Abstract
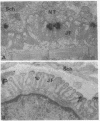
The turnover rates of junctional acetylcholine receptors were measured in innervated and denervated mouse sternomastoid neuromuscular junctions by 125I-labeled alpha-bungarotoxin binding. First, we determined that the density of labeled toxin initially bound to the neuromuscular junction was essentially unchanged up to 16 days after denervation. Innervated muscles and muscles that had been denervated 8 days previously were then saturated with labeled toxin, and the specific label at the endplate regions was compared by gamma counting 7 days later. At that time, the residual junctional label seen in innervated muscle was 3.2 times greater than in denervated muscle. Electron microscope autoradiography further showed that, after saturation with unlabeled toxin, new binding sites appeared rapidly at the specialized receptive region of the postsynaptic membrane with an apparent half-time of turnover of 2-3 days. At innervated junctions, the half-time of turnover was about 10 days. These data show that the mechanisms that control receptor turnover rates are different from those that control high-density receptor clustering. The slow turnover rate of junctional receptors appears to be more directly dependent on the presence of the nerve than is the clustering of junctional receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Barnard E. A., Porter C. W., Warnick J. E. The density of acetylcholine receptors and their sensitivity in the postsynaptic membrane of muscle endplates. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2818–2822. doi: 10.1073/pnas.71.7.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., McIsaac R. J. Fast and slow mammalian muscles after denervation. Exp Neurol. 1970 Jan;26(1):183–202. doi: 10.1016/0014-4886(70)90099-3. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Cohen M. W., Zorychta E. Effects of innervation on the distribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):731–756. doi: 10.1113/jphysiol.1977.sp011879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. K., Hall Z. W. Loss of alpha-bungarotoxin from junctional and extrajunctional acetylcholine receptors in rat diaphragm muscle in vivo and in organ culture. J Physiol. 1975 Nov;252(3):771–789. doi: 10.1113/jphysiol.1975.sp011169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden S. J., Sargent P. B., McMahan U. J. Acetylcholine receptors in regenerating muscle accumulate at original synaptic sites in the absence of the nerve. J Cell Biol. 1979 Aug;82(2):412–425. doi: 10.1083/jcb.82.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden S. Acetylcholine receptors at the neuromuscular junction: developmental change in receptor turnover. Dev Biol. 1977 Nov;61(1):79–85. doi: 10.1016/0012-1606(77)90343-8. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Chuang S. T., Huang M. C. Effects of chronic treatment with various neuromuscular blocking agents on the number and distribution of acetylcholine receptors in the rat diaphragm. J Physiol. 1975 Aug;250(1):161–173. doi: 10.1113/jphysiol.1975.sp011047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Huang M. C. Turnover of junctional and extrajunctional acetylcholine receptors of the rat diaphragm. Nature. 1975 Feb 20;253(5493):643–644. doi: 10.1038/253643a0. [DOI] [PubMed] [Google Scholar]
- Christian C. N., Daniels M. P., Sugiyama H., Vogel Z., Jacques L., Nelson P. G. A factor from neurons increases the number of acetylcholine receptor aggregates on cultured muscle cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4011–4015. doi: 10.1073/pnas.75.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. P., Vogel Z. Immunoperoxidase staining of alpha-bungarotoxin binding sites in muscle endplates shows distribution of acetylcholine receptors. Nature. 1975 Mar 27;254(5498):339–341. doi: 10.1038/254339a0. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Turnover of acetylcholine receptors in skeletal muscle. Cold Spring Harb Symp Quant Biol. 1976;40:237–251. doi: 10.1101/sqb.1976.040.01.025. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Localization of acetylcholine receptor by 125I-labeled alpha-bungarotoxin binding at mouse motor endplates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1376–1378. doi: 10.1073/pnas.71.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976 Apr;69(1):144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Sensitivity in electron microscope autoradiography for 125I. J Histochem Cytochem. 1974 Feb;22(2):80–87. doi: 10.1177/22.2.80. [DOI] [PubMed] [Google Scholar]
- Fertuck H. C., Woodward W., Salpeter M. M. In vivo recovery of muscle contraction after alpha-bungarotoxin binding. J Cell Biol. 1975 Jul;66(1):209–213. doi: 10.1083/jcb.66.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Cohen S. A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev Biol. 1973 Mar;31(1):147–162. doi: 10.1016/0012-1606(73)90326-6. [DOI] [PubMed] [Google Scholar]
- Frank E., Gautvik K., Sommerschild H. Cholinergic receptors at denervated mammalian motor end-plates. Acta Physiol Scand. 1975 Sep;95(1):66–76. doi: 10.1111/j.1748-1716.1975.tb10026.x. [DOI] [PubMed] [Google Scholar]
- Gardner J. M., Fambrough D. M. Acetylcholine receptor degradation measured by density labeling: effects of cholinergic ligands and evidence against recycling. Cell. 1979 Mar;16(3):661–674. doi: 10.1016/0092-8674(79)90039-4. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann S., Merlie J., Lindstrom J. Modulation of acetylcholine receptor in rat diaphragm by anti-receptor sera. Nature. 1978 Jul 6;274(5666):65–68. doi: 10.1038/274065a0. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Siegel R. E., Fischbach G. D. Induction of acetylcholine receptors on cultured skeletal muscle by a factor extracted from brain and spinal cord. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5397–5401. doi: 10.1073/pnas.76.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Chang S. L., Kau S. T., Luh S. H. Chromatographic separation of the venom of Bungarus multicinctus and characterization of its components. J Chromatogr. 1972 Oct 5;72(1):71–82. doi: 10.1016/0021-9673(72)80009-8. [DOI] [PubMed] [Google Scholar]
- Lentz T. L., Mazurkiewicz J. E., Rosenthal J. Cytochemical localization of acetylcholine receptors at the neuromuscular junction by means of horseradish peroxidase-labeled alpha-bungarotoxin. Brain Res. 1977 Sep 2;132(3):423–442. doi: 10.1016/0006-8993(77)90192-5. [DOI] [PubMed] [Google Scholar]
- Linden D. C., Fambrough D. M. Biosynthesis and degradation of acetylcholine receptors in rat skeletal muscles. Effects of electrical stimulation. Neuroscience. 1979;4(4):527–538. doi: 10.1016/0306-4522(79)90129-5. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L. M., Sanes J. R., McMahan U. J. Reinnervation of original synaptic sites on muscle fiber basement membrane after disruption of the muscle cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3073–3077. doi: 10.1073/pnas.74.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews-Bellinger J., Salpeter M. M. Distribution of acetylcholine receptors at frog neuromuscular junctions with a discussion of some physiological implications. J Physiol. 1978 Jun;279:197–213. doi: 10.1113/jphysiol.1978.sp012340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Sanes J. R., Marshall L. M. Cholinesterase is associated with the basal lamina at the neuromuscular junction. Nature. 1978 Jan 12;271(5641):172–174. doi: 10.1038/271172a0. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Changeux J. P., Gros F. Acetylcholine receptor degradation measured by pulse chase labelling. Nature. 1976 Nov 4;264(5581):74–76. doi: 10.1038/264074a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M., Bayse G. S. Catalysis of iodination by lactoperoxidase. Biochemistry. 1970 Jul 21;9(15):2995–3000. doi: 10.1021/bi00817a010. [DOI] [PubMed] [Google Scholar]
- Podleski T. R., Axelrod D., Ravdin P., Greenberg I., Johnson M. M., Salpeter M. M. Nerve extract induces increase and redistribution of acetylcholine receptors on cloned muscle cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2035–2039. doi: 10.1073/pnas.75.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. W., Barnard E. A. Distribution and density of cholinergic receptors at the motor endplates of a denervated mouse muscle. Exp Neurol. 1975 Sep;48(3 Pt 1):542–556. doi: 10.1016/0014-4886(75)90012-6. [DOI] [PubMed] [Google Scholar]
- REGER J. F. Studies on the fine structure of normal and denervated neuromuscular junctions from mouse gastrocnemius. J Ultrastruct Res. 1959 Mar;2(3):269–282. doi: 10.1016/s0022-5320(59)80001-0. [DOI] [PubMed] [Google Scholar]
- Reiness C. G., Weinberg C. B., Hall Z. W. Antibody to acetylcholine receptor increases degradation of junctional and extrajunctional receptors in adult muscle. Nature. 1978 Jul 6;274(5666):68–70. doi: 10.1038/274068a0. [DOI] [PubMed] [Google Scholar]
- Rubin L. L., Schuetze S. M., Fischbach G. D. Accumulation of acetylcholinesterase at newly formed nerve--muscle synapases. Dev Biol. 1979 Mar;69(1):46–58. doi: 10.1016/0012-1606(79)90273-2. [DOI] [PubMed] [Google Scholar]
- SALPETER M. M., BACHMANN L. AUTORADIOGRAPHY WITH THE ELECTRON MICROSCOPE. A PROCEDURE FOR IMPROVING RESOLUTION, SENSITIVITY, AND CONTRAST. J Cell Biol. 1964 Aug;22:469–477. doi: 10.1083/jcb.22.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze S. M., Frank E. F., Fischbach G. D. Channel open time and metabolic stability of synaptic and extrasynaptic acetylcholine receptors on cultured chick myotubes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):520–523. doi: 10.1073/pnas.75.1.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H., Merlie J., Heinemann S., Bloch R. Degradation of junctional and extrajunctional acetylcholine receptors by developing rat skeletal muscle. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3547–3551. doi: 10.1073/pnas.76.7.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytkowski A. J., Vogel Z., Nirenberg M. W. Development of acetylcholine receptor clusters on cultured muscle cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):270–274. doi: 10.1073/pnas.70.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]