Abstract
MicroRNAs are fine tuners of diverse biological responses and are expressed in various cell types of the liver. Here we hypothesized that circulating microRNAs (miRNAs) may serve as biomarkers of liver damage and inflammation. We studied miRNA-122 that is abundant in hepatocytes and miR-155, -146a and -125b that regulate inflammation in immune cells in mouse models of alcoholic liver disease (ALD), drug (acetaminophen; APAP)-induced liver injury (DILI), and Toll-like receptor (TLR) 9+4 ligands-induced inflammatory cell-mediated liver damage. We found that serum/plasma miR-122 correlated with ALT increases in the liver damage caused by alcohol, APAP and TLR9 (CpG)+4 (LPS) ligands. MiR-155, a regulator of inflammation, was increased in serum/plasma in alcoholic and inflammatory liver injury. Alcohol failed to increase serum miR-122 in TLR4-deficient and p47phox-deficient mice that were protected from ALD. We found the most robust increase in plasma miR-122 in DILI and it correlated with the highest ALT levels. Consistent with the massive inflammatory cell infiltration in the liver, plasma miR-155 and miR-146a were significantly elevated after CpG+LPS administration. We show for the first time that, depending on the type of liver injury, circulating miRNAs show association either with the exosome-rich or protein-rich compartments. In ALD and in inflammatory liver injury, serum/plasma miR-122 and miR-155 were predominantly associated with exosome-rich fraction while in DILI/APAP injury these miRNAs were present in the protein-rich fraction. In conclusion, our results suggest that circulating miRNAs may serve as biomarkers to differentiate between hepatocyte injury and inflammation and the exosome versus protein association of miRNAs may provide further specificity to mechanisms of liver pathology.
Keywords: Biomarker, miR-122, miR-155, TLR9, LPS, acetaminophen, ALT, TNF
MicroRNAs (miRNAs) are a class of RNA molecules (18–21 nucleotides), which do not encode for any protein but regulate ~ 30% of total genes in the body [1]. After their discovery in 1993, various roles of miRNAs have been described in development, immunity, stem cell differentiation and cancer [1, 2]. Recent studies suggest that miRNAs are not only localized within the cell (cellular miRNA) but are also present in circulation making them attractive for biomarker discovery [3].
Various agents ranging from chemicals to viruses trigger liver damage that is clinically evaluated by biochemical markers including serum aminotransferases (ALT and AST). However, there are several limitations of these clinically utilized markers of liver damage including requirement of fresh blood samples, lack of tissue specificity and inability to distinguish between hepatocyte damage and inflammation; two important determinants of liver pathology leading to chronic liver disease. Circulating miRNAs possess several advantages over current clinical “standards”. MiRNAs have tremendous stability in extreme conditions (low pH acidic environment, RNAase resistance) and are considered alternative non-invasive biomarkers [3, 4]. Moreover, various disease-specific circulating miRNA signatures have been reported in recent years [4, 5].
MiR-122 is abundant in hepatocytes and recent studies have demonstrated that increased circulating miR-122 is associated with hepatitis C virus infection, hepatocellular carcinoma, acetaminophen (APAP) and acute alcohol-induced liver injury [6–8]. MiR-122 has diverse functions in hepatocytes of which regulation of cholesterol metabolism is highly relevant to liver steatosis [9]. MiR-155, a master regulator of inflammation is upregulated in inflammation-related diseases [10–12]. MiRNA-155 targets multiple components of the pro-inflammatory cytokine production cascade, TLR signaling and intracellular signaling pathways determining pro-and anti-inflammatory cytokines [13]. MiR-146a and miR-125b are also involved in regulation of inflammation [13].
The extent of hepatocyte damage and inflammation can differ between various etiologies of liver disease and may also change during the course of chronic liver disease. For example, prolonged/chronic alcohol induces fatty liver and additional inflammation promotes progression of chronic liver disease [14]. APAP-related drug-induced liver injury (DILI) is characterized by massive and coordinated hepatocyte death and little inflammation [15]. In contrast to APAP-induced DILI, liver damage induced by endogenous and exogenous danger molecules such as TLR9 ligand [unmethylated DNA rich in cytidine-phosphate-guanosine (CpG motif)] and TLR4 ligand [lipopolysaccharide (LPS)] results in robust inflammation and liver injury in mice [16].
In this study, we hypothesized that circulating microRNAs could be markers of liver damage and/or inflammation. We tested circulating miR-122 as a potential marker of hepatocyte damage and miR-155 as a marker of inflammation in different forms of liver injuries induced by alcohol, acetaminophen and CpG+LPS treatment. Our novel data show that increases in circulating miR-122 and miR-155 correlate with liver injury and inflammation respectively. Furthermore, we show for the first time that the association of miRNAs with exosomes could be characteristic for the type of injury causing liver damage.
Methods
Additional methods are provided in the supporting information.
Animal studies
Mice were housed in the UMASS animal facility and cared in compliance with the protocols approved by the Institutional Animal Use and Care Committee of the University of Massachusetts Medical School.
Serum and plasma microRNA analysis
Prior to total RNA isolation, equal volume of plasma or serum samples were thawed on ice, mixed with QIAzole (Qiagen), vortexed and incubated at RT for 5mins. Synthetic C. elegans (cel)-miR-39 was spiked and after this step miRNeasy kit protocol was followed as per instructions (Qiagen). TaqMan miRNA Assay (Applied Biosystems) was used to analyze the miRNA from serum or plasma samples. Cel-miR-39 was used to normalize the technical variation between the samples.
Exosome isolation
100ul of plasma/serum was mixed with ExoQuick exosome precipitation solution and exosome isolation was performed in accordance with the manufacturer’s protocol (SBI System Biosciences cat # EXOQ5A). Briefly, after incubation at 4°C for 30min, the samples were centrifuged at 1500g for 30min. The supernatant was referred as the protein-rich fraction whereas the pellet was denoted as the exosome-rich fraction. The exosome fraction was washed twice with PBS and lysed with QIAzole (Qiagen) or NP-40 buffer (1%NP-40, 150mM NaCl, EDTA plus protease inhibitors) for total RNA or protein isolation respectively. Of note, in our study, we expect microparticles (~200–1000nm) to be in protein-rich fraction as the exosome precipitation solution precipitates up to 90nm size particles.
Statistical analysis
For correlation analysis, Pearson’s correlation test was performed in GraphPad Prism software. Non-parametric Mann-Whitney test or two-tailed T test was employed for statistical analysis. P<0.05 was considered statistically significant.
Results
Increased circulating miR-122 and miR-155 in alcoholic liver disease
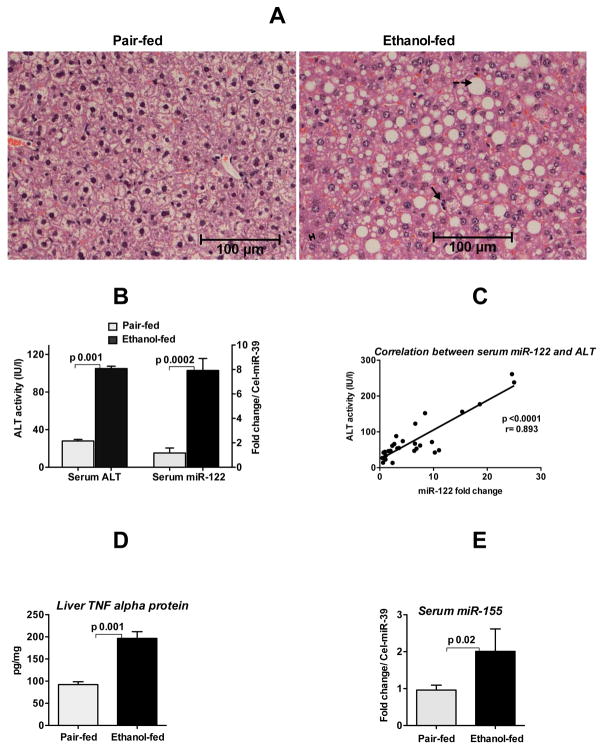
Alcoholic liver disease is characterized by fat accumulation and activation of the pro-inflammatory cytokine cascade in the liver [14]. Alcohol-fed mice showed significant fat accumulation in hepatocytes, mild necrosis and inflammatory infiltrate and increased serum ALT compared to isocaloric diet-fed controls (Fig. 1A &B left panel). To test the hypothesis that circulating miRNAs may serve as biomarkers of liver injury, we evaluated miR-122 that is uniquely abundant in hepatocytes. We found a profound increase in serum miR-122 levels in alcohol-fed mice (Fig. 1B right panel). Furthermore, a positive linear correlation was present between serum ALT and serum miR-122 levels (Fig. 1C).
Fig. 1. Increased circulating miR-122 and miR-155 in alcoholic liver disease.
Eight-week old C57BL/6 female mice received Liber De-Carli diet either with 5% alcohol (alcohol-fed) or isocaloric diet (pair-fed) for 5weeks. A. Hematoxylin and eosin (H&E) staining of liver sections fixed in formalin. Solid arrow indicates immune cells infiltrate, broken arrow indicates fat accumulation in hepatocytes and double arrow indicates mild necrosis of hepatocytes. B. ALT and miR-122 levels (TaqMan qRT-real time PCR) were measured in the serum as described in methods (n=6–8). C. Correlation between serum ALT and serum miR-122 was determined by Pearson method (n=30). D. The protein level of TNFα was measured from the whole cell lysate of liver homogenates by ELISA and normalized to protein concentration (n=6–8). E. MiR-155 expression was detected in the serum by TaqMan qRT-real time PCR (n=5). Synthetic C. elegans miR-39 was used to normalize Ct values. Fold change was calculated compared to pair-fed mice. Data represent mean ± SEM. Statistical analysis was performed with Two-tailed t-test (B&D) or non-parametric Mann-Whitney test (E).
Because the distribution of miRNA may change during serum collection [17], we evaluated miR-122 levels both in serum and plasma fractions in mice after alcohol feeding and found comparable levels (Supporting Fig. 1A&B). We also found that the extent of alcohol-induced increase in miR-122 was similar regardless of the internal controls (miR-16 or miR-223) in both fractions (Supporting Fig 1. A–D). These results suggested that chronic alcohol consumption resulted in miR-122 increase in the circulation.
Alcohol consumption not only causes fat accumulation in the liver but also results in inflammation via induction of pro-inflammatory cytokines such as TNFα, IL-6 and IL-1β [14]. Recently, we reported that alcohol increases miR-155 in macrophages, regulating TNFα production [18]. Consistent with this, TNFα levels were increased in the liver of alcohol-fed mice (Fig. 1D). Furthermore, we found increased serum miR-155 levels in alcohol-fed mice (Fig. 1E). Serum levels of miR-125b and miR-146a that also regulate inflammation were not significantly changed (data not shown) suggesting that alcohol-induced liver inflammation correlates with increased serum miR-155 levels.
TLR4 deficiency or disruption of oxidative stress prevents increased circulating miR-122 in ALD
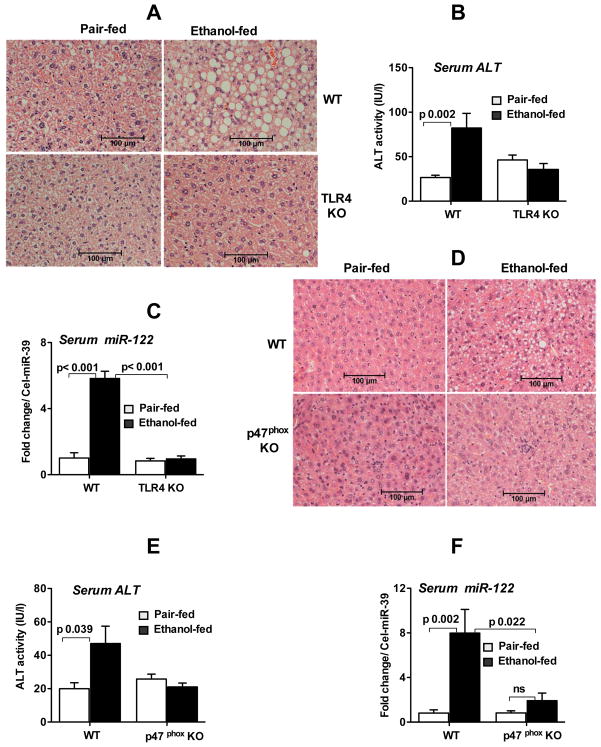
LPS, a TLR4 ligand, plays a key role in the pathogenesis of ALD. Previously, our laboratory demonstrated the critical role of TLR4 in ALD and showed that TLR4-deficient (TLR4KO) mice were protected from ALD [19]. Since we found increased serum miR-122 in alcohol-fed mice, we hypothesized that miR-122 increase correlates with liver damage rather than the alcohol intake itself. We found that wild-type but not TLR4KO mice showed significant liver damage, steatosis and serum ALT increase after alcohol feeding (Fig. 2A&B) and importantly, there was no increase in serum miR-122 induction in TLR4KO mice after alcohol feeding (Fig. 2C). These results imply that serum miR-122 increase after alcohol administration occurred only in the presence of liver damage.
Fig. 2. TLR4 deficiency or disruption of oxidative stress prevents increased serum miR-122 in ALD.
Wild type (WT) or TLR4- or p47phox - deficient mice (KO) were fed with isocaloric (pair-fed) or alcohol (ethanol-fed) containing Liber De-Carli diet for 5 weeks. A. H&E staining of formalin fixed liver sections from WT and TLR4KO mice. B. Serum ALT analysis of WT and TLR4KO mice (n=6–7). C. The expression of miR-122 in the serum isolated from WT and TLR4KO mice was quantified by TaqMan qRT-real time PCR (n=5–7). D. Histopathology (H&E) of liver sections from WT and p47phox KO mice. E. Serum ALT analysis of WT and p47phox KO mice (n=6–7). F. The expression of miR-122 in the serum was determined by TaqMan qRT-real time PCR in WT and p47phox KO mice (n=6). Spiked C. elegans miR-39 was used to normalize Ct values. Fold change was calculated compared to pair-fed mice. Data represent mean ± SEM. Two-tailed T-Test (B,C&E) or non-parametric Mann-Whitney test (F) was employed for statistical analysis.
Increased reactive oxygen species (ROS) generated after alcohol metabolism or LPS contribute to the pathogenesis of ALD. Specifically, the NADPH oxidase enzyme complex plays a role in ROS generation in ALD and mice deficient in the p47phox subunit (p47phoxKO) of NADPH oxidase are protected from alcoholic liver injury [20, 21]. Therefore, we next evaluated the role of ROS in the induction of serum miR-122 levels. Histopathological and biochemical studies indicated no significant fat accumulation or ALT increase in p47phoxKO mice compared to wild type mice after alcohol feeding (Fig. 2D&E). While alcohol-fed wild type mice showed significant upregulation of serum miR-122, there was no significant increase in serum miR-122 in p47phoxKO mice compared to pair-fed controls (Fig. 2F) implying that ALT and serum miR-122 levels mirror one another and serum miR-122 could be a marker of liver damage in ALD.
APAP administration results in increased circulating miRNA-122, -155, -125b and -146a levels
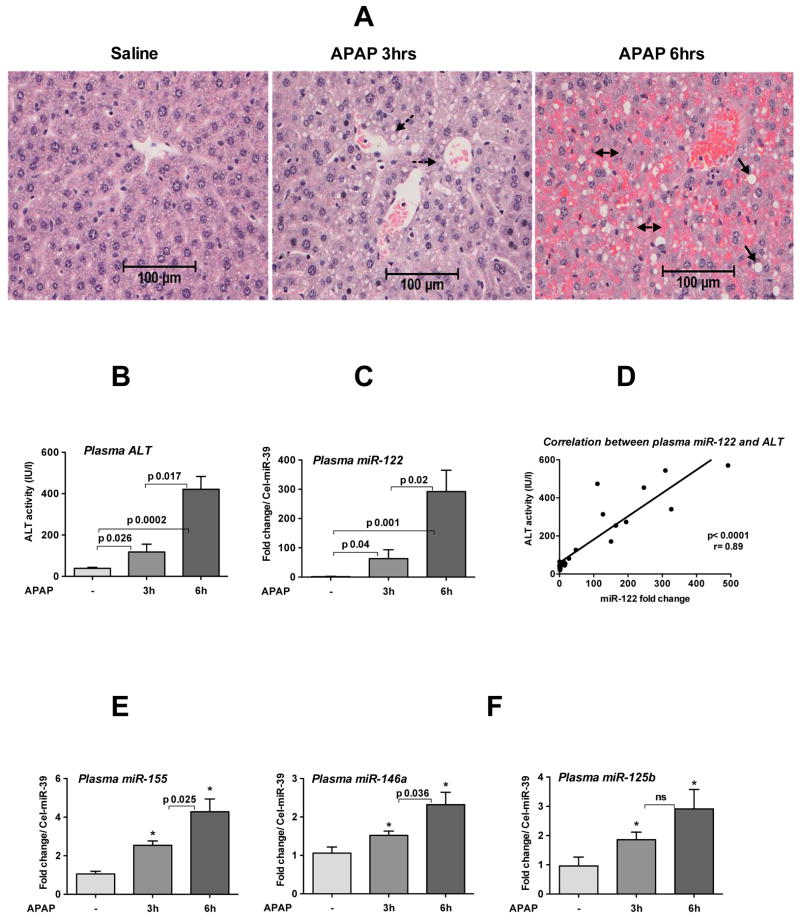
APAP-induced acute liver failure is a significant cause of DILI and mortality worldwide [22]. ALT and AST are routinely used methods to assess liver damage after APAP overdose and here we evaluated the utility of circulating miRNAs. APAP administration (500mg/kg; lethal dose) for 3h resulted in a moderate degeneration of hepatocytes with cytoplasmic vacuoles accompanied by extensive centrilobular necrosis, parenchymal hemorrhages and large cytoplasmic vacuoles after 6h (Fig. 3A). Furthermore, we found a robust time-dependent increase in ALT that correlated with increases in circulating miR-122 after 3h and 6h of APAP treatment (Fig. 3B–D). Since APAP administration induces necrosis that could be accompanied by inflammation, we next examined the inflammatory miRNA, miR-155. We found a time dependent increase in miR-155 in the plasma of mice treated with APAP (Fig. 3E) and other miRNAs involved in inflammation, miR-146a and miR-125b were also increased compared to saline controls (Fig. 3F left and right). No changes were observed in plasma miR-181d expression after 3h of APAP treatment (data not shown). These results indicated that APAP administration results in a robust increase in hepatocyte specific miR-122 and a modest increase of inflammatory miRNAs (miR-155,-146a, and -125b) in the plasma and these miRNA signatures could potentially represent biomarkers of APAP-induced liver injury.
Fig. 3. APAP administration induces a time-dependent increase of miRNA in the plasma.
Wild type female mice (8 weeks old) were fasted overnight as describes in methods and next day, some mice received saline or APAP (500mg/kg, lethal dose) for 3h or 6h. A. H&E staining of formalin fixed liver sections. Broken arrows indicate centrilobular cytoplasmic vacuoles, solid arrows indicate large cytoplasmic vacuoles and double arrows indicate extensive parenchymal hemorrhages. B. Plasma ALT levels (n=8). C. The expression of miR-122 in the plasma was quantified by TaqMan qRT-real time (n=8). D. Correlation between plasma ALT and miR-122 was determined by Pearson method (n=23). E-F. The relative expression of miR-155 (E), -146a (F left) and miR-125b (F right) in plasma was determined by TaqMan qRT-real time PCR (n=8). Spiked C. elegans miR-39 was used as internal control. Fold change over saline treated mice is shown. Data represent mean ± SEM. Non-parametric Mann-Whitney test was employed for statistical analysis.
Increased plasma miRNA-122, -155 and -146a in inflammation-induced liver injury
Emerging evidence suggests that both pathogen- and damage-associated molecular pattern molecules (PAMPs and DAMPs) contribute to liver inflammation. DAMPs include mitochondrial DNA and cells undergoing apoptosis that harbor CpG motif and is recognized by TLR9 and contribute to the activation of downstream signaling cascade [16]. Because of the emerging role of TLR9 in sterile inflammation, autoimmunity and in liver diseases [16, 23, 24], we evaluated the efficacy of circulating miRNA as a marker of liver injury and inflammation after TLR9+TLR4 ligand administration.
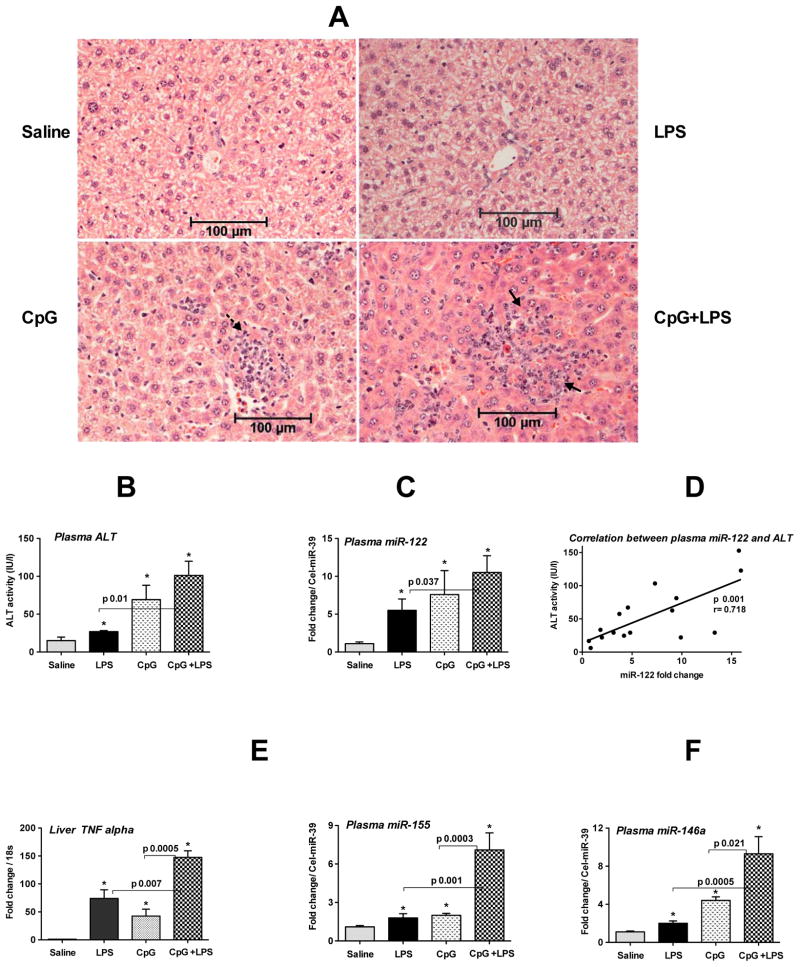
Consistent with our previous report, CpG administration resulted in intense mononuclear inflammatory infiltrates in the liver and a further increase in inflammatory foci were observed when mice were challenged with LPS (Fig. 4A) [24]. Both plasma ALT and miR-122 were increased in LPS- or CpG-treated mice and a further augmentation in plasma ALT and miR-122 was observed in CpG-primed-LPS challenged mice (Fig. 4B&C). Moreover, a linear correlation existed between plasma ALT and miR-122 levels (Fig. 4D).
Fig. 4. Increased plasma miRNA-122, -155 and -146a in CpG+LPS-induced liver inflammation.
Wild type female mice (10–12 weeks old) were injected with 2.5mg/kg CpG DNA (i.p.) once a day for three days and on day 4 some mice received 0.5mg/kg LPS or saline for 3h and sacrificed. A. H&E staining of formalin fixed liver sections. Broken arrow indicates mononuclear inflammatory cells and solid arrows indicate inflammatory foci B. Plasma ALT levels (n=4–6). C. The expression of miR-122 in the plasma was quantified by TaqMan qRT-real time (n=4–6). D. Correlation between plasma ALT and miR-122 was determined by Pearson method (n=17). E. The expression of TNFα was measured by real time PCR using TNFα gene-specific primer and normalized to 18S. F. The relative expression of miR-155 (left) and miR-146a (right) in plasma was determined by TaqMan qRT-real time PCR and spiked C. elegans miR-39 was used as internal control (n=4–6). Fold change over saline treated mice is shown. Data represent mean ± SEM. Non-parametric Mann-Whitney test was employed for statistical analysis.
As there was increased infiltration of immune cells in CpG and in CpG-primed-LPS challenged mice, we next evaluated the level of inflammatory cytokine, TNFα, and its positive regulator, miR-155. Both plasma miR-155 and hepatic TNFα were increased in LPS- or CpG-treated mice with even higher levels in CpG-primed LPS challenged mice (Fig. 4E, right & left). We found comparable levels of miR-155 both in plasma and serum fractions (Supporting Fig. 2). A linear correlation was observed between hepatic miR-155 and TNFα levels (Supporting Fig. 3). We also found increases in plasma miR-146a in the presence of liver inflammation in the CpG model (Fig. 4F) but no change in miR-125b expression (data not shown). These observations suggested that plasma miR-122 and miR-155 mirror hepatocyte damage and inflammation, respectively.
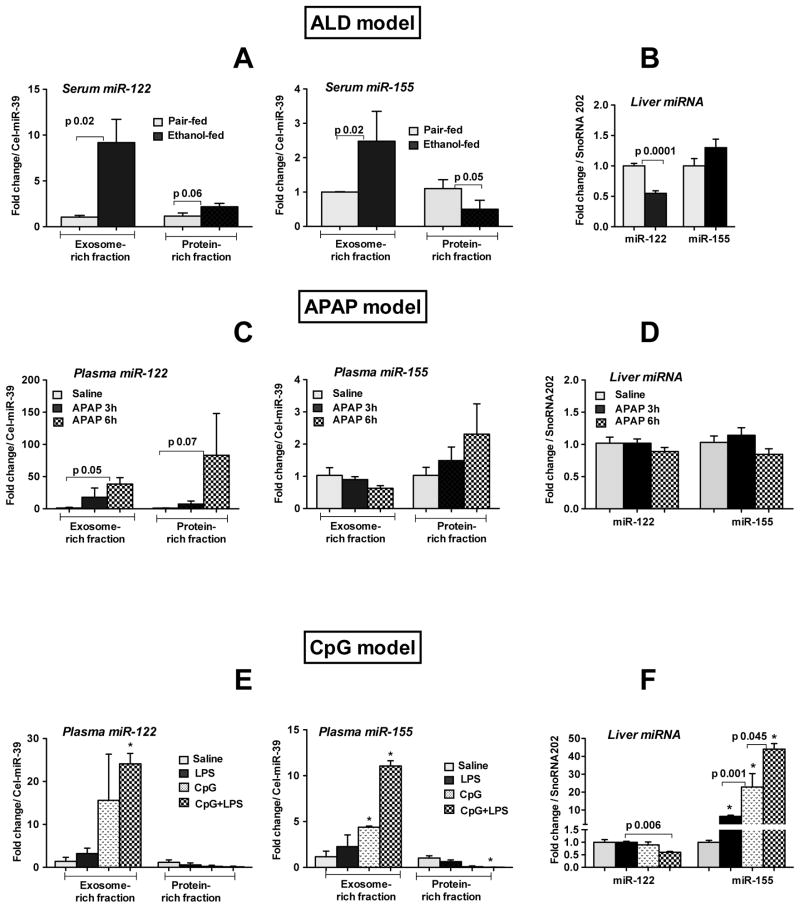
Circulating miRNAs localize to the exosome-rich or protein-rich compartments in a liver injury-specific manner
Recent studies showed that extracellular miRNAs are associated with Ago complexes or packaged inside exosomes in cell lines or in healthy human plasma [17, 25] but little is known about the fate of extracellular miRNAs after liver injury. Here, we examined exosome-rich or protein-rich (exosome free) fractions from the serum or plasma for miR-122 and miR-155 expression in the different liver injury models. We found that in alcohol-induced liver damage which results in fat accumulation and increased inflammatory cytokines, both miR-122 and miR-155 were mostly associated with the exosome-rich fraction (Fig. 5A left & right). A minimal increase in miR-122 and a decrease in miR-155 were observed in protein-rich fraction (Fig. 5A left & right). Because of the induction of miR-122 and miR-155 in the circulation, we next tested total liver levels of these miRNAs and found that the hepatic expression of miR-122 was decreased in alcohol-fed mice whereas miR-155 levels were moderately increased (Fig. 5B).
Fig. 5. Circulating miRNAs localize to the exosome-rich or protein-rich compartments in a liver injury-specific manner.
Exosome-rich or protein-rich fractions were isolated with ExoQuick Exosome Precipitation solution (SBI, System Biosciences) from serum or plasma as described in methods and lysed with QIAzole (Qiagen). Total RNA was isolated with miRNeasy kit (Qiagen) and used for miRNA detection. The expression of miR-122 and miR-155 was quantified in exosome-rich or protein-rich fractions isolated from serum (A left & right; ALD model) and plasma (C left & right; APAP model and E left & right; CpG model). B&D&F Expression of miR-122 and miR-155 was quantified in the liver after alcohol (B), APAP (D) and CpG+LPS (F) treatments (n=4–8). Spiked C. elegans miR-39 (serum or plasma) and SnoRNA202 (liver) was used as internal control. The fold change over pair-fed (5A,B) or saline (5C–F) treated mice is shown. Data represent mean ± SEM. * p<0.05 compared to saline treated mice. Non-parametric Mann-Whitney test was employed for statistical analysis.
In APAP-induced liver injury associated with massive hepatocyte necrosis, miR-122 was more abundant in the plasma protein-rich fraction compared to the exosome-rich fraction (Fig. 5C left). Moreover, a time-dependent increase in miR-155 was found in the protein-rich fraction with a concurrent decrease in exosome fraction (Fig. 5C right). However no significant changes were found in the hepatic miR-122 and miR-155 expression (Fig. 5D).
Finally, in the CpG+LPS-induced inflammation model that mainly causes inflammation, miR-122 and miR-155 were predominately increased in the exosome-rich fraction and decreased in the plasma protein-rich fraction (Fig. 5E left&right). The hepatic expression of miR-122 was unchanged in LPS or CpG treated mice, however CpG priming followed by LPS challenge resulted in its reduction (Fig. 5F). There was a robust induction of miR-155 by LPS and CpG treatment, which was further augmented after LPS challenge in CpG-primed mice (Fig. 5F).
To rule out the possibility that freeze thaw interferes with miRNA levels in exosome- or protein-rich fractions, the expression of miR-122 and miR-155 were compared between fresh and frozen plasma samples after APAP treatment. The expression of these miRNAs in the protein- or exosome-rich fraction was comparable both in fresh and frozen samples (Supporting Fig. 4). The purity of the exosome fraction was confirmed by CD81 (exosome marker) expression and an increase in its level was observed only in the exosome-rich fraction (Supporting Fig. 5). Taken together, our results suggest that the distribution and extent of miR-122 and miR-155 (exosomes versus proteins) differs between ALD, APAP and CpG-induced liver damage.
Discussion
MicroRNAs have emerged as fine regulators of gene function and their presence in various body fluids identifies them as attractive potential biomarkers of disease [3, 5]. In this report, we show that serum/plasma miR-122 and miR-155 may serve as biomarkers of liver damage and inflammation, respectively. Our results suggest that increase in circulating miR-122 correlates with liver damage regardless of the etiology of hepatocyte injury. We also identified miR-155 as a candidate biomarker of liver inflammation. Our novel data demonstrate that miR-122 and miR-155 are present in the serum/plasma in exosome- or protein-rich fractions and show for the first time that miR-122 and miR-155 are associated with exosome-rich fraction in alcoholic and inflammatory liver damage while they are predominantly in the protein-rich fraction in APAP-induced liver necrosis.
Current clinical practice utilizes serum ALT/AST as markers of liver “injury”, however, these cannot distinguish between hepatocyte damage and inflammation. Serum cytokines are surrogate markers of liver inflammation but are not practical in routine clinical practice. Moreover, ALT and serum cytokines are not stable in extreme conditions and lack tissue specificity, creating a need for more sensitive, stable and specific biomarkers of liver injury. Induction of miR-122 has been reported in HCC, HCV and APAP-induced liver damage [6–8] and our results further extend the feasibility of plasma/serum miR-122 as a marker of liver injury. Because our results indicated that increased serum/plasma miR-122 is universally associated with liver disease of different etiologies, the limitation of this potential biomarker is that it alone cannot distinguish between various etiologies of hepatocyte damage. Similarly our results suggest that increase of plasma/serum miR-155 cannot be used to distinguish inflammation caused by various etiologies i.e. alcohol uptake or DAMPs and or drug overdose, however the extent of its induction in the circulation varies between different etiologies. Future studies with broader miRNA profiling may reveal etiology-specific miRNA signatures in liver diseases.
Upregulation of the inflammatory cascade has been attributed to Kupffer cell activation by gut-derived endotoxin in ALD [14]. We recently reported that alcohol-induced miR-155 sensitizes KCs to LPS-induced TNFα production in ALD [18]. Here we found increased serum and liver miR-155 levels in alcohol-fed mice. Increased circulating miR-155 levels were also reported in rheumatoid arthritis and various cancer types [26, 27] and our results support the increase of extracellular miR-155 during inflammation and its use as a marker of inflammation [27]. Furthermore, we demonstrated that serum miR-122 increase in ALD was indeed a marker of liver disease rather than a direct effect of alcohol since mice deficient in TLR4 and p47phox subunit of NADPH oxidase enzyme complex (p47phoxKO) showed no increase in serum miR-122. Relevant to our finding, a recent study reported increased miR-122 in plasma after 6h of acute ethanol gavage in mice [8]. To our knowledge our study is the first to demonstrate the induction of miR-122 in the circulation after prolonged alcohol feeding.
APAP-induced acute liver injury is a major cause of liver failure in western countries [22]. Here, we showed that plasma miR-122 which mirrored ALT levels could be exploited as a marker of APAP-induced liver injury. Our observation is consistent with a previous report, however the dose and duration of APAP administration were different in our study [7]. Similarly, a recent study demonstrated increased miR-122 in the plasma of APAP overdosed humans [28]. However, in our study we found that not only the levels of plasma miR-122 but miR-155, miR-146a and miR-125b were also increased after APAP treatment in a time-dependent manner. Our observation of increased miR-155, miR-146a and miR-125b was somewhat unexpected because APAP injury is not associated with significant inflammation. Unlike in the alcohol-induced or inflammatory liver injury (CpG+LPS) model, where liver miR-155 levels were increased, we found no upregulation of miR-155 expression in APAP liver injury leading us to the speculation that the increase in plasma miR-155, miR-146a and miR-125b could be a result of APAP-induced massive death of hepatocytes and immune cells in the liver rather than induction of the inflammatory miRNA repertoire. Further investigation of miRNA profile of APAP liver injury between lethal and sublethal doses may lead to more specific markers.
Emerging evidence suggests that endogenous danger signals (DAMPs) from dying cells, including DNA, induce sterile inflammation and autoimmunity [23]. Endogenous danger signals such as mitochondrial DNA (TLR9 ligand) are likely to act on the liver in combination with gut-derived endotoxin (TLR4 ligand) present in various forms of liver diseases such as ALD, NASH and portal hypertension [19, 29, 30]. In agreement with this, increased expressions of TLR9 and serum endotoxin have been reported in alcohol-fed mice [31, 18]. Our finding of induction of miR-122 in the plasma after CpG treatment further extends the utility of circulating miR-122 as a marker of liver injury caused by DAMPs. Consistent with CpG- and/or LPS-induced inflammation, plasma levels of miR-155 were significantly increased. Induction of circulatory miR-155 has been reported in various cancers [26, 27] and our finding indicates its induction in liver inflammation. Interestingly, miR-146a but not miR-125b was also increased in the plasma after CpG+LPS treatment. Finding increased miR-146a in plasma after CpG+LPS treatment was surprising. In support of our data, increased levels of miR-146a have been reported in patients with type 2 diabetes idiopathic pulmonary hypertension and in patients with IgA nephropathy [32, 33]. The significance of miR-146a induction in plasma in our study could be attributed to its role in limiting inflammation, however further studies are warranted to rule out this possibility. Interestingly, only a modest increase in hepatic levels of miR-146a was observed after CpG treatment (Supporting Fig. 6), indicating its specific release into circulation. Although alcohol, APAP and CpG all resulted in liver injury as indicated by ALT, the circulating miRNA signature and extent of miR-122 increases (Supporting Fig. 7) varied between the etiologies. To compare the characteristics of ALT versus miR-122 to discriminate between mice with liver injury (alcohol or APAP) and control mice, we calculated area under the receiving operating characteristics curve (AUROC). While our analysis indicated that ALT and miR-122 have similar AUROC values in the ALD model, a slightly higher AUROC was found in APAP model for miR-122 (Supporting Table 1). In future, study with large clinical sample size will be able to predict the superior sensitivity, diagnostics and prognostics values of miR-122 over ALT.
Recent studies highlight the importance of extracellular miRNA in cell-to-cell communication and may also provide a mode of genetic information transfer [34, 35]. Extracellular miRNA can be released from cells via different processes such as exosomes, microparticles and proteins or unidentified mechanisms [36]. The association of miR-122 with protein fractions has been demonstrated in healthy human plasma samples [17]. However, a recent study suggests that majority of miRNAs in the serum and saliva is concentrated in exosomes [37]. Our results indicate that extracellular distribution of miRNA is distinct in different pathological conditions in the liver. In ALD and in inflammation (CpG/LPS)-induced liver damage both miR-122 and miR-155 increases were associated with exosome-rich fraction. In contrast, miR-122 and miR-155 were predominantly increased in the protein-rich fractions in APAP/DILI suggesting injury-specific distribution of miRNA in the circulation. There could be multiple mechanisms via which miRNA enriches in exosomes versus protein fractions after different types of liver injury. For instance, in APAP-induced liver injury, the damage is severe and rapid and miRNAs released primarily because of leakage from dying/damaged cells which we speculate to explain why most miRNAs (miR-122 and -155) are found in the protein fraction (as miRNA normally bound in the RISC complexe with in the cell). On the other hand, in more subtle injuries such as ethanol and CPG+LPS models, the cellular injury is slower and less severe and perhaps altered miRNA processing and release via exosomes which could be the reason of enrichment in the exosome-rich fraction.
The tissue and cellular sources of the miR-122 and miR-155 increase are yet to be fully indentified in liver disease. MiR-122 is abundant in hepatocytes representing about 70% of the total miRNAs and mechanisms underlying changes in liver expression of miR-122 are yet to be understood. MiRNA-155 is present but not limited to immune cells and our observation indicates that it is also present in hepatocytes [38]. Based on the observation that liver miR-122 levels were decreased only in ALD and after CpG+LPS administration irrespective of its increase in circulation (ALD, APAP and CpG+LPS), it is tempting to speculate that multiple hits may contribute to downregulation of hepatic miR-122 levels, where as damaged hepatocytes contribute to increased circulating miR-122 levels. However, further studies are required to discern the pathways responsible for decreased hepatic levels of miR-122 after liver injury. Contrary to our observation, Wang et al [7] reported decreased hepatic miR-122 levels after APAP treatment and this could be due to different APAP doses and timing.
In contrast to miR-122, miR-155 appears to be an inducible miRNA in alcoholic and inflammation (CpG+LPS)-induced liver disease as its levels were increased not only in the serum/plasma but also in the liver. This is not unexpected because TLR activation was shown to upregulate miR-155 in immune cells and our study now shows that it can also be upregulated in the liver. In immune cells and in KC, miR-155 induction involves NF-κB activation [18] and NF-κB activation has been reported both in alcohol-induced liver disease and CpG+LPS-induced liver inflammation [18, 39]. The lack of miR-155, miR-146a and miR-125b increase in the liver along with increase in the plasma after APAP intervention may be the result of cellular damage/death in the immune cell compartment as APAP was shown to induce immune cell as well as hepatocyte death [15]. Collectively, our results indicate stimuli-specific miRNA signatures both in the liver and in the plasma/serum. It will be interesting to explore what signal is required for the assembly of extracellular miRNA into exosomes versus protein compartments in liver injury.
Supplementary Material
Acknowledgments
Supported by NIAAA grants # AA017729, AA020744 and DK075635
List of Abbreviations
- ALT
Alanine aminotransferase
- AST
Asparate aminotransferase
- ALD
Alcoholic liver disease
- DILI
Drug-induced liver injury
- APAP
Acetaminophen
- CpG
Cytidine-phosphate-guanosine
- LPS
Lipopolysaccharide
- H&E
Hematoxylin and Eosin
- ROS
Reactive oxygen species
- KO
Knock out
- NADPH
Nicotinamide adenine dinucleotide phosphate-oxidase
- KC
Kupffer cells
- HCC
hepatocellular carcinoma
- HCV
hepatitis C Virus
References
- 1.Ambros V. The functions of animal microRNAs. Nature. 2004;7006:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 2.Bala S, Marcos M, Szabo G. Emerging role of microRNAs in liver diseases. World J Gastroenterol. 2009;45:5633–5640. doi: 10.3748/wjg.15.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;10:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;30:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji F, Yang B, Peng X, Ding H, You H, Tien P. Circulating microRNAs in hepatitis B virus-infected patients. J Viral Hepat. 2011;7:e242–51. doi: 10.1111/j.1365-2893.2011.01443.x. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;2:136–142. doi: 10.1002/mc.20712. [DOI] [PubMed] [Google Scholar]
- 7.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;11:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, et al. Plasma MicroRNA-122 as a Biomarker for Viral-, Alcohol-, and Chemical-Related Hepatic Diseases. Clin Chem. 2010;12:1830–1838. doi: 10.1373/clinchem.2010.147850. [DOI] [PubMed] [Google Scholar]
- 9.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;2:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Kurowska-Stolarska M, Alivernini S, Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci U S A. 2011;27:11193–11198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tili E, Michaille JJ, Wernicke D, Alder H, Costinean S, Volinia S, et al. Mutator activity induced by microRNA-155 (miR-155) links inflammation and cancer. Proc Natl Acad Sci U S A. 2011;12:4908–4913. doi: 10.1073/pnas.1101795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;4:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol. 2011 doi: 10.1038/cmi.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabo G, Mandrekar P, Petrasek J, Catalano D. The Unfolding Web of Innate Immune Dysregulation in Alcoholic Liver Injury. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2010.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;1:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrasek J, Dolganiuc A, Csak T, Kurt-Jones EA, Szabo G. Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterology. 2011;2:697–708.e4. doi: 10.1053/j.gastro.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;12:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P, et al. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;2:1436–1444. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;4:1224–31. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin I, Petrasek J, Szabo G. The Presence of p47phox in Liver Parenchymal Cells is a Key Mediator in the Pathogenesis of Alcoholic Liver Steatosis. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;7:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig DG, Lee A, Hayes PC, Simpson KJ. Review article: the current management of acute liver failure. Aliment Pharmacol Ther. 2010;3:345–358. doi: 10.1111/j.1365-2036.2009.04175.x. [DOI] [PubMed] [Google Scholar]
- 23.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;2:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velayudham A, Hritz I, Dolganiuc A, Mandrekar P, Kurt-Jones E, Szabo G. Critical role of toll-like receptors and the common TLR adaptor, MyD88, in induction of granulomas and liver injury. J Hepatol. 2006;6:813–824. doi: 10.1016/j.jhep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;10:e13247. doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth C, Rack B, Muller V, Janni W, Pantel K, Schwarzenbach H. Circulating microRNAs as blood-based markers for patients with primary and metastatic breast cancer. Breast Cancer Res. 2010;6:R90. doi: 10.1186/bcr2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng D, Haddadin S, Wang Y, Gu LQ, Perry MC, Freter CE, et al. Plasma microRNAs as novel biomarkers for early detection of lung cancer. Int J Clin Exp Pathol. 2011;6:575–586. [PMC free article] [PubMed] [Google Scholar]
- 28.Starkey Lewis PJ, Dear J, Platt V, Simpson KJ, Craig DG, Antoine DJ, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;5:1767–1776. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- 29.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;1:323–34.e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin HC, Yang YY, Tsai TH, Huang CM, Huang YT, Lee FY, et al. The relationship between endotoxemia and hepatic endocannabinoids in cirrhotic rats with portal hypertension. J Hepatol. 2011;6:1145–1153. doi: 10.1016/j.jhep.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 31.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, et al. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;5:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 32.Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;1:61–69. doi: 10.1007/s00592-010-0226-0. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Kwan BC, Lai FM, Chow KM, Li PK, Szeto CC. Elevated levels of miR-146a and miR-155 in kidney biopsy and urine from patients with IgA nephropathy. Dis Markers. 2011;4:171–179. doi: 10.3233/DMA-2011-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;23:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;4:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salminen WF, Yang X, Shi Q, Mendrick DL. Using microRNA as Biomarkers of Drug-Induced Liver Injury. J Mol Biomark Diagn. 2011;2 (5):119. [Google Scholar]
- 37.Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;3:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bala S, Szabo G. MicroRNA Signature in Alcoholic Liver Disease. Int J Hepatol. 2012:498232. doi: 10.1155/2012/498232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LR, Kodys K, Dolganiuc A, Graham L, Velayudham A, Mandrekar P, et al. Diverse regulation of NF-kappaB and peroxisome proliferator-activated receptors in murine nonalcoholic fatty liver. Hepatology. 2004;2:376–85. doi: 10.1002/hep.20304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.