GLT1 was also found to be associated with the attenuation of relapse to cocaine-seeking behavior. Together, we suggest that GLT1 is a potential target for the treatment of drugs of abuse, including alcohol.
Acknowledgments
The research project described was supported by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health. The authors would also like to thank Charisse Montgomery for editing this manuscript.
ABBREVIATIONS
- CNS
Central Nervous System
- NAc
Nucleus Accumbens
- GABA
γ-aminobutyric acid
- NMDA
N-methyl D-aspartate receptor
- GLT1
Glutamate Transporter 1
- VS
Ventral Striatum
- PFC
Prefrontal Cortex
- BLA
Basal Lateral Amygdala
- HP
Hippocampus
- VP
Ventral Pallidum
- VTA
Ventral Tegmental Area
- DMT
Dorsomedial Thalamus
- SNr
Substantia Nigra reticulata
- MT
Motor Thalamus
- MC
Motor Cortex
- ALS
Amyotrophic Lateral Sclerosis
- DA
Dopamine
- D1
Dopamine type I receptors
- D2
Dopamine type II receptors
- EAAT1
Excitatory Amino Acid Transporter 1
- EAAT2
Excitatory Amino Acid Transporter 2
- EAAT3
Excitatory Amino Acid Transporter 3
- EAAT4
Excitatory Amino Acid Transporter 4
- EAAT5
Excitatory Amino Acid Transporter 5
- VGLUT1
Vesicular glutamate transporter 1
- VGLUT2
Vesicular glutamate transporter 2
- VGLUT3
Vesicular glutamate transporter 3
Footnotes
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
References
- 1.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 2.Diana M, Brodie M, Muntoni A, Puddu MC, Pillolla G, Steffensen S, Spiga S, Little HJ. Enduring effects of chronic ethanol in the CNS: basis for alcoholism. Alcohol Clin Exp Res. 2003;27(2):354–361. doi: 10.1097/01.ALC.0000057121.36127.19. [DOI] [PubMed] [Google Scholar]
- 3.Basavarajappa BS, Hungund BL. Role of the endocannabinoid system in the development of tolerance to alcohol. Alcohol Alcohol. 2005;40(1):15–24. doi: 10.1093/alcalc/agh111. [DOI] [PubMed] [Google Scholar]
- 4.Karcz-Kubicha M, Liljequist S. Effects of post-ethanol administration of NMDA and non-NMDA receptor antagonists on the development of ethanol tolerance in C57B1 mice. Psychopharmacology (Berl) 1995;120(1):49–56. doi: 10.1007/BF02246144. [DOI] [PubMed] [Google Scholar]
- 5.Camarini R, Marcourakis T, Teodorov E, Yonamine M, Calil HM. Ethanol-induced sensitization depends preferentially on D1 rather than D2 dopamine receptors. Pharmacol Biochem Behav. 2011;98(2):173–180. doi: 10.1016/j.pbb.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Tarragon E, Balino P, Aragon CM, Pastor R. Ethanol drinking-in-the-dark facilitates behavioral sensitization to ethanol in C57BL/6J, BALB/cByJ, but not in mu-opioid receptor deficient CXBK mice. Pharmacol Biochem Behav. 2012;101(1):14–23. doi: 10.1016/j.pbb.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Kotlinska J, Bochenski M, Danysz W. N-methyl-D-aspartate and group I metabotropic glutamate receptors are involved in the expression of ethanol-induced sensitization in mice. Behav Pharmacol. 2006;17(1):1–8. doi: 10.1097/01.fbp.0000181600.95405.c7. [DOI] [PubMed] [Google Scholar]
- 8.Carrara-Nascimento PF, Griffin WC, 3rd, Pastrello DM, Olive MF, Camarini R. Changes in extracellular levels of glutamate in the nucleus accumbens after ethanol-induced behavioral sensitization in adolescent and adult mice. Alcohol. 2011;45(5):451–460. doi: 10.1016/j.alcohol.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243(4899):1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 10.Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11(6):775–793. doi: 10.1017/S1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Michaelis ML, Michaelis EK. Effects of chronic ethanol treatment on the expression of calcium transport carriers and NMDA/glutamate receptor proteins in brain synaptic membranes. J Neurochem. 1997;69(4):1559–1569. doi: 10.1046/j.1471-4159.1997.69041559.x. [DOI] [PubMed] [Google Scholar]
- 12.Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176(3):289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- 13.Sanna E, Serra M, Cossu A, Colombo G, Follesa P, Cuccheddu T, Concas A, Biggio G. Chronic ethanol intoxication induces differential effects on GABAA and NMDA receptor function in the rat brain. Alcohol Clin Exp Res. 1993;17(1):115–123. doi: 10.1111/j.1530-0277.1993.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 14.Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res. 1996;40(1):71–78. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- 15.Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283(1–3):177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- 16.Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De Lecea L. Glutamatergic transmission in opiate and alcohol dependence. Ann N Y Acad Sci. 2003;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- 17.Carboni S, Isola R, Gessa GL, Rossetti ZL. Ethanol prevents the glutamate release induced by N-methyl-D-aspartate in the rat striatum. Neurosci Lett. 1993;152(1–2):133–136. doi: 10.1016/0304-3940(93)90501-b. [DOI] [PubMed] [Google Scholar]
- 18.Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24(7):1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Littleton JM. Acamprosate in alcohol dependence: implications of a unique mechanism of action. J Addict Med. 2007;1(3):115–125. doi: 10.1097/ADM.0b013e318156c26f. [DOI] [PubMed] [Google Scholar]
- 20.Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153(1):329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 22.Sari Y, Prieto AL, Barton SJ, Miller BR, Rebec GV. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington’s disease. J Biomed Sci. 2010;17:62. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29(29):9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46(3):239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- 26.Yin HH, Knowlton BJ. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 27.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers CE, Gluck MA. Context, conditioning, and hippocampal rerepresentation in animal learning. Behav Neurosci. 1994;108(5):835–847. doi: 10.1037//0735-7044.108.5.835. [DOI] [PubMed] [Google Scholar]
- 29.Yaniv D, Desmedt A, Jaffard R, Richter-Levin G. The amygdala and appraisal processes: stimulus and response complexity as an organizing factor. Brain Res Brain Res Rev. 2004;44(2–3):179–186. doi: 10.1016/j.brainresrev.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Groenewegen HJ, Galis-de Graaf Y, Smeets WJ. Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. J Chem Neuroanat. 1999;16(3):167–185. doi: 10.1016/s0891-0618(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 31.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacol. 2010;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4(1):23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156(1):11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168(1–2):44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 36.Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22(3):1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21(14):RC155–159. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant KA, Lovinger DM. Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci. 1995;3(3):155–164. [PubMed] [Google Scholar]
- 41.Minami K, Gereau RWt, Minami M, Heinemann SF, Harris RA. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;53(1):148–156. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- 42.Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology. 2007;190(4):415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- 43.Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25(30):7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ticku MK. The effects of acute and chronic ethanol administration and its withdrawal on gamma-aminobutyric acid receptor binding in rat brain. Br J Pharmacol. 1980;70(3):403–410. doi: 10.1111/j.1476-5381.1980.tb08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Criswell HE, Simson PE, Duncan GE, McCown TJ, Herbert JS, Morrow AL, Breese GR. Molecular basis for regionally specific action of ethanol on gamma-aminobutyric acidA receptors: generalization to other ligand-gated ion channels. J Pharmacol Exp Ther. 1993;267(1):522–537. [PubMed] [Google Scholar]
- 46.Schwartz RD, Suzdak PD, Paul SM. gamma-Aminobutyric acid (GABA)- and barbiturate-mediated 36Cl- uptake in rat brain synaptoneurosomes: evidence for rapid desensitization of the GABA receptor-coupled chloride ion channel. Mol Pharmacol. 1986;30(5):419–426. [PubMed] [Google Scholar]
- 47.Hillbom M, Pieninkeroinen I, Leone M. Seizures in alcohol-dependent patients: epidemiology, pathophysiology and management. CNS Drugs. 2003;17(14):1013–1030. doi: 10.2165/00023210-200317140-00002. [DOI] [PubMed] [Google Scholar]
- 48.Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63(1):53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 49.Mhatre MC, Pena G, Sieghart W, Ticku MK. Antibodies specific for GABAA receptor alpha subunits reveal that chronic alcohol treatment down-regulates alpha-subunit expression in rat brain regions. J Neurochem. 1993;61(5):1620–1625. doi: 10.1111/j.1471-4159.1993.tb09795.x. [DOI] [PubMed] [Google Scholar]
- 50.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochemical Pharmacology. 2008;75(1):218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossetti ZL, Carboni S, Fadda F. Glutamate-induced increase of extracellular glutamate through N-methyl-D-aspartate receptors in ethanol withdrawal. Neuroscience. 1999;93(3):1135–1140. doi: 10.1016/s0306-4522(99)00250-x. [DOI] [PubMed] [Google Scholar]
- 52.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45(5):647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Levy AD, Murphy JM, McBride WJ, Lumeng L, Li TK. Microinjection of sulpiride into the nucleus accumbens increases ethanol drinking in alcohol-preferring (P) rats. Alcohol Alcohol Suppl. 1991;1:417–420. [PubMed] [Google Scholar]
- 54.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 55.Adelmann G, Jonas P, Monyer H. Ionotropic glutamate receptors in the CNS. Springer; Berlin ; New York: 1999. [Google Scholar]
- 56.Beart PM, O’Shea RD. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007;150(1):5–17. doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14(9):5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: Opportunities for developing novel therapeutics. J cell physiol. 2010;226:2484–2493. doi: 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 60.Maycox PR, Deckwerth T, Jahn R. Bacteriorhodopsin drives the glutamate transporter of synaptic vesicles after co-reconstitution. EMBO J. 1990;9(5):1465–1469. doi: 10.1002/j.1460-2075.1990.tb08263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moriyama Y, Yamamoto A. Glutamatergic chemical transmission: look! Here, there, and anywhere. J Biochem. 2004;135(2):155–163. doi: 10.1093/jb/mvh018. [DOI] [PubMed] [Google Scholar]
- 62.Liguz-Lecznar M, Skangiel-Kramska J. Vesicular glutamate transporters (VGLUTs): the three musketeers of glutamatergic system. Acta Neurobiol Exp(Wars) 2007;67(3):207–218. doi: 10.55782/ane-2007-1649. [DOI] [PubMed] [Google Scholar]
- 63.Rothstein JD. Excitotoxicity and neurodegeneration in amyotrophic lateral sclerosis. Clin Neurosci. 1995;3(6):348–359. [PubMed] [Google Scholar]
- 64.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38(1):73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 65.Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J Neurosci. 2003;23(18):7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312(1):60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- 67.Pappas TC, Alagarsamy S, Pollard RB, Nokta M. HIV decreases glutamate transport in SK-N-MC neuroblastoma cells. J Neurovirol. 1998;4(1):69–79. doi: 10.3109/13550289809113483. [DOI] [PubMed] [Google Scholar]
- 68.Trotti D, Aoki M, Pasinelli P, Berger UV, Danbolt NC, Brown RH, Jr, Hediger MA. Amyotrophic lateral sclerosis-linked glutamate transporter mutant has impaired glutamate clearance capacity. J Biol Chem. 2001;276(1):576–582. doi: 10.1074/jbc.M003779200. [DOI] [PubMed] [Google Scholar]
- 69.Li S, Mallory M, Alford M, Tanaka S, Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol. 1997;56(8):901–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Martin LJ, Brambrink AM, Lehmann C, Portera-Cailliau C, Koehler R, Rothstein J, Traystman RJ. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997;42(3):335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- 71.Utsunomiya-Tate N, Endou H, Kanai Y. Tissue specific variants of glutamate transporter GLT-1. FEBS Lett. 1997;416(3):312–316. doi: 10.1016/s0014-5793(97)01232-5. [DOI] [PubMed] [Google Scholar]
- 72.Munch C, Schwalenstocker B, Knappenberger B, Liebau S, Volkel H, Ludolph AC, Meyer T. 5′-heterogeneity of the human excitatory amino acid transporter cDNA EAAT2 (GLT-1) Neuroreport. 1998;9(7):1295–1297. doi: 10.1097/00001756-199805110-00007. [DOI] [PubMed] [Google Scholar]
- 73.Meyer T, Munch C, Knappenberger B, Liebau S, Volkel H, Ludolph AC. Alternative splicing of the glutamate transporter EAAT2 (GLT-1) Neurosci Lett. 1998;241(1):68–70. doi: 10.1016/s0304-3940(97)00973-7. [DOI] [PubMed] [Google Scholar]
- 74.Guo H, Lai L, Butchbach ME, Lin CL. Human glioma cells and undifferentiated primary astrocytes that express aberrant EAAT2 mRNA inhibit normal EAAT2 protein expression and prevent cell death. Mol Cell Neurosci. 2002;21(4):546–560. doi: 10.1006/mcne.2002.1198. [DOI] [PubMed] [Google Scholar]
- 75.Hoogland G, van Oort RJ, Proper EA, Jansen GH, van Rijen PC, van Veelen CW, van Nieuwenhuizen O, Troost D, de Graan PN. Alternative splicing of glutamate transporter EAAT2 RNA in neocortex and hippocampus of temporal lobe epilepsy patients. Epilepsy Res. 2004;59(2–3):75–82. doi: 10.1016/j.eplepsyres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Munch C, Zhu BG, Leven A, Stamm S, Einkorn H, Schwalenstocker B, Ludolph AC, Riepe MW, Meyer T. Differential regulation of 5′ splice variants of the glutamate transporter EAAT2 in an in vivo model of chemical hypoxia induced by 3-nitropropionic acid. J Neurosci Res. 2003;71(6):819–825. doi: 10.1002/jnr.10536. [DOI] [PubMed] [Google Scholar]
- 77.Munch C, Ebstein M, Seefried U, Zhu B, Stamm S, Landwehrmeyer GB, Ludolph AC, Schwalenstocker B, Meyer T. Alternative splicing of the 5′-sequences of the mouse EAAT2 glutamate transporter and expression in a transgenic model for amyotrophic lateral sclerosis. J Neurochem. 2002;82(3):594–603. doi: 10.1046/j.1471-4159.2002.01012.x. [DOI] [PubMed] [Google Scholar]
- 78.Castaldo P, Magi S, Gaetani S, Cassano T, Ferraro L, Antonelli T, Amoroso S, Cuomo V. Prenatal exposure to the cannabinoid receptor agonist WIN 55,212-2 increases glutamate uptake through overexpression of GLT1 and EAAC1 glutamate transporter subtypes in rat frontal cerebral cortex. Neuropharmacology. 2007;53(3):369–378. doi: 10.1016/j.neuropharm.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 79.Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326(22):1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 80.Rothstein JD, Tsai G, Kuncl RW, Clawson L, Cornblath DR, Drachman DB, Pestronk A, Stauch BL, Coyle JT. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990;28(1):18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- 81.Spreux-Varoquaux O, Bensimon G, Lacomblez L, Salachas F, Pradat PF, Le Forestier N, Marouan A, Dib M, Meininger V. Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. J Neurol Sci. 2002;193(2):73–78. doi: 10.1016/s0022-510x(01)00661-x. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Sattler R, Yang EJ, Nunes A, Ayukawa Y, Akhtar S, Ji G, Zhang PW, Rothstein JD. Harmine, a natural beta-carboline alkaloid, upregulates astroglial glutamate transporter expression. Neuropharmacology. 2011;60(7–8):1168–1175. doi: 10.1016/j.neuropharm.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Colton CK, Kong Q, Lai L, Zhu MX, Seyb KI, Cuny GD, Xian J, Glicksman MA, Lin CL. Identification of translational activators of glial glutamate transporter EAAT2 through cell-based high-throughput screening: an approach to prevent excitotoxicity. J Biomol Screen. 2010;15(6):653–662. doi: 10.1177/1087057110370998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xing X, Chang LC, Kong Q, Colton CK, Lai L, Glicksman MA, Lin CL, Cuny GD. Structure-activity relationship study of pyridazine derivatives as glutamate transporter EAAT2 activators. Bioorg Med Chem Lett. 2011;21(19):5774–5777. doi: 10.1016/j.bmcl.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino Acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283(19):13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum HI, Wiedau-Pazos M, Schubert D, Maher P, Methner A. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone. in vitro J Neurochem. 2009;111(2):332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- 87.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67(1):81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thone-Reineke C, Neumann C, Namsolleck P, Schmerbach K, Krikov M, Schefe JH, Lucht K, Hortnagl H, Godes M, Muller S, Rumschussel K, Funke-Kaiser H, Villringer A, Steckelings UM, Unger T. The beta-lactam antibiotic, ceftriaxone, dramatically improves survival, increases glutamate uptake and induces neurotrophins in stroke. J Hypertens. 2008;26(12):2426–2435. doi: 10.1097/HJH.0b013e328313e403. [DOI] [PubMed] [Google Scholar]
- 89.Ramos KM, Lewis MT, Morgan KN, Crysdale NY, Kroll JL, Taylor FR, Harrison JA, Sloane EM, Maier SF, Watkins LR. Spinal upregulation of glutamate transporter GLT-1 by ceftriaxone: therapeutic efficacy in a range of experimental nervous system disorders. Neuroscience. 2010;169(4):1888–1900. doi: 10.1016/j.neuroscience.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993;90(14):6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chandrasekar PH, Rolston KV, Smith BR, LeFrock JL. Diffusion of ceftriaxone into the cerebrospinal fluid of adults. J Antimicrob Chemother. 1984;14(4):427–430. doi: 10.1093/jac/14.4.427. [DOI] [PubMed] [Google Scholar]
- 92.Lutsar I, Friedland IR. Pharmacokinetics and pharmacodynamics of cephalosporins in cerebrospinal fluid. Clin Pharmacokinet. 2000;39(5):335–343. doi: 10.2165/00003088-200039050-00003. [DOI] [PubMed] [Google Scholar]
- 93.Nau R, Prange HW, Muth P, Mahr G, Menck S, Kolenda H, Sorgel F. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother. 1993;37(7):1518–1524. doi: 10.1128/aac.37.7.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spector R. Ceftriaxone transport through the blood-brain barrier. J Infect Dis. 1987;156(1):209–211. doi: 10.1093/infdis/156.1.209. [DOI] [PubMed] [Google Scholar]
- 95.Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav Brain Res. 2005;156(2):233–239. doi: 10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 96.Rawls SM, Cavallo F, Capasso A, Ding Z, Raffa RB. The beta-lactam antibiotic ceftriaxone inhibits physical dependence and abstinence-induced withdrawal from cocaine, amphetamine, methamphetamine, and clorazepate in planarians. Eur J Pharmacol. 2008;584(2–3):278–284. doi: 10.1016/j.ejphar.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 97.McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodent. Crit Rev Neurobiol. 1998;12(4):339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- 98.Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res. 2002;26(11):1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- 99.Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J Pharmacol Exp Ther. 2005;315(2):648–657. doi: 10.1124/jpet.105.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction biology. 2006;11(3–4):270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 101.Bell RH, rodd ZA, Murphy JM, McBride WJ. In: Comprehensive handbook of alcohol related pathology. Preedy VR, Watson RR, editors. Vol. 3. New York: Academic Press; 2005. pp. 1515–1533. [Google Scholar]
- 102.Li TK, Lumeng L, McBride WJ, Murphy JM. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol. 1987;(Suppl 1):91–96. [PubMed] [Google Scholar]
- 103.Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006;83(1):35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 104.Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3(5):331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- 105.Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res. 2001;25(8):1140–1150. [PubMed] [Google Scholar]
- 106.Stewart RB, McBride WJ, Lumeng L, Li TK, Murphy JM. Chronic alcohol consumption in alcohol-preferring P rats attenuates subsequent conditioned taste aversion produced by ethanol injections. Psychopharmacology (Berl) 1991;105(4):530–534. doi: 10.1007/BF02244375. [DOI] [PubMed] [Google Scholar]
- 107.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology (Berl) 2008;196(2):303–313. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- 109.Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16(4):1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 111.Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, Grinspan JB, Rothstein JD, Robinson MB. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol Pharmacol. 2000;57(4):667–678. doi: 10.1124/mol.57.4.667. [DOI] [PubMed] [Google Scholar]
- 112.Tai YH, Tsai RY, Wang YH, Cherng CH, Tao PL, Liu TM, Wong CS. Amitriptyline induces nuclear transcription factor-kappaB-dependent glutamate transporter upregulation in chronic morphine-infused rats. Neuroscience. 2008;153(3):823–831. doi: 10.1016/j.neuroscience.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 113.Wu X, Kihara T, Akaike A, Niidome T, Sugimoto H. PI3K/Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochem Biophys Res Commun. 2010;393(3):514–518. doi: 10.1016/j.bbrc.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 114.Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146(2):617–629. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 115.Kalandadze A, Wu Y, Robinson MB. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J Biol Chem. 2002;277(48):45741–45750. doi: 10.1074/jbc.M203771200. [DOI] [PubMed] [Google Scholar]
- 116.Lamb HM, Ormrod D, Scott LJ, Figgitt DP. Ceftriaxone: an update of its use in the management of community-acquired and nosocomial infections. Drugs. 2002;62(7):1041–1089. doi: 10.2165/00003495-200262070-00005. [DOI] [PubMed] [Google Scholar]
- 117.Kanai Y, Smith CP, Hediger MA. The elusive transporters with a high affinity for glutamate. Trends Neurosci. 1993;16(9):365–370. doi: 10.1016/0166-2236(93)90094-3. [DOI] [PubMed] [Google Scholar]
- 118.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32(1):1–14. [PubMed] [Google Scholar]
- 119.Schreiber R, Freund WD. Glutamate transport is downregulated in the cerebral cortex of alcohol-preferring rats. Med Sci Monit. 2000;6(4):649–652. [PubMed] [Google Scholar]
- 120.Kryger R, Wilce PA. The effects of alcoholism on the human basolateral amygdala. Neuroscience. 2010;167(2):361–371. doi: 10.1016/j.neuroscience.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 121.Othman T, Sinclair CJ, Haughey N, Geiger JD, Parkinson FE. Ethanol alters glutamate but not adenosine uptake in rat astrocytes: evidence for protein kinase C involvement. Neurochem Res. 2002;27(4):289–296. doi: 10.1023/a:1014955111742. [DOI] [PubMed] [Google Scholar]
- 122.Smith TL, Zsigo A. Increased Na(+)-dependent high affinity uptake of glutamate in astrocytes chronically exposed to ethanol. Neurosci Lett. 1996;218(2):142–144. doi: 10.1016/s0304-3940(96)13123-2. [DOI] [PubMed] [Google Scholar]
- 123.Smith TL. Regulation of glutamate uptake in astrocytes continuously exposed to ethanol. Life Sci. 1997;61(25):2499–2505. doi: 10.1016/s0024-3205(97)00985-5. [DOI] [PubMed] [Google Scholar]
- 124.Buckman JF, Meshul CK, Finn DA, Janowsky A. Glutamate uptake in mice bred for ethanol withdrawal severity. Psychopharmacology (Berl) 1999;143(2):174–182. doi: 10.1007/s002130050933. [DOI] [PubMed] [Google Scholar]
- 125.Devaud LL. Ethanol dependence has limited effects on GABA or glutamate transporters in rat brain. Alcohol Clin Exp Res. 2001;25(4):606–611. [PubMed] [Google Scholar]
- 126.Park HY, Kim JH, Zuo Z, Do SH. Ethanol increases the activity of rat excitatory amino acid transporter type 4 expressed in Xenopus oocytes: role of protein kinase C and phosphatidylinositol 3-kinase. Alcohol Clin Exp Res. 2008;32(2):348–354. doi: 10.1111/j.1530-0277.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 127.Kim JH, Do SH, Kim YL, Zuo Z. Effects of chronic exposure to ethanol on glutamate transporter EAAT3 expressed in Xenopus oocytes: evidence for protein kinase C involvement. Alcohol Clin Exp Res. 2005;29(11):2046–2052. doi: 10.1097/01.alc.0000187594.92476.07. [DOI] [PubMed] [Google Scholar]
- 128.Zhao H, Mayhan WG, Arrick DM, Xiong W, Sun H. Dose-related influence of chronic alcohol consumption on cerebral ischemia/reperfusion injury. Alcohol Clin Exp Res. 2011;35(7):1265–1269. doi: 10.1111/j.1530-0277.2011.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Flatscher-Bader T, Wilce PA. Impact of alcohol abuse on protein expression of midkine and excitatory amino acid transporter 1 in the human prefrontal cortex. Alcohol Clin Exp Res. 2008;32(10):1849–1858. doi: 10.1111/j.1530-0277.2008.00754.x. [DOI] [PubMed] [Google Scholar]
- 130.Kim JH, Lim YJ, Ro YJ, Min SW, Kim CS, Do SH, Kim YL, Zuo Z. Effects of ethanol on the rat glutamate excitatory amino acid transporter type 3 expressed in Xenopus oocytes: role of protein kinase C and phosphatidylinositol 3-kinase. Alcohol Clin Exp Res. 2003;27(10):1548–1553. doi: 10.1097/01.ALC.0000092061.92393.79. [DOI] [PubMed] [Google Scholar]
- 131.Zhou FC, Sahr RN, Sari Y, Behbahani K. Glutamate and dopamine synaptic terminals in extended amygdala after 14-week chronic alcohol drinking in inbred alcohol-preferring rats. Alcohol. 2006;39(1):39–49. doi: 10.1016/j.alcohol.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 132.Noda Y, Nabeshima T. Opiate physical dependence and N-methyl-D-aspartate receptors. Eur J Pharmacol. 2004;500(1–3):121–128. doi: 10.1016/j.ejphar.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 133.Ozawa T, Nakagawa T, Sekiya Y, Minami M, Satoh M. Effect of gene transfer of GLT-1, a glutamate transporter, into the locus coeruleus by recombinant adenoviruses on morphine physical dependence in rats. Eur J Neurosci. 2004;19(1):221–226. doi: 10.1111/j.1460-9568.2004.03101.x. [DOI] [PubMed] [Google Scholar]
- 134.Nakagawa T, Satoh M. Involvement of glial glutamate transporters in morphine dependence. Ann N Y Acad Sci. 2004;1025:383–388. doi: 10.1196/annals.1307.047. [DOI] [PubMed] [Google Scholar]
- 135.Rawls SM, Zielinski M, Patel H, Sacavage S, Baron DA, Patel D. Beta-lactam antibiotic reduces morphine analgesic tolerance in rats through GLT-1 transporter activation. Drug Alcohol Depend. 2010;107(2–3):261–263. doi: 10.1016/j.drugalcdep.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rawls SM, Baron DA, Kim J. beta-Lactam antibiotic inhibits development of morphine physical dependence in rats. Behav Pharmacol. 2010;21(2):161–164. doi: 10.1097/FBP.0b013e328337be10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rawls SM, Tallarida R, Robinson W, Amin M. The beta-lactam antibiotic, ceftriaxone, attenuates morphine-evoked hyperthermia in rats. Br J Pharmacol. 2007;151(7):1095–1102. doi: 10.1038/sj.bjp.0707309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rawls SM, Robinson W, Patel S, Baron A. Beta-lactam antibiotic prevents tolerance to the hypothermic effect of a kappa opioid receptor agonist. Neuropharmacology. 2008;55(5):865–870. doi: 10.1016/j.neuropharm.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 139.Rasmussen B, Unterwald EM, Rawls SM. Glutamate transporter subtype 1 (GLT-1) activator ceftriaxone attenuates amphetamine-induced hyperactivity and behavioral sensitization in rats. Drug Alcohol Depend. 2011;118(2–3):484–488. doi: 10.1016/j.drugalcdep.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schroeder JA, Quick KF, Landry PM, Rawls SM. Glutamate transporter activation enhances nicotine antinociception and attenuates nicotine analgesic tolerance. Neuroreport. 2011;22(18):970–973. doi: 10.1097/WNR.0b013e32834d87eb. [DOI] [PMC free article] [PubMed] [Google Scholar]