Abstract
Plasma membrane vesicles from red beet (Beta vulgaris L.) storage tissue contain two prominent major intrinsic protein species of 31 and 27 kD (X. Qi, C.Y Tai, B.P. Wasserman [1995] Plant Physiol 108: 387–392). In this study affinity-purified antibodies were used to investigate their localization and biochemical properties. Both plasma membrane intrinsic protein (PMIP) subgroups partitioned identically in sucrose gradients; however, each exhibited distinct properties when probed for multimer formation, and by limited proteolysis. The tendency of each PMIP species to form disulfide-linked aggregates was studied by inclusion of various sulfhydryl agents during tissue homogenization and vesicle isolation. In the absence of dithiothreitol and sulfhydryl reagents, PMIP27 yielded a mixture of monomeric and aggregated species. In contrast, generation of a monomeric species of PMIP31 required the addition of dithiothreitol, iodoacetic acid, or N-ethylmaleimide. Mixed disulfide-linked heterodimers between the PMIP31 and PMIP27 subgroups were not detected. Based on vectorial proteolysis of right-side-out vesicles with trypsin and hydropathy analysis of the predicted amino acid sequence derived from the gene encoding PMIP27, a topological model for a PMIP27 was established. Two exposed tryptic cleavage sites were identified from proteolysis of PMIP27, and each was distinct from the single exposed site previously identified in surface loop C of a PMIP31. Although the PMIP31 and PMIP27 species both contain integral proteins that appear to occur within a single vesicle population, these results demonstrate that each PMIP subgroup responds differently to perturbations of the membrane.
The PM of higher plants has been the subject of extensive research focusing on the areas of recognition, water and ion transport, signal transduction, and cell wall polymer biosynthesis (Chrispeels and Maurel, 1994; Sussman, 1994; Delmer and Amor, 1995; Maurel, 1997; Schaffner, 1998). However, the PM remains one of the least-understood membrane systems in higher plants. Although PM vesicles are relatively simple to isolate and characterize, the hydrophobicity and low abundance of plant PM polypeptides have complicated purification and characterization efforts. Thus, only a limited number of plant PM proteins have been definitively identified. These include the H+-ATPase (Anthon and Spanswick, 1986; Katz and Sussman, 1987; Schaller and Sussman, 1988), a Ca2+-ATPase (Bonza et al., 1998), a Suc-binding polypeptide (Overvoorde and Grimes, 1994), and a group of PMIPs. Many of the PMIPs studied in plants have been shown to function as aquaporins, channels that permit the bidirectional passage of water through cellular membranes (Daniels et al., 1994; Kammerloher et al., 1994; Qi et al., 1995; Johansson et al., 1996; Schaffner, 1998). Molecular models based on deduced amino acid sequences suggest that PMIPs share a common membrane topology, with six hydrophobic membrane-spanning domains, connected by five hydrophilic loops (Chrispeels and Maurel, 1994; Nielsen and Agre, 1995; Lee et al., 1997; Maurel, 1997; Agre et al., 1998; Schaffner, 1998). The molecular structure of the mammalian aquaporin-1 from erythrocyte membranes has been described as a tilted hourglass, with a narrow pathway through the center of oligomerized tetrameric CHIP subunits (Jung et al., 1994; Cheng et al., 1997; Walz et al., 1997; Agre et al., 1998).
PM vesicle fractions isolated from storage tissue of red beet (Beta vulgaris L.) contain two highly abundant bands migrating at 31 and 27 kD. Both bands were identified to contain one or more MIPs by comparison of tryptic peptides with known MIP/aquaporin sequences (Qi et al., 1995). More recently, cDNA clones encoding storage tissue PMIPs were obtained (Qi et al., 1996). Collectively, these data show that the PMIP species of 31 and 27 kD both contain polypeptides sharing a high degree of sequence similarity with known PM aquaporins, such as tomato pTOM75 (Fray et al., 1994), pea clone 7a (Guerrero et al., 1990), and the Arabidopsis PIP proteins (Kammerloher et al., 1994). From a biochemical perspective, our laboratory has shown that the PMIP species of 31 and 27 kD are resistant to extraction with salts or chaotropic agents, largely insoluble in Triton X-100, and partially soluble in the detergents 3-[(cholamidopropyl)dimethylammonio]-1-propanesulfonic acid, digitonin, and octylglucoside (Wasserman et al., 1992). Each PMIP subgroup is highly prone to aggregation, and reducing agents are required to minimize disulfide-linked aggregation (Qi et al., 1995). In PMIP31 addition of exogenous Hg2+ leads to a conformational change characterized by exposure of a previously inaccessible proteolytic site immediately preceding the highly conserved Gly-Gly-Gly-Ala-Asn-X-X-X-X-Gly-Tyr motif. Based on this information, the topological orientation of surface loop C in PMIP31 was directly established (Barone et al., 1997).
The existence of two structurally related PMIPs, PMIP31 and PMIP27, within a single PM vesicle fraction presented a unique opportunity to directly compare their biochemical and topological properties. To explore the comparative properties of PMIP31 and PMIP27, affinity-purified antibodies specifically recognizing each PMIP species were generated. In this study the antibodies were used to assess the differential responses of PMIP31 and PMIP27 to proteolysis, detergent extraction, and exposure to sulfhydryl modification reagents. We also sought to determine if PMIP31 and PMIP27 were able to form mixed disulfide-linked heterodimers within the PM vesicle system. Our results show that PMIP31 and PMIP27 are topologically similar, but each responds differently to various perturbations placed upon the PM.
MATERIALS AND METHODS
RNA Gel-Blot Analysis
Twenty micrograms of total RNA isolated from various tissues was denatured and separated in a 1% agarose gel (Chang et al., 1993). The RNA was transferred to nylon membranes (Hybond N, Amersham), and hybridization was conducted in 50% formamide with α-32P-labeled full-length cDNAs of BPM2 or BPM3. Washes were conducted in 5× SSC and 0.1% SDS at 42°C.
Membrane Isolation
Microsomal membranes were isolated from red beet (Beta vulgaris L.) storage tissue by differential centrifugation (Wasserman et al., 1989, 1996). Aqueous two-phase partitioning was used to prepare a PM vesicle fraction in the right-side-out orientation (Wu et al., 1991; Wu and Wasserman, 1993). Protein content of the vesicle fractions was determined by dye binding using BSA as a standard (Bradford, 1976).
Carboxymethylated microsomal membranes were prepared by substitution of 5 mm I-Ac for DTT in homogenization buffers as described previously (Umbach and Siedow, 1996). All other steps remained unchanged.
Electrophoresis and Immunoblotting
SDS-PAGE was performed using 9% to 18% gradient gels (Laemmli, 1970; Porzio and Pearson, 1976). Sample loading buffers contained 8 m urea, 4% SDS, 20% glycerol, 100 mm DTT (freshly added), and 100 mm Tris-HCl, pH 8.0. To prevent heat-induced aggregation of membrane proteins, samples were not heated before electrophoresis.
Affinity-purified antibodies to PMIP31 and PMIP27 were prepared as described previously (Barone and Wasserman, 1996; Barone et al., 1997). Immunoblotting with development by enhanced chemiluminescence was conducted as described previously (Wasserman et al., 1996; Barone et al., 1997). Mouse monoclonal antibodies that recognized the 60-kD subunit B of the vacuolar H+-ATPase (Ward et al., 1992) were provided by Heven Sze (University of Maryland, College Park). Unless otherwise indicated, each antibody was used at a dilution of 1:2000.
Suc Gradient Centrifugation
For subcellular distribution studies, microsomal fractions (3.4 mg of protein) were loaded onto a 15% to 45% (w/w) linear Suc gradient and centrifuged overnight at 80,000g in a SW28.1 rotor (Beckman). Fractions were assayed for callose synthase activity (Wu and Wasserman, 1993), and screened for PMIP31, PMIP27, and the 60-kD subunit B of the vacuolar H+-ATPase by immunoblotting.
Protease Treatment of PM Vesicles
Protease treatment was performed essentially as described previously (Wu and Wasserman, 1993; Wasserman et al., 1996). Unless indicated otherwise, standard reaction mixtures of 120 μL contained PM vesicles (18 μg), Pronase E (40 μg mL−1) or trypsin (40 μg mL−1), and 50 mm Tris-HCl, pH 7.5, in the absence or presence of 0.01% or 0.1% digitonin, as indicated. Mixtures were held for 20 min at room temperature and were terminated with PMSF or soybean trypsin inhibitor on ice. Mixtures were diluted to 2 mL with 50 mm Tris-HCl, pH 7.5, and centrifuged at 40,000g for 40 min. This wash was repeated to ensure removal of proteases and protease inhibitors that migrate in the range of 20 to 22 kD. Membrane pellets were suspended in 100 μL of 250 mm Suc, 1 mm DTT, 1 mm EDTA, and 10 mm Hepes-NaOH, pH 7.2 (buffer A). Aliquots of 24 μL were then brought to 100 mm DTT, incubated for 15 min, and combined with an equal volume of SDS-PAGE sample buffer. Samples were directly applied to SDS-PAGE gels without heating.
Sequence Analysis of Proteolytic Fragments
The 25- and 22-kD proteolytic fragments were excised from Coomassie blue-stained gels and the protein was electroeluted from the gel slices. Electroeluted proteins were then re-electrophoresed (with 100 mm DTT) and transferred onto PVDF membranes (Bio-Rad) using 10 mm 3-(cyclohexylamino)-1-propane-sulfonic acid and 0.05% SDS in 30% methanol, pH 11. Blots were stained with 0.5% Ponceau S in 1% acetic acid for 10 s and destained with 1% acetic acid. The 25- and 22-kD species were each excised and subjected to N-terminal sequence analysis.
RESULTS
PMIP27 Is the Gene Product of BPM3
Direct amino acid sequence analysis of the N-terminal and internal sequences from electropurified PMIP31 and PMIP27 were used to correlate each species with at least one respective gene. Previous work has demonstrated that the PMIP31 band contains the product of BPM2 (Barone et al., 1997). Direct sequence analysis of PMIP27 demonstrates that it corresponds to the predicted BPM3 gene product. A 22-amino acid internal tryptic fragment Phe-Gln-Pro-Thr-Pro-Tyr-Met-Thr-Ala-Gly-Gly-Gly-Ala-Asn-Tyr-Val-His-His-Gly-Tyr-Thr-Lys exhibited complete identity with the BPM3 deduced amino acid sequence beginning at position 152. This tryptic fragment contains the Gly-Gly-Gly-Ala-Asn-X-X-X-X-Gly-Tyr motif characteristic of plant PM aquaporins (Barone et al., 1997). The hydropathy plot of the BPM3 gene product predicts six transmembrane segments, which is similar to numerous MIPs with demonstrated water-channel activity (Daniels et al., 1996). BPM3 was shown to facilitate water uptake in a water permeability assay using Xenopus oocytes (data not shown), thereby indicating that it functions as an aquaporin.
Tissue Distribution of the BPM Transcripts
RNA gel-blot analysis demonstrated tissue-specific differences in expression patterns of the BPM2 and BPM3 genes (Fig. 1). A single 1.2-kb BPM2 transcript was strongly detected in storage tissue, with barely detectable levels in leaf and stem tissue. Similarly, the 1.3-kb BPM3 transcript was most strongly expressed in storage tissue; however, moderate levels were also present in stem tissue. These observations demonstrate that BPM2 and BPM3 exhibit differential expression patterns.
Figure 1.
Tissue distribution of BPM2 and BPM3 by RNA gel-blot analysis.
Membrane Distribution of PMIP31 and PMIP27
Polyclonal antibodies were generated using electropurified PMIP31 and PMIP27 obtained from PM preparations isolated by aqueous two-phase partitioning. Both sets of affinity-purified antibodies were highly specific and did not cross-react with other PM proteins. To maximize the dissociation of PMIP aggregates into monomeric species, 100 mm DTT was added to membrane vesicles before protein extraction with SDS sample buffer (Qi et al., 1995). Freshly prepared vesicles were generally 90% latent for the PM marker enzyme callose synthase, and were therefore mainly in the right-side-out orientation.
Microsomal membranes were applied to a continuous gradient of 15% to 45% (w/w) Suc. Fractions were collected and analyzed for PM and tonoplast marker enzymes. The PM marker callose synthase (Fig. 2, top) was localized as a well-defined peak at 35% to 42% Suc. However, the majority of the 60-kD subunit of the tonoplast H+-ATPase was located in lower-density fractions (Fig. 2, middle). These results are consistent with previous work, in which PM vesicles partitioned at 38% to 40% Suc, whereas tonoplast vesicles are found at 25% to 28% Suc (Poole et al., 1984; Wasserman et al., 1985).
Figure 2.
Suc-gradient centrifugation of a microsomal vesicle fraction. A microsomal membrane fraction (3.4 mg of protein) was centrifuged overnight at 80,000g on a continuous gradient of Suc (15%–45%, w/w). Top, Distribution of the PM marker callose synthase and Suc. Middle, Immunoblot probed with a monoclonal antibody to the 60-kD vacuolar H+-ATPase (1:1,000 dilution). Bottom, Immunoblot dually probed with affinity-purified polyclonal antibodies specific for the PMIP31 and PMIP27 species (1:2,000 dilution). Each lane contained 20 μL from each gradient fraction and was prepared for SDS-PAGE in 100 mm DTT.
To demonstrate the association of PMIP31 and PMIP27 with the PM, gradient fractions were probed with each respective affinity-purified antibody. Both PMIP species distributed with the PM marker (Fig. 2, bottom) and neither correlated with the distribution of the tonoplast H+-ATPase subunit. Moreover, PMIP31 and PMIP27 were distributed similarly within the gradient, suggesting that the two PMIP subgroups are associated with the same population of membrane vesicles.
Effects of Sulfhydryl-Modification Reagents on PMIP Aggregation State
Our earlier study established that PMIP31 and PMIP27 are each highly prone to forming disulfide-linked oligomeric species. In the absence of sufficient reducing agent, the PMIPs appear as a disulfide-linked species spanning the molecular mass range of 47 to 42 kD (Qi et al., 1995). Dose-dependent conversion of the disulfide-linked oligomers to the corresponding monomeric forms occurs upon addition of DTT to membrane-isolation buffers and to individual membrane fractions immediately preceding protein extraction for SDS-PAGE.
To determine the importance of sulfhydryl-modification agents during membrane preparation with respect to PMIP aggregation states, PM vesicles were prepared in tissue homogenization buffer containing I-Ac or NEM, with control membranes prepared in the absence of these reducing agents. The effect of diamide, which promotes sulfhydryl-group oxidation, was also examined (Umbach and Siedow, 1996).
PMIP31 and PMIP27 were differently affected by each of these treatments. With PMIP27, the disulfide-linked 43-kD species was dominant; however, the monomeric form of PMIP27 was clearly present (Fig. 3A, lanes 1, 3, and 5). Consistent with the formation of disulfide-linked species, addition of diamide promoted the oxidation of the monomeric form of PMIP27 to the 43-kD disulfide-linked species (Fig. 3A, lanes 2, 4, and 6). In contrast to PMIP27, the monomeric form of PMIP31 was markedly less visible when membranes were prepared in the absence of reducing agent. In the absence of DTT, only the anomalously migrating 45-kD aggregated species was observed (Fig. 3B).
Figure 3.
Effects of Cys modification reagents. PM vesicles were isolated in the presence (+) and absence (−) of I-Ac (5 mm) and NEM (5 mm). Samples were treated with (+) or without (−) diamide (3 mm) before SDS-PAGE. Immunoblotting was performed using anti-PMIP27 (1:2500, A) and anti-PMIP31 (1:2000, B).
To determine if disulfide-linked aggregation results from oxidation reactions during homogenization and subsequent centrifugation steps, free sulfhydryl groups were blocked immediately upon tissue disruption by inclusion of I-Ac (Fig. 3, A and B, lanes 3 and 4) or NEM (Fig. 3, A and B, lanes 5 and 6) in homogenization buffers. Neither sulfhydryl reagent significantly enhanced monomer formation. Assuming that the reaction of free thiol groups with I-Ac or NEM is rapid, these results imply that the 27- and 31-kD monomers are already linked by disulfide bonds before tissue disruption.
Homodimeric Nature of the Aggregated Species
Before antibodies became available, it had not been possible to ascertain the monomeric makeup of the 45- and 43-kD disulfide-linked species observed previously (Qi et al., 1995). Therefore, we sought to distinguish between self-aggregation and the formation of mixed aggregates consisting of the 31- and 27-kD monomeric species. If mixed aggregates were present, the antibodies would recognize both the 45- and 43-kD species. However, such cross-reactivity was not observed (Fig. 3). The PMIP31 antibody recognized the 45-kD disulfide-linked species only, whereas the PMIP27 antibody was specific for the 43-kD disulfide-linked species. This demonstrates that members of the 31- and 27-kD PMIP subgroups do not covalently associate with each other to form heterodimeric species. The 45- and 43-kD species, therefore, must be self-aggregates of each respective PMIP subgroup. However, the possibility that these could also be dimeric species formed between each monomer and a nonimmunogenic PM protein cannot be excluded.
Proteolytic Fragmentation Patterns
To further probe PMIP topology, PM vesicles were subjected to proteolysis by trypsin and Pronase E, and fragmentation patterns of the PMIP31 and PMIP27 species were compared. The rationale for this experiment was to determine if PMIP31 and PMIP27 possess similar sets of exposed proteolytic cleavage sites. The PM vesicle fractions used for this study were freshly prepared. Latency was determined by assaying for callose synthase in the absence and presence of 0.1% digitonin (Wu et al., 1991). The calculated latency value of 94% indicates that the vesicles were sealed and in the right-side-out orientation.
Figure 4 shows that each PMIP species responded uniquely to proteolysis. In the absence of added detergent, PMIP31 was completely refractory to proteolysis by either trypsin or Pronase E (Fig. 4, lanes 2 and 5). Addition of 0.01% digitonin caused a dose-dependent decrease of PMIP31, with its complete disappearance at 40 μg mL−1 Pronase E (Fig. 4, lane 3) and partial disappearance with 112 μg mL−1 trypsin (Fig. 4, lane 6). No distinct intermediary proteolytic fragments were produced when digitonin was present.
Figure 4.
Different fragmentation patterns of PMIP31 and PMIP27. Limited proteolysis was conducted with PM vesicles treated with 0.01% digitonin. Proteases used were Pronase E (left) and trypsin (right). Membrane-bound proteolytic products were extracted, electrophoresed, and blotted with anti-PMIP27 and anti-PMIP31, as indicated.
In contrast to PMIP31, PMIP27 yielded two proteolytic fragments in the absence of added detergent. Immunoreactive fragments of 25 and 21 kD were generated by Pronase E, and to a greater degree by trypsin (Fig. 4, lanes 5 and 6). The addition of digitonin did not greatly enhance proteolytic degradation of PMIP27. Thus, PMIP31 and PMIP27 each responded uniquely to limited proteolysis of PM vesicles.
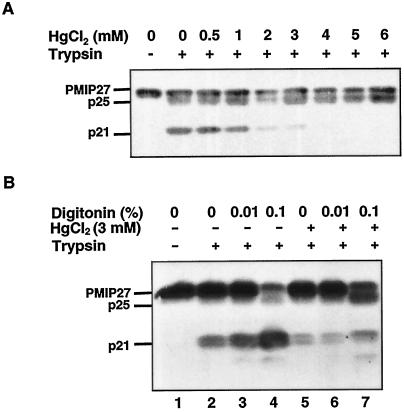
Effect of Hg2+ and Placement of the Proteolytic Sites
Unlike PMIP31, PMIP27 did not produce unique, divalent cation-induced proteolytic fragments (Barone et al., 1997). However, while screening the effect of divalent cations on proteolytic fragmentation patterns to determine possible conformational changes, we noted that Hg2+ led to a concentration-dependent decline of trypsin-generated p21 (Fig. 5A). This was accompanied by the concomitant accumulation of a proteolytic intermediate of p25.
Figure 5.
Occlusion of tryptic site II in the presence of HgCl2. A, PM vesicles were incubated with increasing levels of HgCl2 and 0.01% digitonin before treatment with trypsin (40 μg mL−1). B, Right-side-out vesicles were treated with trypsin in the absence (−) and presence (+) of 3 mm HgCl2 and digitonin, as indicated. The immunoblots were probed with anti-PMIP27.
A topological model based on the hydropathy plot of BPM3 is depicted in Figure 6. Tryptic sites I and II are defined as the cleavage sites giving rise to p25 and p21, respectively. The location of tryptic site I within the BPM3 gene product was directly established by N-terminal sequence analysis of p25 obtained from a carboxymethylated membrane preparation (Barone et al., 1997). The amount of protein recovered was low, but p25 yielded the N-terminal sequence Asp-Tyr-Val-Glu-Pro-Val-Gly-Ala-Asp-Leu-Phe. Although not a perfect match, this sequence generally corresponds to the predicted BPM3 sequence of Asp-Tyr-Lys-Glu-Glu-Pro-Pro-Pro-Ala-Pro-Leu-Phe beginning at residue 29. This sequence immediately follows an Arg residue, a well-known recognition site for trypsin, and demonstrates that tryptic cleavage site I is located within the N-terminal domain of PMIP27.
Figure 6.
Topological model of a PMIP27. This model is based on a hydropathy plot (Kyte and Doolittle, 1982) of BPM3 (Qi et al., 1996) and the topological data provided here. The conserved domains located at surface loops C and E are shown. The locations of tryptic cleavage sites I and II are indicated. Conserved Cys residues and the two NPA domains of BPM3 are also shown.
Despite several attempts, it was not possible to obtain the N-terminal sequence from p21; therefore it was not possible to directly ascertain the location. However, if we make the assumption that the proteolytic cleavage site on PMIP27 giving rise to p21 is located at a similar position to the Hg2+-induced cleavage site on PMIP31, which generates a 22-kD fragment (Barone et al., 1997), this would place tryptic cleavage site II of PMIP27 near the interface of transmembrane segment 3 and surface loop C (Fig. 6). The assignment of this location is supported by the fact that occlusion of tryptic cleavage site II is observed when added Hg2+ binds in close proximity to the four conserved Cys residues.
To establish the orientation of the tryptic cleavage sites we probed the effects of Hg2+ and digitonin on a highly latent (94%) right-side-out vesicle fraction (Fig. 5). In the absence of Hg2+ and digitonin, p21 was the favored product, with lesser amounts of p25 formed (Figs. 4 and 5B, lane 7).
Hg2+ suppressed formation of p21, but simultaneously enhanced the formation of p25 in this vesicle fraction consisting of primarily right-side-out vesicles (Fig. 5). This experiment suggests that the formation of p25 and p21 are conformationally linked. In the absence of Hg2+, the buildup of p21 with increasing levels of digitonin (Fig. 5B, lane 4) indicates that tryptic site II is the preferred site of attack. When Hg2+ was present, however, there was a marked buildup of p25 at 0.1% digitonin (Fig. 5B, lane 7). Suppression of p21 formation is consistent with occlusion of tryptic site II attributable to the binding of Hg2+ near the four conserved Cys residues. Simultaneously, tryptic site I showed increased susceptibility to tryptic attack in the presence of Hg2+. This would suggest a Hg2+-induced conformational change that renders tryptic site I more accessible to proteolytic attack. Because the formation of p21 was significantly enhanced by the addition of digitonin (Figs. 4 and 5), this indicates that tryptic cleavage site II may lie at the interface between surface loop C and transmembrane segment 3.
In summary, tryptic site II of the PMIP27 subgroup exhibits greater sensitivity to proteolytic attack than tryptic site I in a predominantly right-side-out vesicle system when detergent is not present. The ready formation of p21 in the absence of detergent is indicative of an apoplastic orientation for tryptic site II. Conversely, the relatively low levels of p25 formed in the absence of detergent is consistent with a cytosolic orientation for tryptic site I. The formation of p25 could reflect a limited population of unsealed or inside-out vesicles. It is interesting that the PMIP27 and PMIP31 subgroups exhibited very different responses when exogenous proteases were applied. The resultant topological findings for both PMIP27 and PMIP31, however, are generally consistent with the models predicted by hydropathy analysis for plant PMIPs.
DISCUSSION
Previous studies have unequivocally demonstrated that the broad protein bands of 31 and 27 kD from PM vesicles of red beet storage tissue contain MIPs with extensive sequence homologies to known PM aquaporins (Wu and Wasserman, 1993; Qi et al., 1995; Barone et al., 1997). Other than the fact that antibodies prepared against the two species did not cross-react, the degree of similarity between the two PMIP species was unclear. One possibility was that PMIP27 was merely a proteolytic fragment derived from PMIP31. If this were true, one would expect to observe similar proteolytic fragmentation patterns. However, the current evidence indicates that PMIP31 and PMIP27 are topologically similar but contain subtle differences in their structure and biochemistry. These differences are noted by (a) the non-cross-reactivity of the polyclonal antibodies, (b) the distinct proteolytic fragmentation patterns in the absence and presence of Hg2+, (c) the different responses to sulfhydryl modification reagents, (d) the inability to form heterodimeric species, (e) the refractory nature of PMIP27 to proteolysis in the presence of detergent, and (d) the presence of a unique tryptic cleavage site in PMIP27 located in proximity to transmembrane segment 1. PMIP31 does not contain an analogous tryptic cleavage site at this position (Barone et al., 1997).
Although PMIP31 and PMIP27 partition similarly in Suc gradients (Fig. 2), the inability of these PMIPs to form heterodimeric species could imply that each PMIP species is associated with a distinct subdomain of the PM. The existence of microdomains within the plant PM provides a possible explanation for the distinct topological properties of PMIP31 and PMIP27. In plants PIP1 from Arabidopsis was found to be localized within the plasmalemmasome (Robinson et al., 1996). These structures protrude into the vacuole and may provide a means for rapid exchange of water with the apoplast. Two kinds of vacuolar subcompartments have been revealed by immunofluorescence microscopy using antibodies raised against two sources of tonoplast intrinsic protein (Paris et al., 1996; Swanson et al., 1998). Small invaginations of the PM known as caveolae exist in mammalian cells (Parton and Simons, 1995; Kurzchalia and Parton, 1996). Caveolin, an integral membrane protein, co-purifies in low-density, detergent-insoluble fractions with proteins involved in signal transduction. It was recently suggested that detergent-insoluble complexes can exist in the absence of caveolae, leading to the idea of the existence of distinct microdomains in the PM. A putative role for these microdomains is to integrate signaling with membrane transport (Schnitzer et al., 1995). The yeast PM also contains low-density Triton X-100-insoluble domains (Kubler et al., 1996) containing proteins involved in signal transduction. However, the PM marker enzyme H+-ATPase was excluded from low-density Triton X-100-insoluble domains (Kubler et al., 1996), indicating the presence of multiple PM subdomains. To our knowledge, the existence of microdomains in the plant PM has not yet been documented. The distinct topological characteristics and different responses to sulfhydryl-modifying reagents by PMIP31 and PMIP27 could signify the presence of distinct microcompartments within the plant PM.
It is generally accepted that MIPs are strongly hydrophobic, and it is now becoming apparent that the outer surface loops of plant MIPs contain a limited number of exposed proteolytic sites. Assuming that none of the proteolytic fragments escape detection, PMIP31 contains one tryptic site that is accessible only in the presence of Hg2+ (Barone et al., 1997). However, PMIP27 yields two tryptic sites. Based mainly on the relative abundance of p25 and p21, we believe that tryptic cleavage site II is partially exposed to the apoplastic face of the PM, and that tryptic site I lies at the cytosolic face (Fig. 6). This would be consistent with predicted orientations. These results could also be reconciled if segment 1 does not fully traverse the PM. Instead, it may form a loop that causes the N-terminal domain to emerge from the PM at the apoplastic face. However, this would run counter to recent crystallographic studies with aquaporin-1 (Cheng et al., 1997; Walz et al., 1997).
There appears to be a conformational linkage between proteolytic sites I and II in PMIP27. The addition of Hg2+ led to a concentration-dependent decline of trypsin-generated p21, which was accompanied by the concomitant accumulation of a proteolytic intermediate of 25 kD (Fig. 5). Our interpretation of this result is that the putative Hg2+-binding site is located proximal to the Cys residues in transmembrane segments 2 and 3 (Fig. 6). Increasing levels of Hg2+ would result in the occlusion of tryptic site II. The concomitant conformational change would then lead to greater tryptic accessibility of site I, resulting in elevated levels of p25.
With at least 23 MIP isoforms occurring in Arabidopsis (Weig et al., 1997), the complexities of biochemical analysis of plant MIPs becomes apparent. The occurrence of two MIP subgroups in beet storage tissue and the availability of the corresponding antibodies have provided a first step toward understanding the membrane topology of this complex group of proteins. This study demonstrates that the PMIP31 and PMIP27 subgroups both display distinct membrane topologies, and do not form mixed heterodimeric species. Moreover, the possibility that each PMIP class is distributed within distinct microdomains of the PM must be further investigated. Specific physiological functions of the PMIP31 and PMIP27 subgroups, as well as regulatory factors, remain to be determined.
ACKNOWLEDGMENT
We thank Dr. Heven Sze for the kind gift of monoclonal antibodies recognizing the 60-kD vacuolar H+-ATPase subunit.
Abbreviations:
- I-Ac
iodoacetic acid
- MIP
major intrinsic protein
- NEM
N-ethylmaleimide
- PM
plasma membrane
- PMIP
PM intrinsic protein
Footnotes
This research was supported by the National Science Foundation (MCB-95-07766), with a Research Experience for Undergraduates supplement (to K.B.K.).
LITERATURE CITED
- Agre P, Bonhivers M, Borgnia MJ. The aquaporins, blueprints for cellular plumbing systems. J Biol Chem. 1998;273:14659–14662. doi: 10.1074/jbc.273.24.14659. [DOI] [PubMed] [Google Scholar]
- Anthon GE, Spanswick RM. Purification and properties of the H+-translocating ATPase from the plasma membrane of tomato roots. Plant Physiol. 1986;81:1080–1085. doi: 10.1104/pp.81.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone LM, Shih C, Wasserman BP. Mercury-induced conformational changes and identification of conserved surface loops in plasma membrane aquaporins from higher plants: topology of PMIP31 from Beta vulgarisL. J Biol Chem. 1997;272:30672–30677. doi: 10.1074/jbc.272.49.30672. [DOI] [PubMed] [Google Scholar]
- Barone LM, Wasserman BP. Generation and application of immunological probes for the study of membrane-bound proteins and enzymes: a review. J Food Biochem. 1996;20:173–192. [Google Scholar]
- Bonza C, Carnelli A, DeMichelis MI, Rasi-Caldogno R. Purification of the plasma membrane Ca2+-ATPase from radish seedlings by calmodulin-agarose affinity chromatography. Plant Physiol. 1998;116:845–851. doi: 10.1104/pp.116.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Cheng AC, Vanhoek AN, Yeager M, Verkman AS, Mitra AK. Three-dimensional organization of a human water channel. Nature. 1997;387:627–630. doi: 10.1038/42517. [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Agre P. Aquaporins: water channel proteins of plant and animal cells. Trends Biochem Sci. 1994;19:421–425. doi: 10.1016/0968-0004(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Maurel C. Aquaporins. The molecular basis of facilitated water movement through living plant cells? Plant Physiol. 1994;105:9–13. doi: 10.1104/pp.105.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MJ, Chaumont F, Mirkov TE, Chrispeels MJ. Characterization of a new vacuolar membrane aquaporin sensitive to mercury at a unique site. Plant Cell. 1996;8:587–589. doi: 10.1105/tpc.8.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MJ, Mirkov TE, Chrispeels MJ. The plasma membrane of Arabidopsis thalianacontains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol. 1994;106:1325–1333. doi: 10.1104/pp.106.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer DP, Amor Y. Cellulose biosynthesis. Plant Cell. 1995;7:987–1000. doi: 10.1105/tpc.7.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray RG, Wallace A, Grierson D, Lycett GW. Nucleotide sequence and expression of a ripening and water stress-related cDNA from tomato with homology to the MIP class of membrane channel proteins. Plant Mol Biol. 1994;24:539–543. doi: 10.1007/BF00024122. [DOI] [PubMed] [Google Scholar]
- Guerrero FD, Jones JT, Mullet JE. Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted: sequence and expression of three inducible genes. Plant Mol Biol. 1990;15:11–26. doi: 10.1007/BF00017720. [DOI] [PubMed] [Google Scholar]
- Johansson I, Larsson C, Ek B, Kjellbom P. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+and apoplastic water potential. Plant Cell. 1996;8:1181–1191. doi: 10.1105/tpc.8.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP: the hourglass model. J Biol Chem. 1994;269:14648–14654. [PubMed] [Google Scholar]
- Kammerloher W, Fischer U, Piechottka GP, Schaffner AR. Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 1994;6:187–189. doi: 10.1046/j.1365-313x.1994.6020187.x. [DOI] [PubMed] [Google Scholar]
- Katz DB, Sussman MR. Inhibition and labeling of the plant plasma membrane H+-ATPase with N-ethylmaleimide. Plant Physiol. 1987;83:977–981. doi: 10.1104/pp.83.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler E, Dohlman HG, Lisanti MP. Identification of Triton X-100 insoluble membrane domains in the yeast Saccharomyces cerevisiae: lipid requirements for targeting of heterotrimeric G-protein subunits. J Biol Chem. 1996;271:32975–32980. doi: 10.1074/jbc.271.51.32975. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Parton RG . And still they are moving: dynamic properties of caveolae. FEBS Lett. 1996;389:52–54. doi: 10.1016/0014-5793(96)00585-6. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee MD, King LS, Agre P. The aquaporin family of water channel proteins in clinical medicine. Medicine. 1997;76:141–156. doi: 10.1097/00005792-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Maurel C. Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:399–429. doi: 10.1146/annurev.arplant.48.1.399. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Agre P. The aquaporin family of water channels in kidney. Kidney Int. 1995;48:1057–1068. doi: 10.1038/ki.1995.389. [DOI] [PubMed] [Google Scholar]
- Overvoorde PJ, Grimes HD. Topographical analysis of the plasma membrane-associated sucrose binding protein from soybean. J Biol Chem. 1994;269:15154–15161. [PubMed] [Google Scholar]
- Paris N, Stanley CM, Jones RL, Rogers JC. Plant cells contain two functionally distinct vacuolar compartments. Cell. 1996;85:563–572. doi: 10.1016/s0092-8674(00)81256-8. [DOI] [PubMed] [Google Scholar]
- Parton RG, Simons K. Digging into caveolae. Science. 1995;269:1398–1399. doi: 10.1126/science.7660120. [DOI] [PubMed] [Google Scholar]
- Poole RJ, Briskin DP, Kratky Z, Johnstone RM. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet. Characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984;74:549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzio MA, Pearson AM. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1976;490:27–34. doi: 10.1016/0005-2795(77)90102-7. [DOI] [PubMed] [Google Scholar]
- Qi X, Harn C, Mu H, Wasserman BP. Nucleotide sequences of three cDNAs encoding major intrinsic proteins from Beta vulgaris L. (accession nos. U60147, U60148, and U60149) (PGR 96-074) Plant Physiol. 1996;112:861. [Google Scholar]
- Qi X, Tai CY, Wasserman BP. Plasma membrane intrinsic proteins of Beta vulgarisL. Plant Physiol. 1995;108:387–392. doi: 10.1104/pp.108.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Sieber H, Kammerloher W, Schaffner AR. PIP1 aquaporins are concentrated in plasmalemmasomes of Arabidopsis thalianamesophyll. Plant Physiol. 1996;111:645–649. doi: 10.1104/pp.111.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner AR. Aquaporin function, structure, and expression: are there more surprises to surface in water relations? Planta. 1998;204:131–139. doi: 10.1007/s004250050239. [DOI] [PubMed] [Google Scholar]
- Schaller GE, Sussman MR. Isolation and sequence of tryptic peptides from the proton-pumping ATPase of the oat plasma membrane. Plant Physiol. 1988;86:512–516. doi: 10.1104/pp.86.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- Sussman MR. Molecular analysis of proteins in the plant plasma membrane. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:211–234. [Google Scholar]
- Swanson SJ, Bethke PC, Jones RL. Barley aleurone cells contain two types of vacuoles: characterization of lytic organelles by use of fluorescent probes. Plant Cell. 1998;10:685–698. doi: 10.1105/tpc.10.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. The reaction of the soybean cotyledon mitochondrial cyanide-resistant oxidase with sulfhydryl reagents suggests that a-keto acid activation involves the formation of a thiohemiacetal. J Biol Chem. 1996;271:25019–25026. doi: 10.1074/jbc.271.40.25019. [DOI] [PubMed] [Google Scholar]
- Walz T, Hirai T, Murata K, Heymann JB, Mitsuoka K, Fujiyoshi Y, Smith BL, Agre P, Engel A. The three-dimensional structure of aquaporin-1. Nature. 1997;387:624–627. doi: 10.1038/42512. [DOI] [PubMed] [Google Scholar]
- Ward JM, Reinders A, Hsu H-T, Sze H. Dissociation and reassembly of the vacuolar H+-ATPase complex from oat roots. Plant Physiol. 1992;99:161–169. doi: 10.1104/pp.99.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman BP, Frost DJ, Lawson SG, Mason TL, Rodis P, Sabin RD, Sloan ME. Biosynthesis of cell wall polysaccharides: membrane isolation, in vitro glycosyl transferase assay and enzyme solubilization. In: Linskens HF, Jackson JF, editors. Modern Methods of Plant Analysis, Vol 10: Plant Fibers. Berlin: Springer-Verlag; 1989. pp. 1–11. [Google Scholar]
- Wasserman BP, Qi X, Barone LM, Wu A. Probing the subunit composition and topology of plasma membrane-bound (1,3)-β-glucan (callose) synthases. In: Linskens HF, Jackson JF, editors. Modern Methods of Plant Analysis, Vol 17: Plant Cell Wall Analysis. Berlin: Springer-Verlag; 1996. pp. 181–197. [Google Scholar]
- Wasserman BP, Ventola CL, Eiberger LL. Glucan biosynthesis in red beet root microsomes and tissue slices. J Agric Food Chem. 1985;33:144–149. [Google Scholar]
- Wasserman BP, Wu A, Harriman RW. Probing the molecular architecture of (1,3)-β-glucan (callose) synthase: polypeptide depletion studies. Biochem Soc Trans. 1992;20:18–22. doi: 10.1042/bst0200018. [DOI] [PubMed] [Google Scholar]
- Weig A, Deswarte C, Chrispeels MJ. The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol. 1997;114:1347–1357. doi: 10.1104/pp.114.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Harriman RW, Frost DJ, Read SM, Wasserman BP. Rapid enrichment of CHAPS-solubilized UDP-glucose:(1,3)-β-glucan (callose) synthase from Beta vulgarisL. by product entrapment. Entrapment mechanisms and polypeptide characterization. Plant Physiol. 1991;97:684–692. doi: 10.1104/pp.97.2.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Wasserman BP. Limited proteolysis of (1,3)-β-glucan (callose) synthase from Beta vulgarisL.: topology of protease-sensitive sites and polypeptide identification using Pronase E. Plant J. 1993;4:683–695. doi: 10.1046/j.1365-313x.1993.04040683.x. [DOI] [PubMed] [Google Scholar]