Abstract
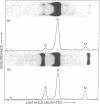
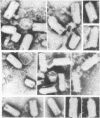
Earlier we reported a reduction to 1/30th-1/100th of the original number of infectious particles in the infectious vesicular stomatitis virus (VSV) released from L cells treated with 10 or 30 reference units of interferon per ml. However, in these cultures virus particle production, as measured by VSV particle-associated viral RNA, virus nucleocapsid protein, and viral transcriptase, was inhibited by less than 10%. Data reported in this paper show that there was a significant reduction in glycoprotein and membrane protein of VSV particles released from interferon-treated cells. Evidence supporting the deficiency of glycoprotein in VSV released from interferon-treated cells was derived from electron microscopic studies. Under conditions where glycoprotein spikes or projections were clearly detectable on the surface of VSV released from cells not treated with interferon, very few spikes were observed on VSV released from interferon-treated cells. These results suggested that interferon-treated cells produced VSV particles with low infectivity and that this low infectivity may be related to the reduced amount of glycoprotein and membrane protein incorporated into such particles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxt B., Sonnabend J. A., Bablanian R. Effects of interferon on vesicular stomatitis virus transcription and translation. J Gen Virol. 1977 May;35(2):325–334. doi: 10.1099/0022-1317-35-2-325. [DOI] [PubMed] [Google Scholar]
- Billiau A., Edy V. G., De Clercq E., Heremans H., De Somer P. Influence of interferon on the synthesis of virus particles in oncornavirus carrier cell lines. III. Survey of effects on A-, B- and C-type oncornaviruses. Int J Cancer. 1975 Jun 15;15(6):947–953. doi: 10.1002/ijc.2910150610. [DOI] [PubMed] [Google Scholar]
- Billiau A., Edy V. G., Sobis H., de Somer P. Influence of interferon on virus-particle synthesis in oncornavirus-carrier lines. II. Evidence for a direct effect on particle release. Int J Cancer. 1974 Sep 15;14(3):335–340. doi: 10.1002/ijc.2910140306. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Jay F. T., Friedman R. M. Physical, morphological, and biochemical alterations in the membrane of AKR mouse cells after interferon treatment. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1859–1863. doi: 10.1073/pnas.75.4.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Mims S. J., Triche T. J., Friedman R. M. Interferon inhibits mouse leukaemia virus release: an electron microscope study. J Gen Virol. 1977 Feb;34(2):363–367. doi: 10.1099/0022-1317-34-2-363. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Antiviral activity of interferons. Bacteriol Rev. 1977 Sep;41(3):543–567. doi: 10.1128/br.41.3.543-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Chang E. H., Ramseur J. M., Myers M. W. Interferon-directed inhibition of chronic murine leukemia virus production in cell cultures: lack of effect on intracellular viral markers. J Virol. 1975 Sep;16(3):569–574. doi: 10.1128/jvi.16.3.569-574.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Ramseur J. M. Inhibition of murine leukemia virus production in chronically infected AKR cells: a novel effect of interferon. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3542–3544. doi: 10.1073/pnas.71.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Miyamoto T., Hanafusa H. A type of chick embryo cell that fails to support formation of infectious RSV. Virology. 1970 Jan;40(1):55–64. doi: 10.1016/0042-6822(70)90378-8. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Weiss R. A. Selective isolation of mutants of vesicular stomatitis virus defective in production of the viral glycoprotein. J Virol. 1979 Apr;30(1):177–189. doi: 10.1128/jvi.30.1.177-189.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari R. K., Friedman R. M. Production of vesicular stomatitis virus with low infectivity by interferon-treated cells. J Gen Virol. 1979 Jul;44(1):261–264. doi: 10.1099/0022-1317-44-1-261. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Engelhardt D. L., Hunt J. M., Sekellick M. J. Interferon action: inhibition of vesicular stomatitis virus RNA synthesis induced by virion-bound polymerase. Science. 1971 Nov 5;174(4009):593–598. doi: 10.1126/science.174.4009.593. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogburn C. A., Berg K., Paucker K. Purification of mouse interferon by affinity chromatography on anti-interferon globulin-sepharose. J Immunol. 1973 Oct;111(4):1206–1218. [PubMed] [Google Scholar]
- Pitha P. M., Rowe W. P., Oxman M. N. Effect of interferon on exogenous, endogenous, and chroniv murine leukemia virus infection. Virology. 1976 Apr;70(2):324–338. doi: 10.1016/0042-6822(76)90275-0. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Staal S. P., Bolognesi D. P., Denny T. P., Rowe W. P. Effect of interferon on murine leukemia virus infection. II. Synthesis of viral components in exogenous infection. Virology. 1977 Jun 1;79(1):1–13. doi: 10.1016/0042-6822(77)90329-4. [DOI] [PubMed] [Google Scholar]
- Schnitzer T. J., Dickson C., Weiss R. A. Morphological and biochemical characterization of viral particles produced by the tsO45 mutant of vesicular stomatitis virus at restrictive temperature. J Virol. 1979 Jan;29(1):185–195. doi: 10.1128/jvi.29.1.185-195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengris V. E., Reynolds F. H., Jr, Hollenberg M. D., Pitha P. M. Interferon action: role of membrane gangliosides. Virology. 1976 Jul 15;72(2):486–493. doi: 10.1016/0042-6822(76)90177-x. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Yuen P. H., MacLeod R., Chang E. H., Myers M. W., Friedman R. M. The effect of interferon on de novo infection of Moloney murine leukemia virus. Cell. 1977 Feb;10(2):245–252. doi: 10.1016/0092-8674(77)90218-5. [DOI] [PubMed] [Google Scholar]
- de Giuli C., Kawai S., Dales S., Hanafusa H. Absence of surface projections of some noninfectious forms of RSV. Virology. 1975 Jul;66(1):253–260. doi: 10.1016/0042-6822(75)90195-6. [DOI] [PubMed] [Google Scholar]