Abstract
Background
Biologic valved grafts are important in cardiac surgery, and although several types of graft are currently available, most commercial xenografts tend to cause early disfiguration due to intimal proliferation and calcification. We studied the graft failure patterns on non-fixed and glutaraldehyde-fixed pulmonary xenograft in vivo animal experiment.
Materials and Methods
Pulmonary valved conduits were obtained from the right ventricular outflow tract of eleven miniature pigs. The grafts were subjected to 2 different preservation methods; with or without glutaraldehyde fixation: glutaraldehyde fixation (n=7) and non-glutaraldehyde fixation (n=4). The processed explanted pulmonary valved grafts of miniature pig were then transplanted into eleven goats. Calcium quantization was achieved in all of the explanted xenograft, hemodynamic, histopathologic and radiologic evaluations were performed in the graft which the transplantation period was over 300 days (n=7).
Results
Grafts treated with glutaraldehyde fixation had more calcification and conduit obstruction in mid-term period. Calcium deposition also appeared much higher in the glutaraldehyde treated graft compared to the non-glutaraldehyde treated graft (p<0.05).
Conclusion
The present study suggests that xenografts prepared using glutaraldehyde fixation alone appeared to have severe calcification compared to the findings of non-glutaraldehyde treated xenografts and to be managed with proper anticalcification treatment and novel preservation methods. This experiment gives the useful basic chemical, histologic data of xenograft failure model with calcification for further animal study.
Keywords: Transplantation, heterologous; Glutaraldehyde
INTRODUCTION
Over the past decade, valved grafts have become an important raw material in cardiac surgery. Heart valves are commonly replaced by mechanical or bioprosthetic valves for the treatment of adult valvular heart disease, though the beneficial aspects of using these valves are under debate.
Allograft valves have the advantages of obviating anticoagulation, a low incidence of thromboembolism, optimal hemodynamics, and resistance to infective endocarditis [1]. On the other hand, allotransplantation is constrained by the availability of human donor organs. Therefore, if we could produce biological valves with characteristics similar to those of allograft valves, they are certain to be of clinical use.
Bioprostheses are widely used for a variety of cardiovascular applications such as heart-valve substitutes and patch materials. Bioprosthetic tissues are conventionally cross-linked with glutaraldehyde (GA) to impart tissue stability, reduce antigenicity and to maintain tissue sterility [2,3]. However, GA-fixed bioprostheses are prone to calcification after long term implantation in humans, and this is one of the limiting factors in the longevity of bioprostheses. The mechanism of calcification of GA-fixed bioprosthetic tissue is complex, but there are evidences that tissue phospholipids, free aldehyde groups of GA [4], and residual antigenicity of the bioprosthetic tissue, all play an important role [5].
The objective of this study was to evaluate the effect and toxicity of GA preservation methods. Different preserving methods, such as GA fixation and other nontoxic cross-linking method such as decellularization, cryopreservation method were tested on extracted porcine pulmonary-valved grafts. GA toxicity was compared with GA fixed and non-GA fixed xonografts.
MATERIALS AND METHODS
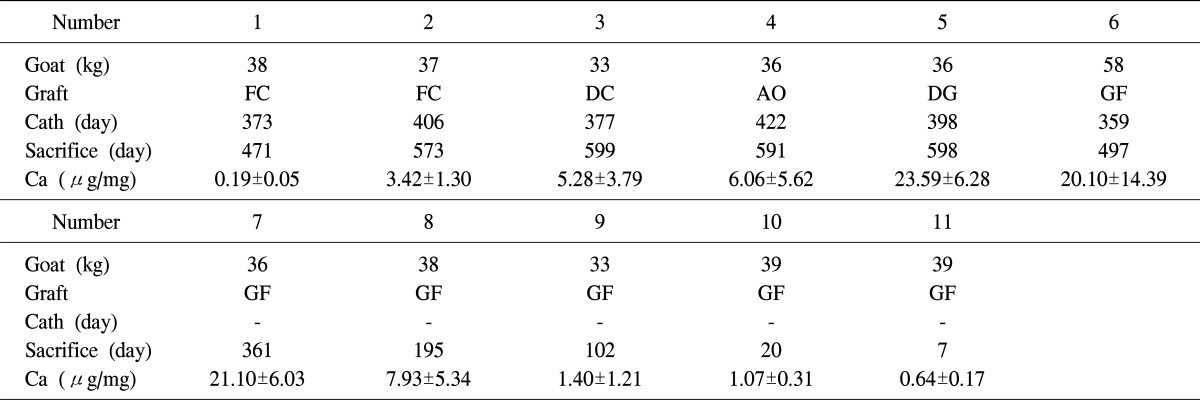
Between December 2005 through January 2009, pulmonary-valved conduits were obtained from eleven Seoul National University (SNU) miniature pigs (weight range, 90 to 110 kg) [6]. Briefly, the procedure used was as follows. After inducing general anesthesia and routine surgical draping, a median sternotomy was performed, and the fresh heart was extracted. The pulmonary xenograft was excised carefully from the root of right ventricular outflow tract (RVOT) and to the main pulmonary artery (PA). The graft was then transferred to a laboratory and washed with phosphate buffered solution (PBS 0.1 M, pH 7.4) supplemented with antibiotic solution (penicillin, streptomycin, and amphotericin B; Sigma-Aldrich Co., St. Louis, MO, USA), and placed in a refrigerator (4℃) for 24 hours. Grafts were then subjected to two different preservation methods such as GA fixed or not: GA fixation (number 6-11), decellularized-glutaraldehyde fixation (number 5), decellularized-cryopreservation (number 3), fresh cryopreservation (number 1-2), or antibiotic solution only (number 4) (Table 1).
Table 1.
Details of the animals enrolled in the study
FC, fresh cryopreservation; DC, decellularized-cryopreservation; AO, antisolution only; DG, decellularized glutaraldehyde fixation; GF, glutaraldehyde fixation; Cath, catheterization; Ca, calcium.
After various periods of transplantation, xenografts were explanted and calcium quantification was achieved. Hemodynamic and radiologic evaluations were performed in the graft which the period of transplantation was around one or two years. Calcium quantization was also compared between the GA fixed graft which the transplantation period was longer than 300 days (LGA) and non-GA fixed graft (NGA). And especially with the GA fixed grafts were sacrificed after few days through 2 years to identify the amount of the calcification process had increased during the transplantation period.
1) Pigs
SNU miniature pigs were bred in a barrier-sustained specific-pathogen-free facility at the Center for Animal Resource Development at SNU. Throughout the breeding period, physical activities and feeding statuses were carefully monitored by a veterinarian. The pigs were maintained under germ free conditions and weights were controlled between 90 and 110 kg. All procedures involving animals were approved by the institutional animal care and use committee of Seoul National University Hospital.
2) Antibiotic solution only
This graft was not preserved, but rather transplanted at the time of extraction after treating it with Sigma solution.
3) Glutaraldehyde fixation
The grafts involved were transferred to 0.625% GA solution for 2 weeks, and then stored in the 0.2% GA for time with identification disc until required.
4) Fresh cryopreservation
These grafts were placed in a cryopreservation bag containing cryoprotective solution (dimethyl sulfoxide, DMSO) and then immediately frozen at a controlled rate in a freezer (CryoMed; Thermo Electron Co., Waltham, MA, USA) to a temperature of -80℃. The valves were stored at -80℃ until the study.
5) Decellularization
Grafts were treated with 1.5 M NaCl solution for 24 hours at 37℃, and then rinsed with PBS solution. They were then treated with 0.5% sodium dodecyl sulfate (SDS) solution for an hour at 37℃ and rinsed with PBS solution for 48 hours. A sample of muscle and vessel in the PA wall were fixed in formalin, and the decellurization procedure was confirmed histologically.
6) Pulmonary xenograft replacement (right ventricular outflow tract replacement)
The transplantation procedure was conducted as follows. Under general anesthesia, a goat was placed in the right decubitus position, and left thoracotomy was performed through the 4th intercostal space. Heparin was injected (300 IU/kg) intravenously. Aortic cannulation was performed through the descending aorta and venous cannulation through the right atrium. Cardiopulmonary bypass was started in body temperature. The entire operative procedure was conducted with a beating heart without cardioplegia. A porcine xenograft was transplanted in the RVOT. Postoperative anticoagulation was not used to avoid interference with assessments of xenograft biocompatibility.
7) Echocardiography, cardiac catheterization
Transthoracic echocardiography was performed at 1, 3, 6, and 12 months post-transplantation to evaluate hemodynamic changes. Conduit diameters of grafts, and the morphologies and competences of leaflet were investigated. Cardiac catheterization was also performed to confirm hemodynamics just before sacrificing the goat.
8) Radiologic confirmations with quantization of calcification
After sacrificing the goat, grafts were tested for radiologic confirmation with simple X-ray. To quantify the degree of calcification of the xenograft, we sectioned 5 or 6 pieces of the wall and washed with saline and dried it under the temperature of 90℃ over 48 hours. Then preparing with 6N hydrochloric acid in a test tube until dissolution, measured the calcium content with inductively coupled plasma-atomic emission spectrometer.
9) Statistical methods
The data of calcium content in this study are expressed as the mean±standard deviation. Statistical analysis of the difference of calcium content was compared with LGA and NGA group and was analyzed by Mann-Whitney method. Statistical analysis was conducted using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Eleven goats of mean weight 38.5±6.8 kg (range, 33 to 58 kg) were used in the experiment and the mean time between transplantation and sacrifice was 364.9±240.3 days (range, 7 to 599 days).
1) Hemodynamic evaluation
Echocardiography was performed after implanting pulmonary-valved grafts. Valvular function and conduit diameters were checked. However, it was difficult to approximate echo probe penetration into the heart as in man, because the heart was in the deep side of the chest wall and covered by the lung. Furthermore, the echo window was not clear enough for us to estimate the exact diameter of conduits and valve functions. We could only obtain general information about the grafts, which was nevertheless useful for determining animal condition during follow-up. Overall, grafts treated with GA, and decellularized-GA had similar appearances. The mobilities of leaflets had decreased, and regurgitation and stenosis were present. Conduit diameters had decreased and all showed right ventricular outflow stenosis. Moderate degrees of regurgitation and decreased leaflet mobility were noted in the graft treated with decellularized-cryopreservation. The fresh cryopreservation graft also showed mild regurgitation, but no leaflet thickening was observed and mobility was near normal.
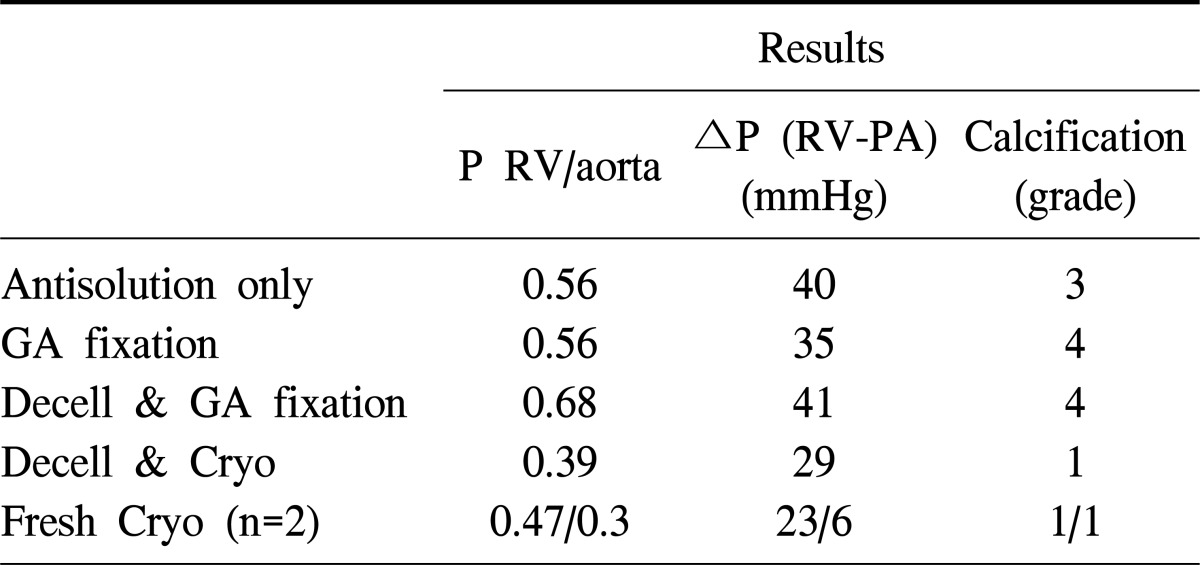
Just before explanting the graft, cardiac catheters were placed through the right ventricle (RV), PA, and aorta in each animal, and pressure differences and gradients were measured and recorded (Table 2). Cardiac catheterization was performed in NGA group and LGA group except xenograft number 7 which had transplantation period over 300 days. For grafts treated with antisolution only and LGA group, the results obtained showed higher RV-aortic pressure gradients and RV-PA pressure differences. Severe calcification and narrowing was also observed in conduits. The decellularized-cryopreservation graft had a smaller pressure gradient and pressure difference as compared with the graft treated with antisolution only and LGA group. Fresh cryopreservation grafts had the smallest RV-PA difference.
Table 2.
Cardiac catheterization data
P, pressure gradient; RV, right ventricle; ΔP, pressure difference; PA, pulmonary artery; GA, glutaraldehyde; Decell, decellularization; Cryo, cryopreservation.
2) Macroscopic, microscopic, and radiologic findings
Explanted valves were visually inspected, and radiographed to quantify the amounts of calcification. We graded calcification in the conduit as follows: grade 1-calcification occupying 1/4 of the conduit, grade 2-calcification occupying >1/4 and <2/4, grade 3-calcification occupying >2/4 and <3/4, and grade 4-calcification occupying >3/4.
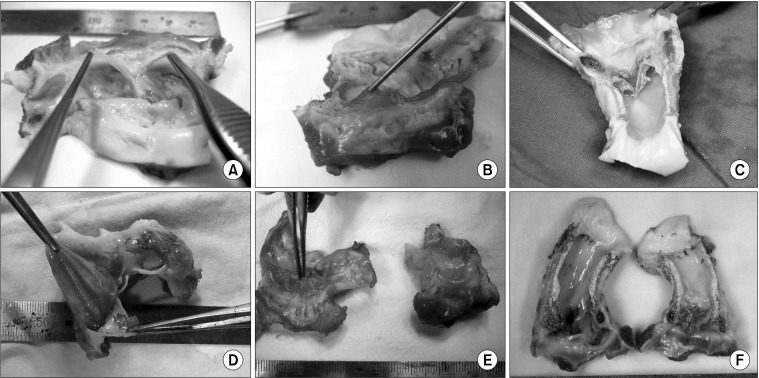
Calcification grades for grafts treated with GA, decellularized-GA, and antisolution only were grades 4, 4 and 3, respectively, and conduits were severely stiffened and calcified. However, fresh cryopreservation (grade 1) and decellularized cryopreservation grafts (grade 1) were in relatively good condition; conduits were soft and little calcification was evident, except at anastomosis sites. Leaflets differed in decellularized-cryopreservation and fresh cryopreservation grafts. Decellularized cryopreservation leaflets showed degeneration, which resulted in a larger RV-PA pressure difference than those of the fresh cryopreservation graft (Figs. 1, 2).
Fig. 1.
Macroscopic xenograft findings. Glutaraldehyde fixed graft had calcification throughout the conduit, leaflet retraction was prominent and severe calcification was observed. (A) Fresh cryopreservation, (B) decellularized-cryopreservation, (C) decellularized-glutaraldehyde fixation, (D) antisolution only, (E) fresh cryopreservation, and (F) glutaraldehyde fixation.
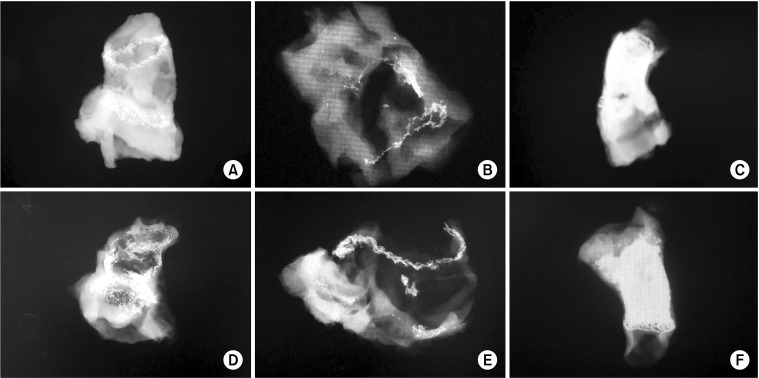
Fig. 2.
Radiologic findings and calcification grade. Both types of cryopreserved grafts showed minimal calcification throughout the conduit except in the anastomosis site. (A) grade 1: fresh cryopreservation, (B) grade 1: decellularized-cryopreservation, (C) grade 4: decellularized-glutaraldehyde fixation, (D) grade 3: antisolution only, (E) grade 1: fresh cryopreservation, and (F) grade 4: glutaraldehyde fixation.
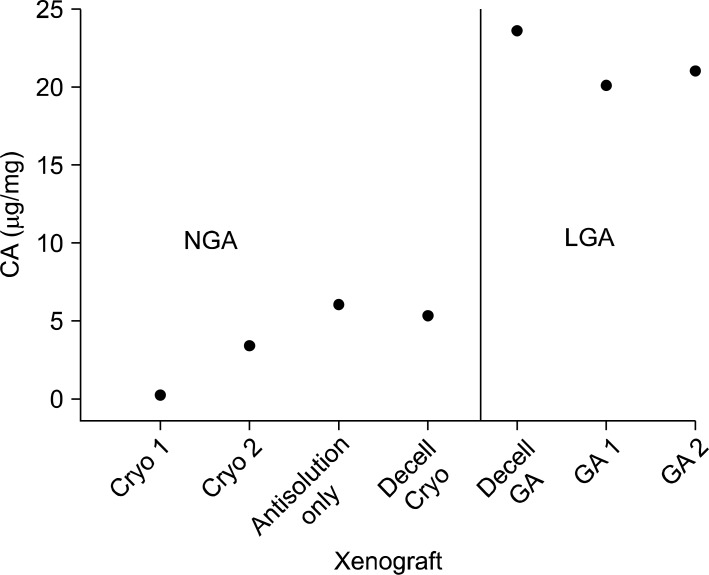
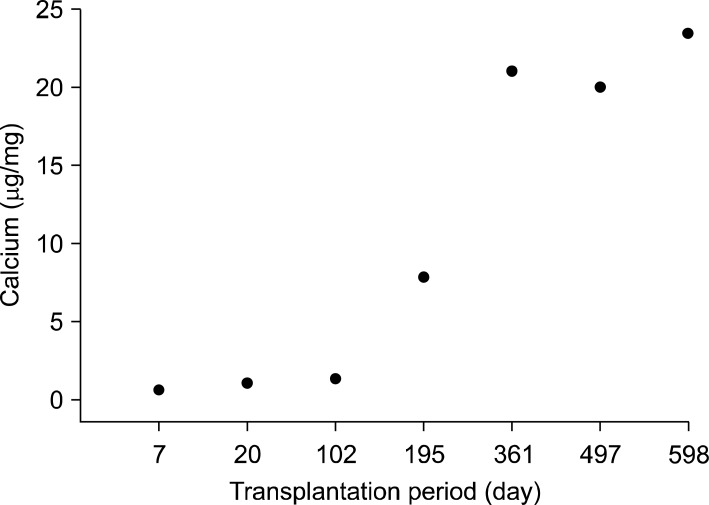
Macroscopic and radiologic examinations inferred that graft treated with GA with or without decellularization triggers calcification of the xenograft. Quantification of calcium content of the xenograft also appeared much higher in LGA group (p<0.01) (Fig. 3). And calcium deposition increased as the transplantation period became longer (Fig. 4).
Fig. 3.
Calcium deposition of the xenograft appeared higher in LGA (xenograft with transplantation period longer than 300 days) (p<0.01). CA, calcium; LGA, longer than 300 days; NGA, non-GA fixed graft; Cryo, cryopreservation; Decell, decellularization; GA, glutaraldehyde.
Fig. 4.
Calcium quantization of the glutaraldehyde fixed xenogarft by the transplantation period. We can see the calcium deposition increases as the transplantation period becomes longer.
DISCUSSION
Ever since GA preservation method in biological tissue has been introduced in 1969 by Carpentier et al. [3], it has been used most commonly to cross-link the xenograft valves and pericardium. GA cross-links decreases immunogenicity for it masks the antigens when it is implanted [7] and forms interfibrillar, intrafibrillar collagen cross-link compounds which reduces biodegradation and increasing biocompatibility which makes mechanical strength and durability to the graft [8,9]. These bioprosthesis has excellent extensibility of leaflet, enabling full leaflet opening without kinking and have similar hemodynamics compare to the native vales [10,11]. Requirement of anticoagulation is not needed for they are non thrombogenic [12] and also produces high defending properties from postoperative infection [13]. For these various advantages it has been implanted into innumerable patients with very high degree of clinical success and is still used world widely [14-16].
But calcification which makes tissue degeneration promotes bioprosthetic valve failure. It gradually calcifies over time and eventually deteriorates and fails to have its normal function. Adult recipients may have valve function well for a long period of time [17]. However young adults and children fail more quickly [18]. There are reports that aldehydes bond with calcium in vivo, which results in endothelial calcification [4]. Especially the pyridinium cross-link which is the common type of GA induced cross-links [8,9] could theoretically cause influx of phosphate into the intrafibrillar spaces and cause calcification [19]. There are various chemical sources which produce calcification process but the exact mechanism of the calcification process is not well known.
In the present study, xenografts prepared using GA with and without decellularization showed severe calcification throughout the conduit and valve leaflets. These findings suggest that a means of minimizing calcification or antitoxicity treatment must be developed.
Xenografts treated with decellularized-cryopreservation or fresh cryoprservation were in relatively good condition as compared to GA treated valves. However, declellularized-cryopreservation explanted leaflets showed severely retracted and degenerated, maybe due to enzymatic degradation without cross-linking.
Liao et al. [20] reported that after decellularization (SDS, trypsin, and Triton X-100), the collagen crimp structure, which is a component of the extracellular matrix, is disrupted in valve tissue, and that this might have a negative effect on long term valve durability. The authors speculate that this may have caused the leaflet degeneration in the present study.
One of the assumptions of decellularization of biological scaffolds is that antigenicity is associated predominantly with the cellular component of the tissue, and that removal of the native cells will remove antigenicity [21].
Considering the complex and multi factorial mechanism of calcification of GA-fixed bioprosthetic tissue, combining anticalcification treatments targeting different steps of calcification process may be beneficial through a synergistic effect.
The conduits and leaflets of fresh cryopreservation grafts were in relatively good functional conditions in this study. The cryopreservation technique was first developed to enable valves to be stored [22], and glycerol and DMSO was first used as cryoprotective agents. These agents had contributed in providing valve availability for replacement efforts [23,24]. O'Brien et al. [25] reported that cryopreserved homografts produce excellent long-term clinical results, but unlike mechanical prostheses, valves eventually deteriorate.
Xenograft destruction was easily detected macroscopically and by using radiologic findings. Quantification of the calcium also informed that, the destruction was mostly due to calcification. Calcification on the GA-fixed graft, the layer of the inner part was mostly calcified and it was scattered on the outer layer, which infers that calcification starts from the intimal layer.
Other than tissue failure due to toxicity, degeneration and calcification, there is a humoral response and also cellular response, with the cells, including macrophages, T cells, and eosinophils, infiltrating the xenografts in tissue graft failure [26]. Especially in viable xenotransplantation, the hyperacute rejection, which is mediated by preexisting anti-GALα1, 3Galβ1, 4GlcNAc (αGal) natural antibodies and the classical complement pathway in animal to human xenografting [27,28], should also be considered before transplantation.
The limitation was, this animal study was time consuming and breeding the animal in a restricted space was difficult. Thus the study was not large and non-GA treated xenograft preservation methods were heterogenous and therefore it does not have statistical significance, but xenografts prepared using GA with and without decellularization showed severe calcification compared to the findings of non-GA treated grafts.
CONCLUSION
The use of xenografts appears to be inevitable, because the number of allotransplantations is severely constrained by the non-availability of human donor organs. Modification of negative effects in decellularization techniques and with anticalcification treatment & novel combination of preservation methods require thorough investigation to overcome current both tissue valve preservation and engineering problems. Developing a combination of nontoxic cross-linking and valid methods of decellularization with removal of xenoreactive antigenicity technique, it may provide an acceptable results in the future. This experiment gives the useful basic chemical, histologic data of xenograft failure model with calcification for further animal study.
ACKNOWLEDGMENTS
This study was supported by the Korean Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (project no: A040004-006). This study was approved by Institutional Animal Care and Use Committee at the Clinical Research Institute, Seoul National University Hospital. This facility had been accredited by the International Association for the Assessment and Accreditation of Laboratory Animal Care.
References
- 1.Yap CH, Yii M. Allograft aortic valve replacement in the adult: a review. Heart Lung Circ. 2004;13:41–51. doi: 10.1016/j.hlc.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Grimm M, Eybl E, Grabenwoger M, et al. Biocompatibility of aldehyde-fixed bovine pericardium. An in vitro and in vivo approach toward improvement of bioprosthetic heart valves. J Thorac Cardiovasc Surg. 1991;102:195–201. [PubMed] [Google Scholar]
- 3.Carpentier A, Lemaigre G, Robert L, Carpentier S, Dubost C. Biological factors affecting long-term results of valvular heterografts. J Thorac Cardiovasc Surg. 1969;58:467–483. [PubMed] [Google Scholar]
- 4.Webb CL, Benedict JJ, Schoen FJ, Linden JA, Levy RJ. Inhibition of bioprosthetic heart valve calcification with aminodiphosphonate covalently bound to residual aldehyde groups. Ann Thorac Surg. 1988;46:309–316. doi: 10.1016/s0003-4975(10)65932-2. [DOI] [PubMed] [Google Scholar]
- 5.Allaire E, Guettier C, Bruneval P, Plissonnier D, Michel JB. Cell-free arterial grafts: morphologic characteristics of aortic isografts, allografts, and xenografts in rats. J Vasc Surg. 1994;19:446–456. doi: 10.1016/s0741-5214(94)70071-0. [DOI] [PubMed] [Google Scholar]
- 6.Kim HI, Lee SY, Jin SM, et al. Parameters for successful pig islet isolation as determined using 68 specific-pathogen-free miniature pigs. Xenotransplantation. 2009;16:11–18. doi: 10.1111/j.1399-3089.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- 7.Okamura K, Chiba C, Iriyama T, et al. Antigen depressant effect of glutaraldehyde for aortic heterografts with a valve, with special reference to a concentration right fit for the preservation of grafts. Surgery. 1980;87:170–176. [PubMed] [Google Scholar]
- 8.Richards FM, Knowles JR. Glutaraldehyde as a protein cross-linkage reagent. J Mol Biol. 1968;37:231–233. doi: 10.1016/0022-2836(68)90086-7. [DOI] [PubMed] [Google Scholar]
- 9.Bowes JH, Cater CW. The interaction of aldehydes with collagen. Biochim Biophys Acta. 1968;168:341–352. doi: 10.1016/0005-2795(68)90156-6. [DOI] [PubMed] [Google Scholar]
- 10.Crofts CE, Trowbridge EA. Local variation in the tearing strength of chemically modified pericardium. Biomaterials. 1989;10:230–234. doi: 10.1016/0142-9612(89)90098-7. [DOI] [PubMed] [Google Scholar]
- 11.Barratt-Boyes BG, Jaffe WM, Ko PH, Whitlock RM. The zero pressure fixed medtronic intact porcine valve: an 8.5 year review. J Heart Valve Dis. 1993;2:604–611. [PubMed] [Google Scholar]
- 12.Bruck SD. Possible causes for the calcification of glutaraldehyde-treated tissue heart valves and blood contacting elastomers during prolonged use in medical devices: a physico-chemical view. Biomaterials. 1981;2:14–18. doi: 10.1016/0142-9612(81)90081-8. [DOI] [PubMed] [Google Scholar]
- 13.Ferrans VJ, Boyce SW, Billingham ME, Spray TL, Roberts WC. Infection of glutaraldehyde-preserved porcine valve heterografts. Am J Cardiol. 1979;43:1123–1136. doi: 10.1016/0002-9149(79)90143-7. [DOI] [PubMed] [Google Scholar]
- 14.Kazui T, Morikawa M, Nakanishi K, et al. Long-term results of mitral valve replacement using glutaraldehyde-treated porcine bioprostheses: comparison of the Hancock and the Liotta valves. Nihon Kyobu Geka Gakkai Zasshi. 1990;38:1023–1029. [PubMed] [Google Scholar]
- 15.Jamieson WR, Miyagishima RT, Munro AI, et al. The Carpentier-Edwards supra-annular porcine bioprosthesis: clinical performance to 8 years of a new generation porcine bioprosthesis. J Card Surg. 1991;6(4 Suppl):562–567. doi: 10.1111/jocs.1991.6.4s.562. [DOI] [PubMed] [Google Scholar]
- 16.Valente M, Minarini M, Ius P, et al. Durability of glutaraldehyde-fixed pericardial valve prostheses: clinical and animal experimental studies. J Heart Valve Dis. 1992;1:216–224. [PubMed] [Google Scholar]
- 17.Ueyama K, Kamiya H, Kanamori T, et al. Long-term follow-up of cardiac valve replacement using bioprosthesis in patients 70 years old and older. Artif Organs. 2002;26:1059–1062. doi: 10.1046/j.1525-1594.2002.07005_4.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams DB, Danielson GK, McGoon DC, Puga FJ, Mair DD, Edwards WD. Porcine heterograft valve replacement in children. J Thorac Cardiovasc Surg. 1982;84:446–450. [PubMed] [Google Scholar]
- 19.Levy RJ, Schoen FJ, Levy JT, Nelson AC, Howard SL, Oshry LJ. Biologic determinants of dystrophic calcification and osteocalcin deposition in glutaraldehyde-preserved porcine aortic valve leaflets implanted subcutaneously in rats. Am J Pathol. 1983;113:143–155. [PMC free article] [PubMed] [Google Scholar]
- 20.Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29:1065–1074. doi: 10.1016/j.biomaterials.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer SR, Nagendran J, Desai LS, et al. Decellularization reduces the immune response to aortic valve allografts in the rat. J Thorac Cardiovasc Surg. 2005;130:469–476. doi: 10.1016/j.jtcvs.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Watts LK, Duffy P, Field RB, Stafford EG, O'Brien MF. Establishment of a viable homograft cardiac valve bank: a rapid method of determining homograft viability. Ann Thorac Surg. 1976;21:230–236. doi: 10.1016/s0003-4975(10)64297-x. [DOI] [PubMed] [Google Scholar]
- 23.Lovelock JE, Bishop MW. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959;183:1394–1395. doi: 10.1038/1831394a0. [DOI] [PubMed] [Google Scholar]
- 24.Smith AU. The effects of glycerol and of freezing on mammalian organs. In: Smith AU, editor. Biological effects of freezing and supercooling. London: Edward Arnold; 1961. pp. 247–269. [Google Scholar]
- 25.O'Brien MF, McGiffin DC, Stafford EG, et al. Allograft aortic valve replacement: long-term comparative clinical analysis of the viable cryopreserved and antibiotic 4 degrees C stored valves. J Card Surg. 1991;6(4 Suppl):534–543. doi: 10.1111/jocs.1991.6.4s.534. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Waer M, Billiau AD. Xenotransplantation: role of natural immunity. Transpl Immunol. 2009;21:70–74. doi: 10.1016/j.trim.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Sprangers B, Waer M, Billiau AD. Xenotransplantation: where are we in 2008? Kidney Int. 2008;74:14–21. doi: 10.1038/ki.2008.135. [DOI] [PubMed] [Google Scholar]
- 28.Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7:519–531. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]