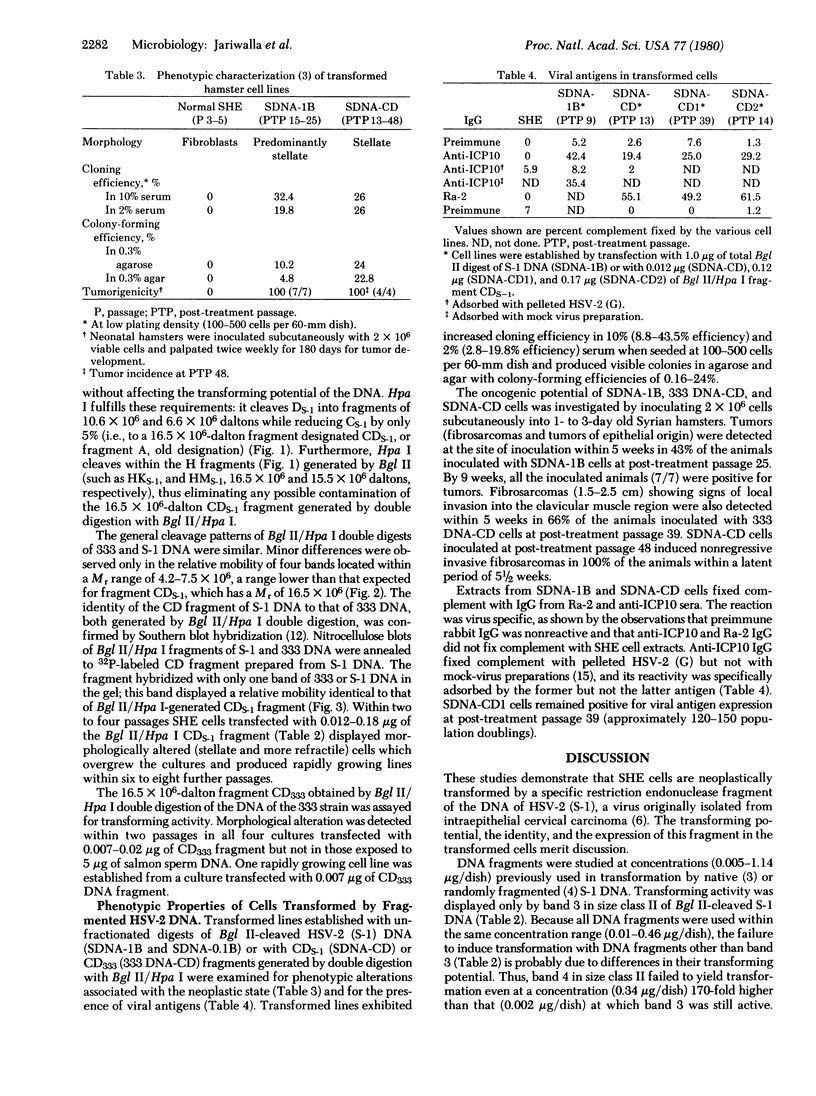
Abstract
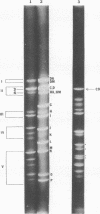
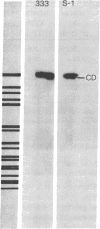
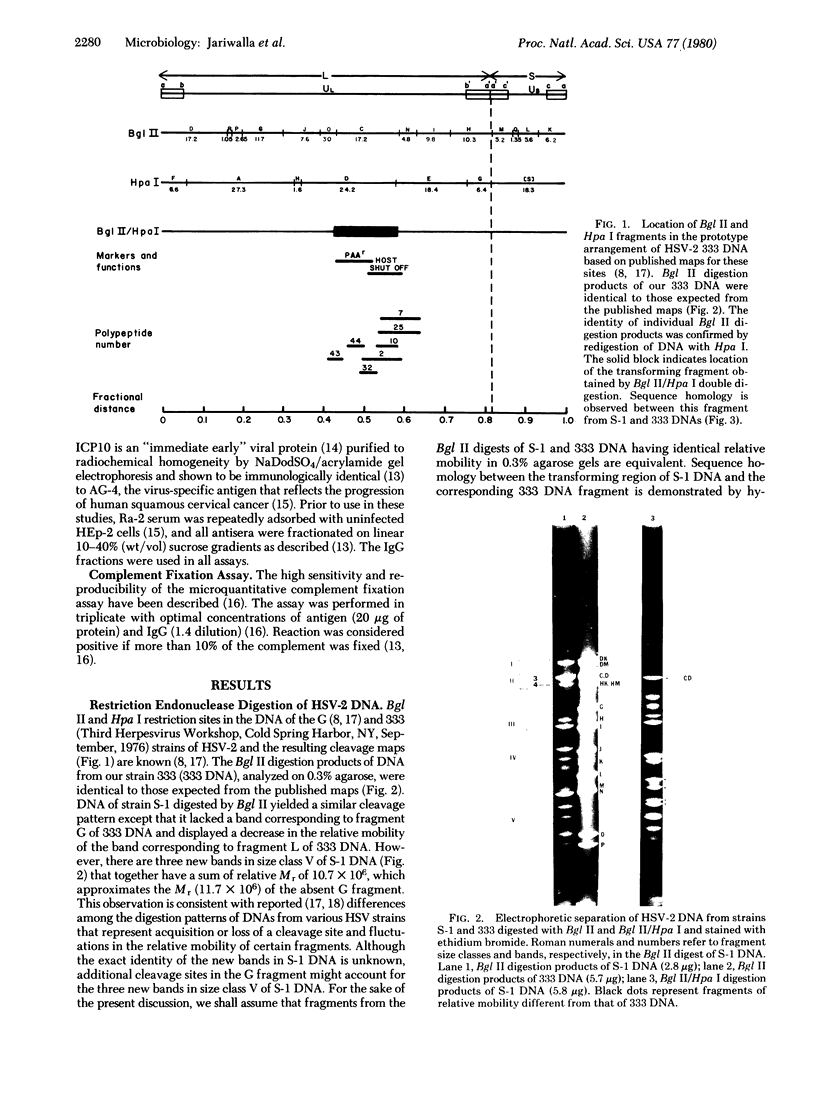
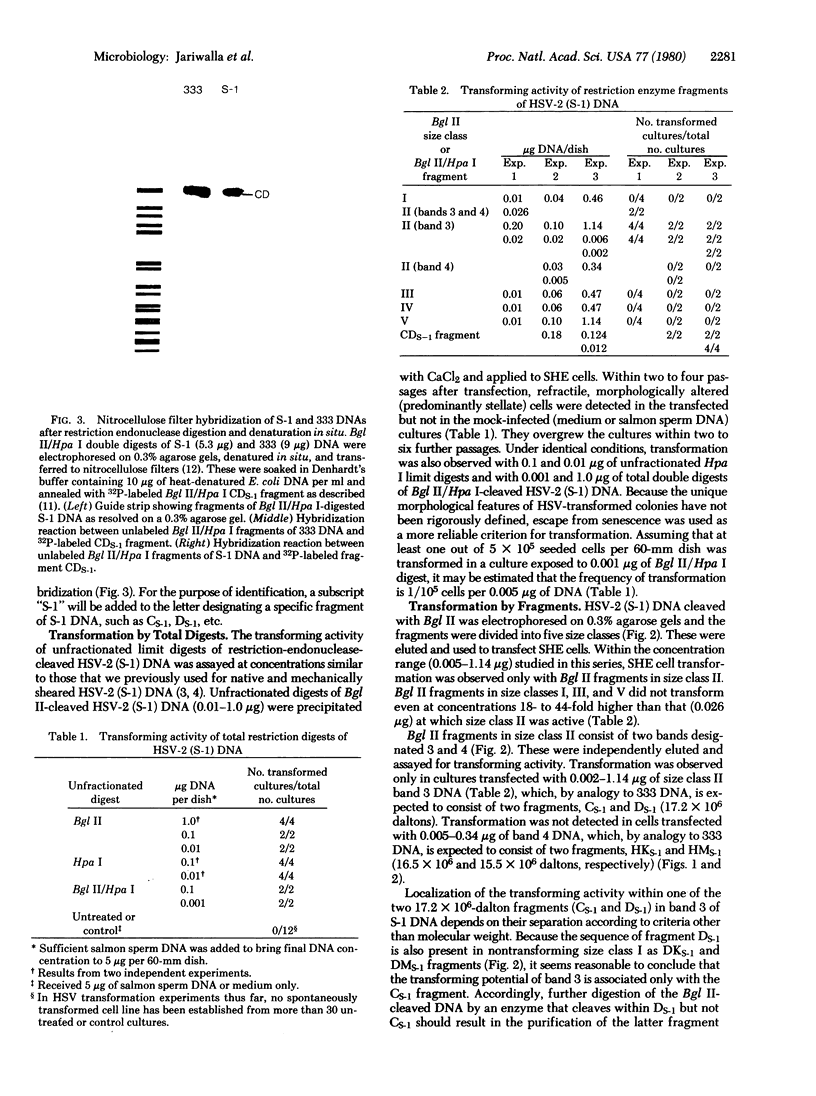
Transfection of Syrian hamster embryo cells with limit digests of Bgl II-, Hpa I-, or Bgl II/Hpa I-cleaved DNA from herpes simplex virus type 2 (strain S-1) but not with salmon sperm DNA resulted in the appearance of refractile, morphologically altered cells at a frequency of 10(-5)/0.005 microgram of viral DNA within two to four passages. Transformed lines manifested reduced serum requirement and anchorage-independent growth and were tumorigenic in newborn hamsters. They expressed ICP10, a viral protein immunologically identical to the cervical-tumor-associated AG-4 antigen. Transforming activity was localized in the 16.5 x 10(6)-dalton Bgl II/Hpa I double-digest fragment CDs-1, which exhibited sequence homology to the Bgl II/Hpa I fragment CD of DNA from herpes simplex virus type 2 strain 333, mapping between coordinates 0.43 and 0.58 on the physical map of strain 333 DNA. This fragment, CD333, was also shown to induce neoplastic transformation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurelian L., Schumann B., Marcus R. L., Davis H. J. Antibody to HSV-2 induced tumor specific antigens in serums from patients with cervical carcinoma. Science. 1973 Jul 13;181(4095):161–164. doi: 10.1126/science.181.4095.161. [DOI] [PubMed] [Google Scholar]
- Aurelian L., Strandberg J. D. Biologic and immunologic comparison of two HSV-2 variants: one an isolate from cervical tumor cells. Arch Gesamte Virusforsch. 1974;45(1-2):27–38. doi: 10.1007/BF01240539. [DOI] [PubMed] [Google Scholar]
- Aurelian L., Strnad B. C., Smith M. F. Immunodiagnostic potential of a virus-coded, tumor-associated antigen (AG-4) in cervical cancer. Cancer. 1977 Apr;39(4 Suppl):1834–1849. doi: 10.1002/1097-0142(197704)39:4+<1834::aid-cncr2820390816>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Camacho A., Spear G. Transformation of hamster embryo fibroblasts by a specific fragment of the herpes simplex virus genome. Cell. 1978 Nov;15(3):993–1002. doi: 10.1016/0092-8674(78)90283-0. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Locker H., Cox B., Roizman B., Rapp F. Herpes simplex virus DNA in transformed cells: sequence complexity in five hamster cell lines and one derived hamster tumor. J Virol. 1976 Jun;18(3):885–893. doi: 10.1128/jvi.18.3.885-893.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Frenkel N., Roizman B. Anatomy of herpes simplex virus DNA: strain differences and heterogeneity in the locations of restriction endonuclease cleavage sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1768–1772. doi: 10.1073/pnas.72.5.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwalla R. J., Aurelian L., Ts'o P. O. Neoplastic transformation of cultured Syrian hamster embryo cells by DNA of herpes simplex virus type 2. J Virol. 1979 Apr;30(1):404–409. doi: 10.1128/jvi.30.1.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera L. S., Gusdon J. P., Edwards I., Herbst G. Oncogenic transformation of rat embryo fibroblasts with photoinactivated herpes simplex virus: rapid in vitro cloning of transformed cells. J Gen Virol. 1977 Jun;35(3):473–485. doi: 10.1099/0022-1317-35-3-473. [DOI] [PubMed] [Google Scholar]
- Kurokawa T., Igarashi K., Sugino Y. Biochemical studies on bovine adenovirus type 3. III. Cleavage maps of viral DNA by restriction endoncleases EcoRI, BamHI, and HindIII. J Virol. 1978 Oct;28(1):212–218. doi: 10.1128/jvi.28.1.212-218.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Buchman T. G., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus DNA. IX. Apparent exclusion of some parental DNA arrangements in the generation of intertypic (HSV-1 X HSV-2) recombinants. J Virol. 1977 Oct;24(1):231–248. doi: 10.1128/jvi.24.1.231-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Rapp F., Reed C. Experimental evidence for the oncogenic potential of herpes simplex virus. Cancer Res. 1976 Feb;36(2 Pt 2):800–806. [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979 Mar;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Aurelian L. Proteins of herpesvirus type 2. II. Studies demonstrating a correlation between a tumor-associated antigen (AG-4) and a virion protein. Virology. 1976 Aug;73(1):244–258. doi: 10.1016/0042-6822(76)90078-7. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Aurelian L. Proteins of herpesvirus type 2. III. Isolation and immunologic characterization of a large molecular weight viral protein. Virology. 1978 Jun 15;87(2):401–415. doi: 10.1016/0042-6822(78)90144-7. [DOI] [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]