Abstract
The term neuroplasticity encompasses structural and functional modifications of neuronal connectivity. Abnormal neuroplasticity is involved in various neuropsychiatric diseases, such as dystonia, epilepsy, migraine, Alzheimer's disease, fronto-temporal degeneration, schizophrenia, and post cerebral stroke. Drugs affecting neuroplasticity are increasingly used as therapeutics in these conditions. Neuroplasticity was first discovered and explored in animal experimentation. However, non-invasive brain stimulation (NIBS) has enabled researchers recently to induce and study similar processes in the intact human brain. Plasticity induced by NIBS can be modulated by pharmacological interventions, targeting ion channels, or neurotransmitters. Importantly, abnormalities of plasticity as studied by NIBS are directly related to clinical symptoms in neuropsychiatric diseases. Therefore, a core theme of this review is the hypothesis that NIBS-induced plasticity can explore and potentially predict the therapeutic efficacy of CNS-acting drugs in neuropsychiatric diseases. We will (a) review the basics of neuroplasticity, as explored in animal experimentation, and relate these to our knowledge about neuroplasticity induced in humans by NIBS techniques. We will then (b) discuss pharmacological modulation of plasticity in animals and humans. Finally, we will (c) review abnormalities of plasticity in neuropsychiatric diseases, and discuss how the combination of NIBS with pharmacological intervention may improve our understanding of the pathophysiology of abnormal plasticity in these diseases and their purposeful pharmacological treatment.

Ulf Ziemann (far left) is clinical director of the Department of Neurology and Stroke, Hertie Institute for Clinical Brain Research, Eberhard-Karls University of Tübingen, Germany. His expertise is in clinical neurophysiology with focus on motor cortical excitability, plasticity, learning and neurorehabilitation. He has developed the field of pharmaco-TMS, which provides important knowledge on the physiology of TMS measures of excitability and the pharmacological regulation of plasticity in human motor cortex. Florian Müller-Dahlhaus (centre left) is resident at the Department of Neurology, Goethe-University Frankfurt, Germany. He has expertise in cellular electrophysiology and imaging as well as pharmaco-TMS. Research focuses on understanding mechanisms of neural plasticity involved in motor learning to enhance rehabilitation post stroke. Walter Paulus (centre right) is clinical director of the Department of Clinical Neurophysiology, University Medical Centre, Germany. He investigates motor cortex physiology by means of transcranial stimulation, further refined by co-application of CNS-active drugs. He was involved in the development of new stimulation methods for induction of neuroplasticity such as tDCS, tACS and tRNS. His clinical focus encompasses Parkinson's disease, restless legs syndrome, epilepsy and pain. Michael A. Nitsche (far right) is a consultant in the same department. His scientific interests are focused at human systems neuroscience, with special dedication to plasticity induction in the human brain by non-invasive brain stimulation tools, neuropsychopharmacology, the motor system, and cognition. His clinical focus is epileptology.
Introduction
Neuroplasticity is the ability of the brain to reorganize its structure and function due to intrinsic or environmental demands. More specifically, this term encompasses the weakening and strengthening of pre-existing synaptic connections as well as the pruning of pre-existing, and the formation of new, synapses. First experimentally demonstrated in 1973 by Bliss and Lømo, neuroplasticity has attracted ever increasing attention in neuroscience research, because it seems to form the physiological basis of cognitive processes such as learning and memory formation. Moreover, neuroplasticity is involved in neuropsychiatric diseases and rehabilitation processes, such as reorganization of neuronal networks in schizophrenia or re-learning of motor functions after stroke. In animal research, protocols suitable to induce plasticity, and mechanisms of plasticity induction, have been extensively studied since its introduction and, conceptually, may be transferred to non-invasive brain stimulation (NIBS) protocols for the induction of plasticity in the human brain. This basic research has helped to clarify mechanisms of plasticity in the human brain in vivo, and its importance for normal and pathological brain function. Importantly, it was demonstrated that plasticity in humans can be significantly modified by pharmacological agents.
Drug development for the treatment of neuropsychiatric diseases in humans is currently burdened by a high rate of marketing failure at late stages due to unsuccessful large-scale clinical trials (Woolf, 2010). For neuropsychiatric diseases, in which alterations of plasticity are causally involved, exploration of the impact of CNS active drugs on neuroplasticity is likely to be a highly attractive intermediate step. Modifying drug effects on abnormal plasticity may constitute novel biomarkers of monitoring or predicting treatment efficacy.
In this paper, we will first review stimulation techniques for the induction of plasticity in animals and humans, summarize the involved mechanisms of plasticity, and review the pharmacology of plasticity. In the next step, we will give an overview about abnormalities of plasticity in neuropsychiatric diseases, as explored with NIBS techniques, and describe pharmacological effects on neuroplasticity and their relation to clinical symptoms in these diseases. Finally, we will give an outlook on the potential of NIBS-induced neuroplasticity to serve as a biomarker for probing the therapeutic efficacy of CNS active drugs.
Plasticity induction protocols
Animal experimentation
Repetitive electrical stimulation
Since the seminal work by Bliss & Lømo (1973) it is known that repetitive electrical stimulation of nerve fibres can induce an immediate and prolonged increase in synaptic transmission. This effect is called long-term potentiation (LTP). First described in the anaesthetized rabbit, high-frequency stimulation (≥10 Hz) of perforant path fibres, the main excitatory input from the entorhinal cortex to the dentate gyrus of the hippocampus, resulted in an increase of the population response recorded from dentate gyrus granule cells for up to 10 h (Bliss & Lømo, 1973). Effective stimulation protocols comprised 10–20 Hz for 10–15 s or 100 Hz for 3–4 s.
Activity-dependent LTP of synaptic efficacy has since been found in virtually all excitatory pathways in the hippocampus (Bliss & Collingridge, 1993), as well as in the neocortex (Feldman, 2009). A multitude of different electrical stimulation protocols has proved to induce LTP. Among the most common are tetanic stimulation, which involves a high-frequency train of 50–100 stimuli at 100 Hz, and theta-burst stimulation (TBS). In TBS, typically several bursts of 3–5 stimuli at 100 Hz are delivered at short (<1 s) inter-burst intervals. In contrast, low-frequency stimulation typically induces long-term depression (LTD) of synaptic efficacy (Lynch et al. 1977; Dunwiddie & Lynch, 1978; Bramham & Srebro, 1987; Mulkey & Malenka, 1992; Kirkwood & Bear, 1994). Thus, synaptic transmission can be modified in a bidirectional manner, the polarity of which depends on the rate and pattern of synaptic activity. There is now compelling evidence for a role of these synaptic mechanisms in many forms of learning and memory as well as neuronal development and circuit reorganization (Katz & Shatz, 1996; Morris et al. 2003; Cooke & Bliss, 2006; Feldman, 2009).
Spike-timing dependent plasticity induction protocols
The plasticity induction protocols reviewed above are rate-based, i.e. they primarily rely on the presynaptic spike frequency. In more recent years, however, a new concept of Hebbian synaptic plasticity (Hebb, 1949) has emerged, known as spike-timing dependent plasticity (STDP). STDP emphasizes the temporal order instead of the frequency of spike trains: both LTP and LTD can be induced at low frequency depending on the precise timing relationships between pre- and postsynaptic firing (Markram et al. 2011). Although the temporal windows for LTP and LTD induction differ significantly in a cell- and synapse-type-specific manner, STDP is well preserved across cortical layers and brain regions. In its classical form, synapses between excitatory neurons are strengthened if presynaptic precedes postsynaptic activity by some tens of milliseconds or less; if this order is reversed, and presynaptic activity does not predict postsynaptic spiking, synaptic efficacy is reduced (Caporale & Dan, 2008). More recent work has focused on parameterizing STDP with respect to factors such as rate, complex spike train motifs, or dendritic localization (Froemke et al. 2010).
Classical LTP/LTD and STDP are complementary models of synaptic plasticity rather than exclusive of each other; rate- and timing-dependent forms of plasticity may co-exist at the same synapse type (Nelson et al. 2002). In addition, synaptic activity (or a lack thereof) may trigger slower non-Hebbian forms of plasticity including homeostatic plasticity (Turrigiano, 2008; Pozo & Goda, 2010) and metaplasticity (Abraham, 2008). The complexity of, and interaction among, the various forms of synaptic plasticity are just about to emerge (Sjostrom et al. 2001; Nelson & Turrigiano, 2008).
DC stimulation
Brain stimulation with direct currents (DC stimulation, DCS) to generate plasticity differs qualitatively from the above-mentioned stimulation protocols. In resting cells stimulation itself does not elicit action potentials; in active cells it modulates stimulation polarity-dependently the resting neuronal membrane potential, resulting in immediate changes of cortical firing rates (Creutzfeldt et al. 1962; Bindman et al. 1964). These changes transfer to long-lasting alterations that are present for some hours after stimulation, if DCS is applied for sufficiently long (at least a few minutes). Anodal DCS of the sensorimotor cortex and the visual cortex of rats and cats enhanced spontaneous neuronal activity, and increased the size of evoked potentials, whereas cathodal DCS had the opposite effects (Creutzfeldt et al. 1962; Bindman et al. 1964; Purpura & McMurtry, 1965). Since the impact of DCS on membrane polarization depends on the orientation of a given neuron relative to the induced electrical field, the effects are not, however, absolutely homogeneous (Faria et al. 2011). They might also differ, for instance, between neurons situated at the crown of cerebral gyri, and deep in the sulci (Creutzfeldt et al. 1962).
For the induction of DCS after-effects, the coupling between membrane polarity alterations and presynaptic neuronal activity seems necessary (Fritsch et al. 2010). Enhancement of intraneuronal calcium concentration occurs after anodal DCS (Islam et al. 1995a), and probably triggers plastic change of synaptic efficacy, as shown for other stimulation protocols (Malenka & Bear, 2004). Moreover, the after-effects of anodal DCS depend on protein synthesis, alter brain-derived neurotrophic factor (BDNF) and tyrosine kinase B activation, and are accompanied by enhanced expression of immediate early genes (Gartside, 1968; Islam et al. 1995b; Fritsch et al. 2010), which are all characteristics of late phase LTP-like processes (Malenka & Bear, 2004).
In accordance with the modulating effects of DCS on spontaneous neuronal activity, numerous animal experiments have demonstrated a polarity-specific alteration of learning and memory formation by DCS (Morrell & Naitoh, 1962; Proctor et al. 1964; Albert, 1966; Rosen & Stamm, 1972). The DCS-induced postsynaptic alteration of membrane polarization in the presence of presynaptic neuronal activity shares some similarities with STDP. In contrast to STDP, which is thought to be restricted to the set of synapses activated by the stimulation protocol, the effects of DCS should, however, be less specific.
Experiments in humans
The above-mentioned plasticity induction protocols are not only able to induce synaptic plasticity in animal slice preparations, but also in human brain tissue. It was demonstrated that rhythmic electrical theta burst stimulation induces glutamatergic LTP in human hippocampal slices, and low frequency stimulation results in depotentiation (Beck et al. 2000). Similar effects were obtained for STDP protocols in human hippocampal slices, but the time window to induce LTP was relevantly broader than that seen in rodent experiments (Testa-Silva et al. 2010). However, the opportunities to obtain human brain tissue for experimental purposes are rare, and in vitro results might not always be completely identical to in vivo results, due to differences in spontaneous activity, neurotransmitter and neuromodulator concentration, and other factors. Thus, during the last 20 years NIBS techniques to elicit neuroplasticity in the intact human brain were developed.
Repetitive transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) is a safe and painless NIBS technique to study and interfere with ongoing neuronal activity in conscious human subjects (Barker et al. 1985). For TMS, a time-varying magnetic field is generated by a brief high-current pulse in a coil of wire placed above the subject's head. The magnetic field passes through the intact scalp unrestricted and induces an electric field in the underlying brain. TMS activates both excitatory and inhibitory neuronal elements in the cerebral cortex (Hallett, 2007), with axons being most likely the primary site of activation (Rotem & Moses, 2008). Thus, different patterns of neuronal activity can be imposed on the stimulated cortex and interconnected brain areas non-invasively.
Repetitive TMS (rTMS) can modulate human cortical excitability for minutes and even hours beyond the stimulation period (Ziemann et al. 2008). In the motor cortex, these after-effects of rTMS can be measured by the size of the electromyographic response evoked by a single standard suprathreshold TMS pulse in the target muscle (motor evoked potential, MEP). In non-motor areas, long-lasting rTMS effects were demonstrated based on neuroimaging techniques (Siebner et al. 2009) and EEG recordings (Rossi et al. 2009; Thut & Pascual-Leone, 2010).
The threshold for inducing changes in cortical excitability is a complex function of the intensity, rate and duration of rTMS. For instance, in motor cortex short trains of high-frequency 5 Hz-rTMS result in only short-lasting increases in MEP size, which can be prevented by blockers of voltage-gated sodium channels (Inghilleri et al. 2004), suggestive of post-tetanic potentiation-like plasticity. In contrast, long trains of high-frequency rTMS (several hundred pulses at frequencies ≥5 Hz) result in long-lasting (>30 min) MEP increases, suggestive of LTP-like mechanisms (Ziemann, 2004). Moreover, it is now well-established that high-frequency rTMS at ≥5 Hz increases human cortical excitability long-lastingly, whereas low-frequency rTMS at around 1 Hz has the opposite effect (Ziemann, 2004; Ziemann et al. 2008). Most studies claiming an excitability increasing effect of 5 Hz have been performed with intervals for the purpose of coil cooling; when applied continuously 5 Hz rTMS turned out to be inhibitory (Rothkegel et al. 2010). Thus, human cortical excitability can be modulated in a bidirectional and rate-dependent manner by rTMS. Although evidence suggests that LTP/LTD-like mechanisms play a role in rTMS-induced long-lasting after-effects on cortical excitability (Ziemann, 2004; Ziemann et al. 2008), the exact mechanisms remain insufficiently understood.
More recently, theta burst stimulation (TBS) as first applied in hippocampal electrophysiology has been successfully transferred to the human brain by means of TMS (Huang et al. 2005). In these experiments, short bursts (3 pulses) of magnetic stimuli were given at high frequency (50 Hz), repeated every 200 ms. TBS of the human motor cortex can produce a rapid bidirectional modulation of cortical excitability as shown by increases and decreases in MEP size, respectively. The direction of these MEP changes is critically dependent on the pattern of TBS. Intermittent TBS (iTBS), in which a 2 s train of TBS is repeated every 10 s for a total of 190 s (600 pulses), increases MEPs for up to 20 min post stimulation. In contrast, continuous TBS (cTBS), in which a 40 s train of uninterrupted 600 TBS pulses is given, depresses MEPs for about 60 min post stimulation. TBS has become an increasingly used NIBS protocol as it offers powerful effects on human motor cortex physiology and behaviour after very short application periods (Huang et al. 2005).
Paired associative stimulation
Recently, convergent evidence has been accumulated that it is also possible to induce plasticity in the human CNS by NIBS, which shares some similarities with STDP (Müller-Dahlhaus et al. 2010).
In paired associative stimulation (PAS) in humans two convergent inputs are given to a cortical area repetitively with strict temporal order and timing at low repetition frequency. In the original experiments, PAS consisted of electrical stimulation of the median nerve at the wrist (MNS), followed by TMS of the contralateral primary motor cortex (M1) after 25 ms (Stefan et al. 2000). At this interstimulus interval, the afferent signal evoked by MNS arrives in M1 nearly synchronously, or even shortly before transsynaptic excitation of corticospinal neurons by the TMS pulse. Ninety pairs of conjoint MNS and TMS applied at a frequency of 0.05 Hz over 30 min significantly increased MEP amplitudes in a resting intrinsic hand muscle for 30–60 min post stimulation (Stefan et al. 2000). This effect was critically dependent on the interval between MNS and TMS because an interstimulus interval of only 10 ms resulted in depression of MEPs (Wolters et al. 2003).
Timing dependent associative plasticity in conscious human subjects has also been demonstrated in primary somatosensory cortex (Wolters et al. 2005; Litvak et al. 2007), and interhemispherically between homologous areas of left and right M1 (Koganemaru et al. 2009; Rizzo et al. 2009b). Recent work extended these findings by demonstrating bidirectional associative plasticity in intrahemispheric cortico-cortical pathways in healthy human subjects (Arai et al. 2011; Buch et al. 2011). Of note, in the study by Arai and colleagues associative plasticity in a human cortical motor network involving the supplementary motor area (SMA) and M1 required priming by near-simultaneous bilateral M1 stimulation to occur, indicating state-dependency of SMA-M1 associative plasticity (Arai et al. 2011). Furthermore, it was demonstrated that motor learning significantly modulates PAS-induced timing dependent plasticity (Ziemann et al. 2004), and, conversely, motor learning can be modulated bidirectionally by prior PAS-induced plasticity (Jung & Ziemann, 2009), providing circumstantial evidence that LTP-like mechanisms may be involved in human motor learning.
Transcranial direct current stimulation
Based on the above-reviewed animal experiments, stimulation protocols were developed which allow the induction of long-lasting cortical excitability alterations in humans via non-invasive application of direct currents through the intact scalp (transcranial direct current stimulation, tDCS). Stimulation is performed by relatively large electrodes (sizes employed so far about 3.5–35 cm2) positioned over the scalp area covering the region of interest. It was shown first for M1, but later also for other areas, such as visual and somatosensory cortices, that tDCS is able to induce prolonged alterations of cortical excitability, which can last for over one hour after the end of stimulation, if its duration is in the range between 10 and 15 min (Nitsche & Paulus, 2000, 2001; Nitsche et al. 2003b; Antal et al. 2004b; Matsunaga et al. 2004). Hereby, the duration and strength of the after-effects depends on stimulation intensity and duration. Moreover, the efficacy of stimulation depends on the position of the return electrode, supporting the relevance of the current flow/electric field relative to neuronal orientation in space (Nitsche & Paulus, 2000). For the motor cortex, anodal tDCS enhances, while cathodal tDCS diminishes, corticospinal excitability, as explored by recruitment curves obtained for MEP amplitudes elicited by single pulse TMS of M1 (Nitsche et al. 2005). This effect might be causally related to an impact of tDCS on indirect waves (I-waves) of the multiple-discharge corticospinal volley (Lang et al. 2011). At the intracortical level, anodal tDCS enhances cortical facilitation, and decreases cortical inhibition, whereas cathodal tDCS results in opposite effects. Apart from its local effects on the areas under the electrodes, tDCS affects activity and excitability of widespread cortical and subcortical areas (Lang et al. 2004). These remote effects of tDCS might, at least partially, be due to effects of tDCS on functional connectivity of cortical and subcortical areas connected with the cortical areas under the electrodes (Keeser et al. 2011). Application of tDCS during learning processes can improve learning performance, an observation that is in favour of overlapping mechanisms of action shared by learning and DCS-induced plasticity (Nitsche et al. 2003c; Antal et al. 2004a; Reis et al. 2009).
Similarities and differences between plasticity induction protocols
The reviewed plasticity induction protocols, both in animal preparations and humans differ qualitatively from each other. Repetitive and associative stimulation protocols elicit action potentials, and induce plasticity via their specific frequency or timing, whereas DCS modulates spontaneous neuronal network activity by subthreshold effects on neuronal resting membrane potentials. However, all of these techniques are suited to generate plasticity at excitatory glutamatergic synapses in animals and humans (see also below). In animal experiments, plasticity at inhibitory GABAergic synapses has also been described (Maffei, 2011), while this form of plasticity is less well explored in humans.
The duration of the after-effects of NIBS in humans is similar to that of early LTP and LTD in animal preparations. While the principal mechanisms of action of the stimulation protocols are similar in animal and human experiments, there are also important specific differences: for repetitive stimulation, the classical stimulation protocols in animal slice preparations stimulate afferent connections of the target area, while in humans axons in the target area itself are stimulated. In addition, input specificity is compromised in human NIBS studies because hundreds of thousands of axons of diverse origin, including axons from excitatory as well as inhibitory neurons, are stimulated simultaneously. For instance, whereas STDP as studied at the cellular level critically depends on coordinated pre- and postsynaptic activity in single neurons (or even in single pre- and postsynaptic elements), experiments at the system level of the human cortex have demonstrated PAS-induced after-effects by near-synchronous activation of the target area by two convergent inputs (e.g. by afferent activation via peripheral nerve stimulation and associative TMS of the target area or an interconnected brain area). The input specificity of these effects is in the order of cubic centimetres of cortex, and thus multiple orders of magnitude higher than the scale at which synaptic plasticity operates. Thus, comparisons between different levels of observation (i.e. cellular vs. system level) should be done with caution. However, physiological properties and modelling of PAS-induced changes in cortical excitability (for review see e.g. Müller-Dahlhaus et al. 2010) are to some degree reminiscent of STDP as observed for excitatory-to-excitatory connections in animal experiments (Caporale & Dan, 2008), suggesting at least some correspondence between these phenomena. Finally, the majority of plasticity induction procedures in animal experimentation were conducted in hippocampal or prefrontal cortex slices, whereas studies in humans have mainly focused on the motor cortex. This further limits direct comparability between both strains of experiments because neuronal architecture and receptor density, which are relevant for neuroplastic processes, differ substantially between these areas.
Taking into account these differences and similarities between plasticity induction procedures applied in animals and humans, the currently available procedures do not allow one-to-one comparability, but due to similar stimulation protocols, modes of action, and physiological characteristics of the induced effects it can be stated that NIBS in humans is capable of inducing LTP- and LTD-like plasticity, similar to LTP/LTD as studied on a cellular level (Cooke & Bliss, 2006). A synopsis of the characteristics, mechanisms of action and effects of the main plasticity-inducing protocols in animal preparations and humans is given in Table 1.
Table 1.
Synopsis of the main plasticity-producing stimulation protocols applied in animal experimentation and their counterparts for non-invasive brain stimulation in humans, including protocol characteristics, effects, and mechanisms of action
| Repetitive stimulation | Associative stimulation | Tonic stimulation with DC | ||||
|---|---|---|---|---|---|---|
| Animals | Humans | Animals | Humans | Animals | Humans | |
| Stimulation | Electrical stimulation of afferents | TMS of axons of cortical neurones | Pre- and postsynaptic electrical | Peripheral nerve electrical and TMS of cortex; dual-coil TMS of cortex | Epidural/intracortical/transcranial electrical stimulation of cortex | Transcranial electrical stimulation of cortex |
| Protocols | High/low frequency, theta-burst | High/low frequency, theta-burst | Near-synchronous/a-synchronous | Near-synchronous | Anodal/cathodal | Anodal/cathodal |
| Effects | LTP: high-frequency, theta-burst | LTP-like: high-frequency, intermittent theta-burst | LTP: near-synchronous (pre-before-post) | LTP-like: near-synchronous; conditioning pulse-before-test pulse in target area | LTP: anodal | LTP-like: anodal |
| LTD: low-frequency | LTD-like: low frequency, continuous theta-burst | LTD: asynchronous; near-synchronous (post-before-pre) | LTD-like: near-synchronous; test pulse in target area-before-conditioning pulse | LTD: cathodal | LTD-like: cathodal | |
| Synapses | Glutamatergic, GABAergic | Glutamatergic | Glutamatergic, GABAergic | Glutamatergic | ? | Glutamatergic |
Note that transcranial magnetic stimulation (TMS) generates its effects via electrical stimulation of axons of cortical neurons. LTP, long term potentiation; LTD, long term depression; GABA, γ-aminobutyric acid.
Pharmacological modulation of plasticity
Animal experimentation
Drivers of plasticity
The glutamatergic system. Neurotransmission at most excitatory synapses in the brain operates through two types of ionotropic glutamate receptors termed α-amino-3-hydroxy-5-methyl-4- isoxazolepropionate (AMPA) and N-methyl-d-aspartate (NMDA) receptors. Whereas NMDA receptors are the main driver for induction of LTP, AMPA receptor-mediated synaptic neurotransmission is the key target of LTP expression. In the following the molecular mechanisms of LTP and LTD are reviewed in more detail.
Induction of long-term plasticity at most excitatory synapses depends on synaptic activation of NMDA receptors and subsequent calcium influx through the NMDA receptor channel (Lynch et al. 1983; Morris et al. 1986; Malenka et al. 1988). However, under resting membrane conditions, NMDA receptors are blocked by magnesium. Only after removal of the magnesium block by sufficient depolarization of the postsynaptic membrane is the NMDA receptor functioning and calcium may flow into the postsynaptic cell. This voltage-sensitivity of the NMDA receptor complex may explain the frequency-dependence of LTP/LTD induction. High-frequency stimulation results in strong postsynaptic depolarization, NMDA receptor activation and high levels of postsynaptic calcium which triggers the molecular pathways for LTP, whereas low-frequency stimulation evokes less NMDA receptor-mediated calcium influx resulting in LTD. In addition, the NMDA receptor voltage-sensitivity serves as a coincidence detector for pre- and postsynaptic activity in associative LTP.
Apart from NMDA receptors, kainate receptors – a third type of ionotropic glutamate receptor – and metabotropic glutamate receptors have also been implicated in the induction of LTP (Bashir et al. 1993; Bortolotto et al. 1999). Furthermore, induction of LTP at excitatory synapses may also depend on neuron–glia interactions, as d-serine from astrocytes is necessary for LTP in nearby synapses (Henneberger et al. 2010).
The intracellular signalling pathways triggered by a strong increase in postsynaptic calcium include activation of protein kinase C, calcium/calmodulin-dependent protein kinase II and tyrosine kinases (Malinow et al. 1989; O’Dell et al. 1991). These molecular events result in phosphorylation of AMPA receptors in the postsynaptic membrane and insertion of new AMPA receptors into it (Malinow & Malenka, 2002). Thus, LTP is primarily expressed as an increase in AMPA receptor-mediated neurotransmission, although the NMDA receptor-mediated component of glutamatergic neurotransmission may also be potentiated (Bashir et al. 1991). In the extreme case, under resting conditions ‘silent’ (i.e. AMPA receptor-lacking) synapses are activated by insertion of functional AMPA receptors upon LTP induction (Liao et al. 1995). In contrast, LTD is mediated by AMPA receptor internalization (Beattie et al. 2000). In addition, there is evidence for presynaptic expression mechanisms of LTP and LTD, which involve diffusion of retrograde messengers such as nitric oxide (Schuman & Madison, 1991), platelet-activating factor (Kato et al. 1994) or endocannabinoids (Sjostrom et al. 2003) from the post- to the presynaptic element. Currently, to what extent even the best-studied form of synaptic plasticity, NMDA receptor-dependent LTP, is expressed pre- or postsynaptically is still a matter of ongoing debate (Lisman, 2009).
LTP at excitatory synapses develops in stages, from a short-lasting (less than one hour), protein kinase-independent phase to three different levels of long-lasting potentiation, requiring protein phosphory lation, protein synthesis, and gene transcription, respectively (Bliss & Collingridge, 1993; Raymond, 2007). These functional alterations of synaptic neurotransmission are paralleled by morphological changes of dendritic spines, the main site of glutamatergic synapses in the brain (Segal, 2005; Alvarez & Sabatini, 2007; Holtmaat & Svoboda, 2009).
The GABAergic system.γ-Aminobutyric acid (GABA) acts as the main inhibitory neurotransmitter in the mammalian brain and operates through two classes of receptors, a ionotropic GABAA and a metabotropic GABAB receptor.
A role for GABAergic inhibition in modulating excitatory synaptic plasticity has long been recognized, as GABA receptor blockers promote LTP of glutamatergic neurotransmission in the hippocampus (Wigstrom & Gustafsson, 1983) and the neocortex (Artola & Singer, 1987). Interestingly, GABAAergic inhibitory control of excitatory synapses is released during high-frequency synaptic transmission by autoinhibition of GABAergic terminals via GABAB receptors, thus allowing for sufficient postsynaptic depolarization to activate NMDA receptors and induce LTP (Davies et al. 1991).
Studies on synaptic plasticity have mainly focused on plasticity of excitatory synapses. However, more recent evidence suggests that GABAergic synapses themselves are also highly dynamic and capable of activity-dependent long-term plasticity (Gaiarsa et al. 2002). Both classical LTP/LTD and STDP may be induced at GABAergic synapses (Maffei, 2011). Some forms of inhibitory synaptic plasticity, e.g. by high-frequency stimulation, also require postsynaptic activation of glutamatergic receptors, while GABAA receptor activity seems to be more involved in maintaining plasticity. Thus, both excitatory and inhibitory inputs are integrated in this form of heterosynaptic inhibitory plasticity, which serves to fine-tune neural networks and maintain circuit stability by dynamically regulating the balance between excitation and inhibition. In addition, inhibitory synaptic plasticity may impact on induction of plasticity between excitatory neurons, thus adding to the complex interplay between different forms of neural plasticity.
The mechanisms of induction and expression of inhibitory synaptic plasticity show significant differences between cell-types, brain areas and developmental stages (Maffei, 2011), but in common with the induction of excitatory synaptic plasticity the rise in intracellular calcium concentration plays a crucial role for triggering inhibitory synaptic plasticity (Gaiarsa et al. 2002). Expression of LTP at GABAergic synapses induced by high-frequency stimulation involves BDNF retrograde signalling in visual cortex and hippocampus, allowing for a spatially restricted action of GABAergic plasticity at specific synapses. However, at synapses in the ventral tegmental area NO signalling is involved in GABAergic plasticity, which has a more widespread influence on multiple presynaptic GABAergic terminals and thereby changes excitability and information processing in a larger portion of the microcircuit. As for excitatory synaptic plasticity, the site of expression of long-term alterations of GABAergic neurotransmission can be both pre- and postsynaptic, depending on the form of induced plasticity (classical LTP/LTD; STD-LTP/-LTD), the type of inhibitory neuron, the brain area, or even the layer within a given cortical area (Maffei, 2011).
Voltage-gated ion channels. As reviewed above, LTP of synaptic efficacy is a calcium-dependent process, which is classically induced by calcium influx through NMDA receptor channels. An alternative way of postsynaptic calcium influx is through voltage-gated calcium channels (VGCCs), which have also been implicated in induction of LTP (Westenbroek et al. 1990). Additionally, VGCCs may be involved in presynaptic expression of LTP (Ahmed & Siegelbaum, 2009).
Transmission and integration of synaptic inputs depends on dendritic excitability. There is increasing evidence for an active role of dendrites in neuronal information processing (Johnston et al. 1996). Modulation of the distribution and/or function of dendritic voltage-gated ion channels may therefore change the neuronal input–output relationship, a form of plasticity termed intrinsic plasticity (Remy et al. 2010). Moreover, there is a complex, reciprocal relationship between dendritic excitability and synaptic plasticity (Sjostrom et al. 2008). Since postsynaptic, dendritic depolarization is critical for the induction of synaptic long-term plasticity (see above), changes in, for example, back-propagating action potential and/or dendritic spike generation may critically modulate the plastic properties of synapses. Conversely, dendritic excitability can be altered by synaptic plasticity, with fundamental implications for information processing and subsequent memory storage in neuronal networks.
Modulators of plasticity
In contrast to the above-mentioned drivers of plasticity, several neurotransmitter systems (dopaminergic, cholinergic, serotonergic and noradrenergic) have modulating roles on plasticity. Activation of these neuromodulating systems is not a necessary precondition to induce plasticity, but these systems have the capacity to modify the amount and direction of plasticity induced. Typically, the impact of neuromodulators is not restricted to a single type of receptor or channel, but involves a diversity of structures, often in a non-linear manner. Thus, the net effect of a neuromodulator on plasticity is often not easily predictable. Although neuromodulators modify plasticity not only at the cortical, but also at subcortical levels, we will restrict our overview to cortical plasticity, which is the focus of action of the plasticity induction protocols applied in humans.
The dopaminergic system. Dopamine (DA) has major modulating effects on glutamatergic synaptic plasticity. DA enhanced LTP and LTD in slice and in vivo animal experiments for repetitive electrical stimulation (Otani et al. 1998; Bailey et al. 2000), and LTP, or the time window for induction of LTP, in the case of STDP (Zhang et al. 2009; Edelmann & Lessmann, 2011). This effect of DA is non-linear and depends on subtype of receptor activation as well as the concentration of background DA. It was proposed that low background DA favours LTD induction, whereas high background DA fosters LTP induction and even further increases of DA result in no plasticity, if combined with phasic, activation-dependent DA increases (Goto et al. 2010). Dopaminergic subtypes of receptors contribute differently to these plasticity-modulating effects: whereas D1 receptor activation enhances LTP and LTD for repetitive stimulation-induced plasticity (Chen et al. 1996; Otmakhova & Lisman, 1996; Bach et al. 1999; Bailey et al. 2000; Gurden et al. 2000; Huang et al. 2004), increasing but also decreasing effects on LTP and LTD occur with D2 receptor activation (Frey et al. 1989; Chen et al. 1996; Otani et al. 1998; Gurden et al. 2000; Spencer & Murphy, 2000; Manahan-Vaughan & Kulla, 2003). For STDP, D1 receptor activity was shown to enhance LTP, or broaden the time window for LTP induction, and converted the effect of LTD-inducing protocols into LTP (Zhang et al. 2009; Edelmann & Lessmann, 2011). However, abolishment of LTD by D1 receptor blockade has been reported in another study (Pawlak & Kerr, 2008). In contrast, D2 receptor activity might reduce LTP (Pawlak & Kerr, 2008). Because DA has a complex, often non-linear and subtype-of-receptor-specific impact on diverse ion channels, receptors and enzyme cascades involved in neuroplasticity, how DA affects plasticity is difficult to predict and incompletely understood (Seamans & Yang, 2004). However, since D1 and D2 receptors have antagonistic effects on NMDA and GABA receptors (D1 receptors enhance the activity of both kinds of receptors, while D2 receptors reduce their activity; Seamans & Yang, 2004), it might be speculated that the balance of the effects of DA subtypes of receptors on NMDA and GABA receptors determines the net impact of DA on neuroplasticity (Goto et al. 2010; Xu & Yao, 2010).
The cholinergic system. The cholinergic system also has a prominent impact on cortical plasticity. For NMDA receptor-dependent LTP induced by repetitive electrical stimulation, a permissive function of the cholinergic system could be demonstrated: cholinergic activation promotes LTP, whereas cholinergic antagonists block it (Blitzer et al. 1990; Brocher et al. 1992; Hasselmo & Barkai, 1995; Auerbach & Segal, 1996). Similarly, cholinergic activation enhances NMDA receptor-dependent LTD (Huerta & Lisman, 1995; Kirkwood et al. 1999). The two major acetylcholine receptor subtypes (muscarinergic (mAChR) and nicotinergic receptors (nAChR)) seem to be involved in LTP and LTD enhancement (Burgard & Sarvey, 1990; Fujii & Sumikawa, 2001; Shinoe et al. 2005; Scheiderer et al. 2008; Jia et al. 2010). One important common mechanism seems to be that activation of both receptor subtypes results in depolarization of the postsynaptic membrane or increase of intracellular calcium level (Sawada et al. 1994; Huerta & Lisman, 1995; Auerbach & Segal, 1996; Gu, 2002). Although a plasticity-enhancing effect of cholinergic activation has been demonstrated in the majority of studies, two studies also showed a dose-dependent reduction at high concentrations of acetylcholine (Maeda et al. 1993; Sugisaki et al. 2011). For STDP the situation might be somewhat different, because here M1 mAChR activation prevented LTP induction, but enhanced and broadened the window for induction of LTD (Seol et al. 2007; Huang et al. 2012).
The serotonergic system. Serotonin (or 5-HT) also had a prominent, but seemingly heterogeneous, impact on LTP and LTD in animal preparations, as induced by repetitive electrical stimulation. A number of studies showed an LTP-reducing or -abolishing effect of serotonin enhancement or 5-HT receptor activation (Staubli & Otaky, 1994; Edagawa et al. 1998; Kojima et al. 2003; Mnie-Filali et al. 2006). However, 5-HT antagonists can also abolish LTP (Sanberg et al. 2006; Huang & Kandel, 2007). In addition a nil effect of 5-HT on LTP was reported (Normann & Clark, 2005), and some studies even showed enhanced LTP under serotonergic activation (Kojic et al. 1997; Mori et al. 2001). Taken together, these results are in favour of a non-linear, dosage-dependent effect of 5-HT on LTP. Moreover, age, cortical area, the types of serotonergic subtypes of receptors, and duration of 5-HT receptor activation all seem to affect the impact of this neuromodulator on LTP (Kojic et al. 1997; Mori et al. 2001; Ohashi et al. 2002; Ryan et al. 2009; Bhagya et al. 2011).
5-HT receptor activation blocks LTD or even converts it into LTP (Kemp & Manahan-Vaughan, 2005; Normann & Clark, 2005; Jang et al. 2010), whereas 5-HT antagonists enhance LTD in brain slices of adult animals (Kemp & Manahan-Vaughan, 2005). However, in the visual cortex of juvenile cats, serotonin enhances LTD (Kojic et al. 1997). Thus, the effects of 5-HT on LTD may also be affected by the specific conditions of the slice preparations.
The adrenergic system. The adrenergic impact on plasticity seems relatively uniform, as can be derived from the results of animal slice experiments. Noradrenergic activity enhances LTP (Hu et al. 2007; Tully et al. 2007; Korol & Gold, 2008). Likewise, adrenaline enhances LTD (Marzo et al. 2010), while noradrenaline blocks LTD (Katsuki et al. 1997). However, the effects of adrenergic subtypes of receptors on LTP and LTD differ significantly. Activation of β-adrenergic receptors uniformly enhances LTP induced by high-frequency stimulation, and by STDP protocols, and moreover, demonstrates an important role in the conversion of early to late LTP (Gelinas & Nguyen, 2005; Tenorio et al. 2010; Wojtowicz et al. 2010). In accordance, blockade of these receptors prevents the induction of LTP (Kemp & Manahan-Vaughan, 2008; Flores et al. 2010). In contrast, the activation of adrenergic α1 and α2 receptors reduces LTP (Mondaca et al. 2004; Takamatsu et al. 2008; Wang et al. 2008), and therefore counteracts the effects of β-adrenergic receptors on plasticity. With regard to LTD, the effects of β-adrenergic receptor activation are conflicting: Kemp & Manahan-Vaughan (2008), as well as Lemon and colleagues (Lemon et al. 2009) described an enhancement of LTD, whereas a diminution or prevention was found in other studies (Katsuki et al. 1997; Lin et al. 2008). For α-adrenergic receptors, α2 receptor activation reduces LTD (DeBock et al. 2003), while activation of α1 receptors enhances it (Marzo et al. 2010).
Taken together, the overall effect of adrenergic activation seems to be an enhancement of LTP, and maybe also LTD. With regard to adrenergic subtypes of receptors, at least partially antagonistic effects have been described. In contrast to the other neuromodulators, however, a non-linear dosage-dependency of the effects has not been described so far.
Experiments in humans
Pharmacological testing of NIBS-induced plasticity in humans is important because it cannot be taken for granted that effects observed in in vitro animal studies do translate one-to-one to the human brain in vivo. The human studies reviewed below were performed in healthy young adults and, typically, the effects of a single drug dose on NIBS-induced plasticity were explored in a randomized placebo-controlled design.
Drivers of plasticity
The glutamatergic system. Memantine, an NMDA receptor antagonist, abolishes the LTP-like plasticity induced by iTBS and the LTD-like plasticity induced by cTBS (Huang et al. 2007). This provides evidence that TBS-induced plasticity depends on activation of NMDA receptors. Similar blocking effects on PAS- and tDCS-induced LTP-like and LTD-like plasticity were obtained under the NMDA receptor antagonist dextromethorphan (Liebetanz et al. 2002; Stefan et al. 2002; Nitsche et al. 2003a; Wolters et al. 2003). In contrast, the partial NMDA receptor agonist d-cycloserine enhances LTP-like plasticity induced by anodal tDCS (Nitsche et al. 2004b).
The GABAergic system. The GABAergic drugs diazepam and tiagabine reduce PAS-induced LTP-like plasticity (Heidegger et al. 2010). Similarly, the GABAB receptor agonist baclofen leads to suppression of PAS-induced LTP-like plasticity (McDonnell et al. 2007). In contrast, lorazepam, a positive allosteric modulator at the GABAA receptor, has no effect on tDCS-induced LTD-like plasticity, while it reduces LTP-like plasticity in the early phase after anodal tDCS, but enhances and prolongs LTP-like plasticity in the late phase (Nitsche et al. 2004c). Magnetic resonance (MR) spectroscopy experiments support a role for GABA in tDCS-induced plasticity, since induction of LTP-like and LTD-like plasticity is associated with a reduction of GABA concentration in the stimulated cortex (Stagg et al. 2009a). Similarly, cTBS reduces GABA concentration in the stimulated cortex (Stagg et al. 2009b). Although it is not clear at the moment whether in vivo concentrations of GABA detected by MR spectroscopy represent synaptic or extrasynaptic GABA levels (Stagg et al. 2011), the above-mentioned studies provide evidence for a regulating role of GABA in NIBS-induced LTP-/LTD-like plasticity in the human cortex.
Voltage-gated ion channels. Since voltage-gated ion channel activity determines neuronal membrane excitability, and calcium influx is prominently involved in plasticity induction in basic experiments, an impact of voltage-gated ion channels on NIBS-induced plasticity is plausible. In accordance with this, the voltage-gated sodium channel blocker lamotrigine reduces PAS-induced LTP-like plasticity (Heidegger et al. 2010), and carbamazepine abolishes anodal tDCS-induced LTP-like plasticity, but not LTD-like plasticity induced by cathodal tDCS (Nitsche et al. 2003a). The most likely explanation for the nil effect on LTD-like plasticity is the fact that cathodal tDCS hyperpolarizes neuronal membranes, which precludes an additional impact of voltage-dependent sodium channel blockers on membrane polarization. Furthermore, L-type calcium channel blockade by nimodipine abolishes TBS-induced LTP-like plasticity (Wankerl et al. 2010) and eliminates PAS-induced LTD-like plasticity (Wolters et al. 2003). Finally, the T-type calcium channel antagonist flunarizine abolishes anodal tDCS-induced LTP-like plasticity but, for the same reasons as indicated above, does not influence cathodal tDCS-induced LTD-like plasticity (Nitsche et al. 2003a).
Taken together, blockade of voltage-gated sodium and calcium channels reduces or abolishes LTP-like plasticity in all stimulation protocols explored, while the effects on LTD-like plasticity seem to depend on the specific plasticity induction protocol.
Modulators of plasticity
The dopaminergic system. Among the neuromodulating neurotransmitter systems, the impact of dopamine on NIBS-induced plasticity has been explored most extensively. The consistent abolition of PAS-, tDCS- and rTMS-induced plasticity by D2 receptor blockade by sulpiride or haloperidol demonstrates that dopaminergic activity is a necessary precondition to inducing plasticity in human motor cortex (Nitsche et al. 2006, 2009a; Monte-Silva et al. 2011).
Global dopaminergic activation (i.e. activation of D1 and D2 receptors) has heterogeneous effects on plasticity, depending on the plasticity induction protocol, and drug dosage. Low-dose and high-dose l-dopa abolishes tDCS- and PAS-induced LTP-/LTD-like plasticity, with the exception of LTP-like plasticity induced by PAS, which converts to LTD-like plasticity. In contrast, medium-dose l-dopa prolongs LTP-like plasticity induced by PAS, but converts it into LTD-like plasticity in the case of tDCS (Monte-Silva et al. 2010). The reasons for these conspicuous differences of drug effects on plasticity induced by different NIBS protocols (see also below) are not fully understood but are certainly linked to differences in the underlying physiology (e.g. STDP-like effects after PAS versus membrane polarization effects after tDCS). The effect of low-dose dopaminergic activation seems to be driven by D2 receptors, because low doses of the D2/D3 receptor agonist ropinirol induce largely identical effects to l-dopa on NIBS-induced plasticity. However, medium doses do not affect plasticity, independent of the NIBS protocol (Monte-Silva et al. 2009).
Taken together, a certain amount of activity of the dopaminergic system is necessary for the induction of plasticity. Further enhancement of dopaminergic activity results in non-linear effects on plasticity, which depend on dosage, the plasticity induction protocol, and the balance of D1 versus D2 receptor activation.
The cholinergic system. Global cholinergic activation (i.e. activation of both mAChR and nAChR) by the cholinesterase inhibitor rivastigmine enhances and prolongs LTP- and LTD-like plasticity induced by PAS, whereas it abolishes LTP-like plasticity induced by anodal tDCS (Kuo et al. 2007). Tacrine, another cholinesterase inhibitor, has no effect on PAS-induced plasticity (Korchounov & Ziemann, 2011). The reason for these conflicting results might be a dose-dependent effect of cholinergic activation on plasticity, which has not yet been explored. With regard to the contribution of mAChR vs. nAChR, nicotine abolishes tDCS-induced, but prolongs PAS-induced LTP-like plasticity, while it abolishes LTD-like plasticity in both stimulation protocols (Thirugnanasambandam et al. 2011). Finally, the mAChR antagonist biperiden reduces LTP-like plasticity induced by PAS (Korchounov & Ziemann, 2011).
In summary, cholinergic activity seems to exert important modulating effects on NIBS-induced plasticity, but the knowledge about subtype-of-receptor and dose-dependent effects is limited.
The serotonergic system. Only one study is available with regard to the impact of serotonin on NIBS-induced plasticity in humans: a single dose of the serotonin reuptake inhibitor citalopram enhances and prolongs LTP-like plasticity induced by anodal tDCS, while it converts LTD-like plasticity induced by cathodal tDCS into LTP-like plasticity (Nitsche et al. 2009b).
The adrenergic system. The monoamine reuptake inhibitor amphetamine enhances the duration of the LTP-like after-effects induced by anodal tDCS (Nitsche et al. 2004a). In contrast, methylphenidate has no effect on PAS-induced LTP-like plasticity (Korchounov & Ziemann, 2011). The LTP-/LTD-like after-effects of anodal and cathodal tDCS are reduced by β-adrenergic receptor blockade accomplished by propanolol (Nitsche et al. 2004a), and LTP-like plasticity induced by PAS is abolished by the α1 receptor antagonist prazosine (Korchounov & Ziemann, 2011). From these results it is clear that the adrenergic system has significant impact on NIBS-induced plasticity in humans, although the specific effects might somewhat differ between stimulation protocols.
Similarities and differences
For the drivers of plasticity, the results of animal and human studies are largely comparable: plasticity at both levels of experimentation depends on activation of the glutamatergic system and calcium influx. Accordingly, anti-glutamatergic drugs and calcium channel blockers have diminishing effects on plasticity in animal and human experiments.
With regard to neuromodulators, the dopaminergic system has been most extensively explored. Dopamine affects glutamatergic plasticity in a complex non-linear fashion. Here the results of experiments in humans and animals are in rough accordance, although not all determinants of the modulating impact of dopamine on plasticity have been identified, and a direct comparison might be difficult due to differences of spontaneous cortical activity, connectivity, transmitter concentration, and other factors. For the cholinergic system, most animal experiments show a plasticity-promoting effect, whereas experiments in humans show that the effects of the cholinergic system on plasticity might depend on the specific stimulation protocol. Furthermore, animal experiments are in favour of a non-linear effect of cholinergic activation on plasticity, which has so far not been studied in humans. The effects of serotonin on plasticity, as so far explored only in animal experiments, seem to be heterogeneous, and depend on subtype of receptor activation and dosage. Noradrenergic and adrenergic activation were shown to have LTP-enhancing effects in both animal and human experiments. A synopsis of the available pharmacological studies on NIBS-induced plasticity in human cortex is provided in Table 2.
Table 2.
Impact of CNS-active drugs on NIBS-induced plasticity in human cortex
| Study | Substance | Pharmacodynamic effect | Dosage (mg) | NIBS protocol | LTP-like plasticity | LTD-like plasticity |
|---|---|---|---|---|---|---|
| Glutamate | ||||||
| Huang et al. (2007) | Memantine | NMDA receptor antagonist | 5 + 5 + 10 | TBS | ⇓ | ⇓ |
| Stefan et al. (2002); Wolters et al. (2003) | Dextromethorphan | NMDA receptor antagonist | 150 | PAS | ⇓ | ⇓ |
| Liebetanz et al. (2002); Nitsche et al. (2003a) | Dextromethorphan | NMDA receptor antagonist | 150 | tDCS | ⇓ | ⇓ |
| Nitsche et al. (2004b) | d-Cycloserine | NMDA receptor agonist | 100 | tDCS | ⇑ | • |
| Teo et al. (2007) | d-Cycloserine | NMDA receptor agonist | 100 | TBS | ⇓, conversion | n.t. |
| to LTD | ||||||
| GABA | ||||||
| Heidegger et al. (2010) | Diazepam Tiagabine | GABAAR: positive allosteric modulator | 20 | PAS | ⇓ | n.t. |
| GABA reuptake inhibitor | 15 | |||||
| Nitsche et al. (2004c) | Lorazepam | GABAAR: positive allosteric modulator | 2 | tDCS | ⇑, initial delay | • |
| McDonnell et al. (2007) | Baclofen | GABABR agonist | 50 | PAS | ⇓ | n.t. |
| Voltage-gated ion channels | ||||||
| Heidegger et al. (2010) | Lamotrigine | Voltage-gated sodium channel blocker | 300 | PAS | ⇓ | n.t. |
| Liebetanz et al. (2002); Nitsche et al. (2003a) | Carbamazepine | Voltage-gated sodium channel blocker | 300 + 300 | tDCS | ⇓ | • |
| Wankerl et al. (2010) | Nimodipine | Voltage-gated calcium channel blocker | 30 | TBS | ⇓ | n.t. |
| Wolters et al. (2003) | Nimodipine | Voltage-gated calcium channel blocker | 30 | PAS | n.t. | ⇓ |
| Nitsche et al. (2003a) | Flunarizine | Voltage-gated calcium channel blocker | 10 | tDCS | ⇓ | • |
| Dopamine | ||||||
| Monte-Silva et al. (2011) | Sulpiride | D2 receptor antagonist | 400 | TBS | ⇓ | ⇓ |
| Nitsche et al. (2009a) | Sulpiride | D2 receptor antagonist | 400 | PAS | • | ⇓ |
| Nitsche et al. (2006) | Sulpiride | D2 receptor antagonist | 400 | tDCS | ⇓ | ⇓ |
| Korchounov & Ziemann (2011) | Haloperidol | D2 receptor antagonist | 2.5 | PAS | ⇓ | n.t. |
| Thirugnanasambandam et al. (2011) | l-Dopa | Dopamine precursor | 25 | PAS | ⇓ | ⇓ |
| Thirugnanasambandam et al. (2011) | l-Dopa | Dopamine precursor | 100 | PAS | ⇑ | • |
| Thirugnanasambandam et al. (2011) | l-Dopa | Dopamine precursor | 200 | PAS | ⇓ conversion | • |
| to LTD | ||||||
| Monte-Silva et al. (2010) | l-Dopa | Dopamine precursor | 25 | tDCS | ⇓ | ⇓ |
| Kuo et al. (2008), Monte-Silva et al. (2010) | l-Dopa | Dopamine precursor | 100 | tDCS | ⇓ conversion | ⇑ |
| to LTD | ||||||
| Monte-Silva et al. (2010) | l-Dopa | Dopamine precursor | 200 | tDCS | ⇓ | ⇓ |
| Monte-Silva et al. (2009) | Ropinirole | D2/3 receptor agonist | 0.125 | PAS | ⇓ | • |
| Monte-Silva et al. (2009) | Ropinirole | D2/3 receptor agonist | 0.5 | PAS | • | • |
| Monte-Silva et al. (2009) | Ropinirole | D2/3 receptor agonist | 1 | PAS | ⇓ | • |
| Monte-Silva et al. (2009) | Ropinirole | D2/3 receptor agonist | 0.125 | tDCS | ⇓ | ⇓ |
| Monte-Silva et al. (2009) | Ropinirole | D2/3 receptor agonist | 0.5 | tDCS | • | • |
| Monte-Silva et al. (2009) | Ropinirole | D2/3 receptor agonist | 1 | tDCS | ⇓ | ⇓ |
| Nitsche et al. (2009a) | l-Dopa + sulpiride | Activation of D1 receptor under D2 receptor blockade | 100 + 400 | tDCS | • | • |
| Acetylcholine | ||||||
| Kuo et al. (2007) | Rivastigmine | Cholinesterase inhibitor | 3 | PAS | ⇑ | ⇑ |
| Kuo et al. (2007) | Rivastigmine | Cholinesterase inhibitor | 3 | tDCS | ⇓ | ⇓ |
| Korchounov & Ziemann (2011) | Tacrine | Cholinesterase inhibitor | 40 | PAS | • | n.t. |
| Korchounov & Ziemann (2011) | Biperiden | Muscarinic receptor antagonist | 8 | PAS | • | n.t. |
| Thirugnanasambandam et al. (2011) | Nicotine | Nicotinic receptor agonist | 15, patch | tDCS | ⇑ | ⇓ |
| Thirugnanasambandam et al. (2011) | Nicotine | Nicotinic receptor agonist | 15, patch | tDCS | ⇓ | ⇓ |
| Serotonin | ||||||
| Nitsche et al. (2009b) | Citalopram | Serotonin reuptake inhibitor | 20 | tDCS | ⇑ | ⇓ conversion |
| Adrenaline/noradrenaline | ||||||
| Nitsche et al. (2004a) | Amfetaminil | Precursor of amphetamine, monoaminergic reuptake inhibitor | 20 | tDCS | ⇑ | • |
| Korchounov & Ziemann (2011) | Methylphenidate | Monoaminergic reuptake inhibitor | 40 | PAS | • | n.t. |
| Nitsche et al. (2004a) | Propanolol | β-Adrenergic receptor antagonist | 80 | tDCS | ⇓ | ⇓ |
| Korchounov & Ziemann (2011) | Prazosine | α-Adrenergic antagonist | 1 | PAS | ⇓ | n.t. |
Summary of the impact of pharmacological interventions affecting the glutamatergic, GABAergic, dopaminergic, cholinergic, serotonergic and adrenergic systems, and ion channel activity, on non-invasive brain stimulation-induced plasticity in healthy subjects. n.t., not tested; tDCS, transcranial direct current stimulation; PAS, paired associative stimulation; TBS, theta burst stimulation; LTP, long-term potentiation; LTD, long-term depression; GABAAR, γ-aminobutyric acid type A receptor; GABABR, γ-aminobutyric acid type B receptor; •, no plasticity; ↓, decrease of plasticity; ↑, increase of plasticity.
Pathological alterations of plasticity in neuropsychiatric diseases, as explored by NIBS techniques, and their modulation by pharmacological interventions
Abnormal neuroplasticity has come increasingly into the focus as a correlate and pathophysiological mechanism in many neuropsychiatric diseases during the last years. Beyond stroke and Alzheimer's disease, where the causal impact of pathological alterations of plasticity for the development and progress of clinical symptoms as well as the therapeutic relevance of plasticity-modifying therapies is relatively well explored (for recent reviews see Floel & Cohen, 2010; Boggio et al. 2011; Dimyan & Cohen, 2011; Freitas et al. 2011), it was demonstrated that neuroplasticity is also altered in dystonia, migraine, depression, schizophrenia and other neuropsychiatric diseases. In the following, we will exemplify currently available knowledge about the involvement of pathologically altered neuroplasticity in Parkinson's disease and schizophrenia, including the effect of pharmacological interventions on plasticity and clinical symptoms. Figure 1 gives an overview of the application of non-invasive brain stimulation (NIBS) for the prediction of drug efficacy in neuropsychiatric disorders.
Figure 1. Application of non-invasive brain stimulation (NIBS) for the prediction of drug efficacy in neuropsychiatric disorders.
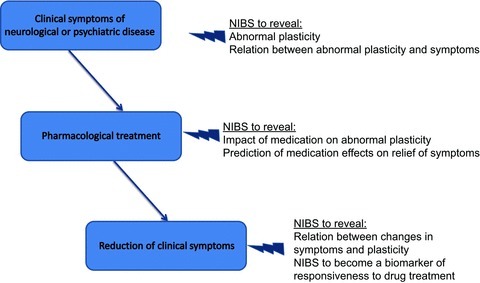
This schematic diagram shows how NIBS can be used for the identification of effective drug therapies in neuropsychiatric disorders. In the first step, NIBS can be applied to identify abnormal neuroplasticity, and to explore its association with clinical symptoms. NIBS can then serve to explore the impact of medication on abnormal plasticity, and to predict medication effects on the relief of symptoms. In the case of a causal relationship between medication-induced clinical response and plasticity, NIBS can serve as biomarker of responsiveness to drug treatment.
Parkinson's disease
M1 is innervated by dopaminergic fibres originating in the ventral tegmental area and contributing to M1 plasticity. On a behavioural level these fibres were demonstrated to be necessary for successful motor skill learning in the rat (Hosp et al. 2011). The same group showed in the rat M1 that antagonists of D2 but not D1 receptors reduce the size of the M1 forelimb representation, and increase movement thresholds and motor response latencies as determined by direct electrical stimulation of M1 (Hosp et al. 2009). Thus, abnormal motor cortical plasticity in patients with Parkinson's disease (PD) may be caused by intrinsic M1 pathology, and/or altered signalling from the basal ganglia. The latter has to be kept in mind in the context of NIBS, since rTMS of M1 can induce dopamine release in the striatum (Strafella et al. 2003). M1 plasticity is abnormal in PD patients as measured by different rTMS protocols. Low-frequency (1 Hz) rTMS applied to premotor cortex normalizes abnormally increased baseline intracortical excitability in M1 in PD patients, while the same rTMS protocol increases M1 excitability in healthy controls (Buhmann et al. 2004). A single dose of l-dopa reverses and thus normalizes the direction of excitability changes along the premotor–M1 connection in PD (Buhmann et al. 2004). Another premotor cortex-to-M1 connectivity study in PD tested short-term plasticity induced by 5 Hz rTMS of M1 before and after conditioning dorsal premotor cortex stimulation with 5 Hz rTMS (Suppa et al. 2010). Short-term facilitation of MEPs occurred only when the PD patients were on dopaminergic therapy (Suppa et al. 2010).
Further examples of abnormal plasticity in PD are reduced LTP-/LTD-like plasticity induced by PAS (Ueki et al. 2006), or TBS (Eggers et al. 2010; Stephani et al. 2011; Suppa et al. 2011; Kishore et al. 2012). Another study, however, did not find a difference in TBS-induced plasticity between PD patients and healthy controls (Zamir et al. 2012). For PAS, however, other data by Bagnato and co-workers (Bagnato et al. 2006) showed enhanced PAS-induced plasticity when the PD patients were off medication, which normalized in the On-state. The reasons for the disparity between those studies are at present unclear. PD patients with either On–Off fluctuations or dyskinesias provide an opportunity to compare plasticity in states with either insufficient or too much dopamine. Since dopamine replacement in PD patients in the Off-state re-establishes the LTP-like plasticity induced by PAS it was claimed that plasticity impairment in PD is caused by dopamine depletion (Ueki et al. 2006). The finding that the LTP-like plasticity induced by PAS was re-established in the non-dyskinetic group but not in the dyskinetic group (Morgante et al. 2006) might be a hint for a non-linear (i.e. inverse U-shaped) effect of dopamine on plasticity in PD patients, similar to such non-linear dose-dependent effects demonstrated in healthy controls (see above, Pharmacological modulation of plasticity/Experiments in humans/Modulators of plasticity/The dopaminergic system). These data demonstrate a clear association between clinical symptoms and NIBS-induced plasticity.
In another disease possibly associated with a dopaminergic deficit, idiopathic restless legs syndrome, PAS does not result in LTP-like plasticity without treatment. PAS-induced plasticity is, however, restored after 4 weeks of dopaminergic treatment (Rizzo et al. 2009a).
Further support for the necessity of a sufficient dopamine concentration for maintaining the capacity for plasticity induction comes from a rather complex newly developed protocol, which explored l-dopa dose effects in PD patients. The authors tested depotentiation (induced by a short TBS protocol) of LTP-like plasticity previously induced by a longer TBS protocol and followed by a 1 min contraction of the target muscle. Patients without l-dopa-induced dyskinesias had normal LTP- and depotentiation-like effects when they took their full dose of l-dopa; however, halving the dose led to the disappearance of the LTP-like plasticity (Huang et al. 2011). With this half dose, however, patients with l-dopa-induced dyskinesias could be successfully potentiated, but they were unresponsive to the depotentiation protocol. These findings suggest that depotentiation is abnormal in M1 of PD patients with levodopa-induced dyskinesias and that LTP-like plasticity is more readily affected by administration of l-dopa than the clinical symptoms in these patients.
In summary, overwhelming evidence in clinical studies of PD patients exists that supports the necessity for the presence of a sufficient level of dopamine for inducing plasticity. Furthermore, there is substantial evidence that the effects of dopamine enhancement on plasticity and clinical symptoms are tightly linked. This underscores the potential of NIBS-induced plasticity to serve as a biomarker for clinical restoration in PD.
Schizophrenia
Beyond positive symptoms like hallucinations and delusions, and negative symptoms (reduction of interests, emotions, missing ability to feel joy, amongst others), cognitive dysfunction in schizophrenia (ScZ) has gained increased attention during the last years, which might be caused by abnormal neuroplasticity. It has been demonstrated that ScZ patients display reduced cortical connectivity (Balu & Coyle, 2011), and also enhanced, but aberrant connectivity (Barbalat et al. 2009; Cole et al. 2011). Hereby different parameters of functional connectivity seem to be altered differently in ScZ: the strength of functional connectivity is reduced, whereas the diversity of functional connections is increased (Lynall et al. 2010). Moreover, dysfunctional NMDA receptors, as well as dopaminergic alterations are critically involved in this disease (Paz et al. 2008; Howes & Kapur, 2009; Balu & Coyle, 2011).
In principal generally, LTP-like plasticity was reduced or abolished in ScZ patients, when induced by high-frequency rTMS, PAS, or anodal tDCS (Oxley et al. 2004; Frantseva et al. 2008; Hasan et al. 2011b). Similarly, LTD-like plasticity induced by either low-frequency rTMS or cathodal tDCS was absent (Fitzgerald et al. 2004; Hasan et al. 2011a). While those studies explored M1 plasticity, Barr and colleagues (Barr et al. 2011) investigated the effects of plasticity induction by high-frequency rTMS of the dorsolateral prefrontal cortex on gamma oscillations. The findings demonstrate enhanced oscillatory activity during the performance of a working memory task before rTMS in ScZ, but a reduction after rTMS, while gamma oscillations were enhanced by rTMS in healthy controls. With the exception of the study conducted by Fitzgerald and colleagues (Fitzgerald et al. 2004), patients in all other studies were explored under anti-psychotic (i.e. anti-dopaminergic) treatment. Since dopamine has a prominent impact on plasticity in humans (see above), this makes it difficult to ascertain whether the alterations of plasticity in ScZ are disease- or medication-related. The finding, however, that medicated and non-medicated patients showed similar plasticity deficits (Fitzgerald et al. 2004) favours a disease-related alteration. Although most of the NIBS-induced plasticity studies conducted so far show reduced plasticity in ScZ patients, the diverse impact of the disease on various aspects of functional connectivity (see above) might hint at the possibility that there is also a less uniform effect of ScZ on plasticity, which might be uncovered by future NIBS experiments.
Unfortunately, no studies are available at present that probe the effects of anti-dopaminergic medication on disturbed plasticity in ScZ, and any possible association with clinical symptoms, including cognitive performance. In one study, at least, an association between the level of impairment of LTP-like plasticity induced by PAS and motor skill learning was described in ScZ patients (Frantseva et al. 2008). Although these patients were tested under anti-dopaminergic medication, this result supports the notion that NIBS-induced LTP-like plasticity is a candidate biomarker for cognitive performance in schizophrenia.
Outlook
Knowledge about the physiological basis of neuroplasticity, and its functional consequences has considerably enhanced during the last decades, not only based on animal experimentation, but also in humans. Due to the recent development of NIBS techniques, it is now possible to induce alterations in cortical excitability in conscious human subjects, which resemble synaptic plasticity as studied at the cellular level. Interestingly, recent studies provide evidence for a causal relationship between pathological alterations in NIBS-induced plasticity and clinical symptoms in a number of neuropsychiatric diseases. Furthermore, many CNS-active drugs affecting synaptic plasticity also show modifying effects on the magnitude and direction of NIBS-induced neuroplasticity. Therefore, it appears a promising approach to explore the suitability of neuroplasticity induced by NIBS as a biomarker for the clinical efficacy of newly developed drugs to treat neuropsychiatric diseases, as well as for individual adjustment of drug type and dosage. This approach might thus help to close the gap between pre-clinical studies and pharmacotherapy in patients by applying CNS-active drugs that modulate synaptic plasticity. However, the number of studies exploring causality between alterations in NIBS-induced neuroplasticity and clinical symptoms in neuropsychiatric diseases is currently limited. This deficit applies to an even larger extent to experiments studying the causal relationship between pharmacological modulations of NIBS-induced plasticity and clinical symptoms. There is an urgent need for this type of study before it can be ultimately decided if, indeed, NIBS-induced neuroplasticity is a reliable and valid biomarker for the exploration of the clinical efficacy of CNS-acting drugs. This review provided a comprehensive rationale in support of continuing research along this direction towards identifying the clinical utility of pharmacological modulation of NIBS-induced plasticity in patients with neuropsychiatric diseases.
Glossary
- BDNF
brain-derived neurotrophic factor
- cTBS
continuous theta burst stimulation
- DA
dopamine
- DCS
direct current stimulation
- iTBS
intermittent theta burst stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- M1
primary motor cortex
- MEP
motor evoked potential
- MNS
median nerve stimulation
- NIBS
non-invasive brain stimulation
- PAS
paired associative stimulation
- PD
Parkinson's disease
- rTMS
repetitive transcranial magnetic stimulation
- ScZ
schizophrenia
- SMA
supplementary motor area
- STDP
spike-timing dependent plasticity
- TBS
theta burst stimulation
- tDCS
transcranial direct current stimulation
- TMS
transcranial magnetic stimulation
- VGCC
voltage-gated calcium channel
References
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Ahmed MS, Siegelbaum SA. Recruitment of N-type Ca2+ channels during LTP enhances low release efficacy of hippocampal CA1 perforant path synapses. Neuron. 2009;63:372–385. doi: 10.1016/j.neuron.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert DJ. The effects of polarizing currents on the consolidation of learning. Neuropsychologia. 1966;4:65–77. [Google Scholar]
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche M, Kincses T, Kruse W, Hoffmann K, Paulus W. Facilitation of visuo-motor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans. Eur J Neurosci. 2004a;19:2888–2892. doi: 10.1111/j.1460-9568.2004.03367.x. [DOI] [PubMed] [Google Scholar]
- Antal A, Varga E, Kincses T, Nitsche M, Paulus W. Oscillatory brain activity and transcranial direct current stimulation in humans. Neuroreport. 2004b;15:1307–1310. doi: 10.1097/01.wnr.0000127460.08361.84. [DOI] [PubMed] [Google Scholar]
- Arai N, Müller-Dahlhaus F, Murakami T, Bliem B, Lu MK, Ugawa Y, Ziemann U. State-dependent and timing-dependent bidirectional associative plasticity in the human SMA-M1 network. J Neurosci. 2011;31:15376–15383. doi: 10.1523/JNEUROSCI.2271-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Auerbach JM, Segal M. Muscarinic receptors mediating depression and long-term potentiation in rat hippocampus. J Physiol. 1996;492:479–493. doi: 10.1113/jphysiol.1996.sp021323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signalling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato S, Agostino R, Modugno N, Quartarone A, Berardelli A. Plasticity of the motor cortex in Parkinson's disease patients on and off therapy. Mov Disord. 2006;21:639–645. doi: 10.1002/mds.20778. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory. Nat Rev Neurosci. 2000;1:11–20. doi: 10.1038/35036191. [DOI] [PubMed] [Google Scholar]
- Balu DT, Coyle JT. Neuroplasticity signalling pathways linked to the pathophysiology of schizophrenia. Neurosci Biobehav Rev. 2011;35:848–870. doi: 10.1016/j.neubiorev.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbalat G, Chambon V, Franck N, Koechlin E, Farrer C. Organization of cognitive control within the lateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry. 2009;66:377–386. doi: 10.1001/archgenpsychiatry.2009.10. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Arenovich T, Chen R, Fitzgerald PB, Daskalakis ZJ. The effect of repetitive transcranial magnetic stimulation on gamma oscillatory activity in schizophrenia. PLoS ONE. 2011;6:e22627. doi: 10.1371/journal.pone.0022627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signalling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Beck H, Goussakov IV, Lie A, Helmstaedter C, Elger CE. Synaptic plasticity in the human dentate gyrus. J Neurosci. 2000;20:7080–7086. doi: 10.1523/JNEUROSCI.20-18-07080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagya V, Srikumar BN, Raju TR, Rao BS. Chronic escitalopram treatment restores spatial learning, monoamine levels, and hippocampal long-term potentiation in an animal model of depression. Psychopharmacology (Berl) 2011;214:477–494. doi: 10.1007/s00213-010-2054-x. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OCJ, Redfearn JWT. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Gil O, Landau EM. Cholinergic stimulation enhances long-term potentiation in the CA1 region of rat hippocampus. Neurosci Lett. 1990;119:207–210. doi: 10.1016/0304-3940(90)90835-w. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Valasek CA, Campanha C, Giglio AC, Baptista NI, Lapenta OM, Fregni F. Non-invasive brain stimulation to assess and modulate neuroplasticity in Alzheimer's disease. Neuropsychol Rehabil. 2011;21:703–716. doi: 10.1080/09602011.2011.617943. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Srebro B. Induction of long-term depression and potentiation by low- and high-frequency stimulation in the dentate area of the anaesthetized rat: magnitude, time course and EEG. Brain Res. 1987;405:100–107. doi: 10.1016/0006-8993(87)90994-2. [DOI] [PubMed] [Google Scholar]
- Brocher S, Artola A, Singer W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 1992;573:27–36. doi: 10.1016/0006-8993(92)90110-u. [DOI] [PubMed] [Google Scholar]
- Buch ER, Johnen VM, Nelissen N, O'Shea J, Rushworth MF. Noninvasive associative plasticity induction in a corticocortical pathway of the human brain. J Neurosci. 2011;31:17669–17679. doi: 10.1523/JNEUROSCI.1513-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhmann C, Gorsler A, Baumer T, Hidding U, Demiralay C, Hinkelmann K, Weiller C, Siebner HR, Munchau A. Abnormal excitability of premotor-motor connections in de novo Parkinson's disease. Brain. 2004;127:2732–2746. doi: 10.1093/brain/awh321. [DOI] [PubMed] [Google Scholar]
- Burgard EC, Sarvey JM. Muscarinic receptor activation facilitates the induction of long-term potentiation (LTP) in the rat dentate gyrus. Neurosci Lett. 1990;116:34–39. doi: 10.1016/0304-3940(90)90382-j. [DOI] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ito K, Fujii S, Miura M, Furuse H, Sasaki H, Kaneko K, Kato H, Miyakawa H. Roles of dopamine receptors in long-term depression: enhancement via D1 receptors and inhibition via D2 receptors. Receptors Channels. 1996;4:1–8. [PubMed] [Google Scholar]
- Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke S, Bliss T. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- DeBock F, Kurz J, Azad SC, Parsons CG, Hapfelmeier G, Zieglgansberger W, Rammes G. α2-Adrenoreceptor activation inhibits LTP and LTD in the basolateral amygdala: involvement of Gi/o-protein-mediated modulation of Ca2+-channels and inwardly rectifying K+-channels in LTD. Eur J Neurosci. 2003;17:1411–1424. doi: 10.1046/j.1460-9568.2003.02544.x. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T, Lynch G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J Physiol. 1978;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edagawa Y, Saito H, Abe K. Serotonin inhibits the induction of long-term potentiation in rat primary visual cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:983–997. doi: 10.1016/s0278-5846(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Edelmann E, Lessmann V. Dopamine modulates spike timing-dependent plasticity and action potential properties in CA1 pyramidal neurons of acute rat hippocampal slices. Front Synaptic Neurosci. 2011;3:6. doi: 10.3389/fnsyn.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers C, Fink GR, Nowak DA. Theta burst stimulation over the primary motor cortex does not induce cortical plasticity in Parkinson's disease. J Neurol. 2010;257:1669–1674. doi: 10.1007/s00415-010-5597-1. [DOI] [PubMed] [Google Scholar]
- Faria P, Hallett M, Miranda PC. A finite element analysis of the effect of electrode area and inter-electrode distance on the spatial distribution of the current density in tDCS. J Neural Eng. 2011;8:066017. doi: 10.1088/1741-2560/8/6/066017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Marston NA, Oxley T, De Castella A, Daskalakis ZJ, Kulkarni J. Reduced plastic brain responses in schizophrenia: a transcranial magnetic stimulation study. Schizophr Res. 2004;71:17–26. doi: 10.1016/j.schres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Floel A, Cohen LG. Recovery of function in humans: cortical stimulation and pharmacological treatments after stroke. Neurobiol Dis. 2010;37:243–251. doi: 10.1016/j.nbd.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores O, Nunez H, Perez H, Morgan C, Soto-Moyano R, Valladares L, Burgos H, Olivares R, Hernandez A. β-Adrenoceptor blockade depresses molecular and functional plasticities in the rat neocortex. Brain Res Bull. 2010;82:284–288. doi: 10.1016/j.brainresbull.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Fitzgerald PB, Chen R, Moller B, Daigle M, Daskalakis ZJ. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill learning. Cereb Cortex. 2008;18:990–996. doi: 10.1093/cercor/bhm151. [DOI] [PubMed] [Google Scholar]
- Freitas C, Mondragon-Llorca H, Pascual-Leone A. Noninvasive brain stimulation in Alzheimer's disease: systematic review and perspectives for the future. Exp Gerontol. 2011;46:611–627. doi: 10.1016/j.exger.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Hartmann S, Matthies H. Domperidone, an inhibitor of the D2-receptor, blocks a late phase of an electrically induced long-term potentiation in the CA1-region in rats. Biomed Biochim Acta. 1989;48:473–476. [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Debanne D, Bi GQ. Temporal modulation of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010;2:19. doi: 10.3389/fnsyn.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Sumikawa K. Nicotine accelerates reversal of long-term potentiation and enhances long-term depression in the rat hippocampal CA1 region. Brain Res. 2001;894:340–346. doi: 10.1016/s0006-8993(01)02058-3. [DOI] [PubMed] [Google Scholar]
- Gaiarsa JL, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci. 2002;25:564–570. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- Gartside IB. Mechanisms of sustained increases of firing rate of neurones in the rat cerebral cortex after polarization: role of protein synthesis. Nature. 1968;220:383–384. doi: 10.1038/220383a0. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. β-Adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci. 2005;25:3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay T. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hasan A, Nitsche MA, Herrmann M, Schneider-Axmann T, Marshall L, Gruber O, Falkai P, Wobrock T. Impaired long-term depression in schizophrenia: a cathodal tDCS pilot study. Brain Stimul. 2011a doi: 10.1016/j.brs.2011.08.004. DOI: 10.1016/j.brs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Hasan A, Nitsche MA, Rein B, Schneider-Axmann T, Guse B, Gruber O, Falkai P, Wobrock T. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behav Brain Res. 2011b;224:15–22. doi: 10.1016/j.bbr.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Hasselmo M, Barkai E. Cholinergic modulation of activity-dependent synaptic plasticity in the piriform cortex and associative memory function in a network biophysical simulation. J Neurosci. 1995;15:6592–6604. doi: 10.1523/JNEUROSCI.15-10-06592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. New York: Wiley & Sons; 1949. The organization of behavior. [Google Scholar]
- Heidegger T, Krakow K, Ziemann U. Effects of antiepileptic drugs on associative LTP-like plasticity in human motor cortex. Eur J Neurosci. 2010;32:1215–1222. doi: 10.1111/j.1460-9568.2010.07375.x. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of d-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Molina-Luna K, Hertler B, Atiemo CO, Luft AR. Dopaminergic modulation of motor maps in rat motor cortex: an in vivo study. Neuroscience. 2009;159:692–700. doi: 10.1016/j.neuroscience.2008.12.056. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci. 2011;31:2481–2487. doi: 10.1523/JNEUROSCI.5411-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Huang S, Trevino M, He K, Ardiles A, Pasquale R, Guo Y, Palacios A, Huganir R, Kirkwood A. Pull–push neuromodulation of LTP and LTD enables bidirectional experience-induced synaptic scaling in visual cortex. Neuron. 2012;73:497–510. doi: 10.1016/j.neuron.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. 5-Hydroxytryptamine induces a protein kinase A/mitogen-activated protein kinase-mediated and macromolecular synthesis-dependent late phase of long-term potentiation in the amygdala. J Neurosci. 2007;27:3111–3119. doi: 10.1523/JNEUROSCI.3908-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Pittenger C, Kandel ER. A form of long-lasting, learning-related synaptic plasticity in the hippocampus induced by heterosynaptic low-frequency pairing. Proc Natl Acad Sci U S A. 2004;101:859–864. doi: 10.1073/pnas.2237201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Lu CS, Chuang WL, Chen RS. Abnormal bidirectional plasticity-like effects in Parkinson's disease. Brain. 2011;134:2312–2320. doi: 10.1093/brain/awr158. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Conte A, Frasca V, Curra A, Gilio F, Manfredi M, Berardelli A. Antiepileptic drugs and cortical excitability: a study with repetitive transcranial stimulation. Exp Brain Res. 2004;154:488–493. doi: 10.1007/s00221-003-1685-0. [DOI] [PubMed] [Google Scholar]
- Islam N, Aftabuddin M, Moriwaki A, Hattori Y, Hori Y. Increase in the calcium level following anodal polarization in the rat brain. Brain Res. 1995a;684:206–208. doi: 10.1016/0006-8993(95)00434-r. [DOI] [PubMed] [Google Scholar]
- Islam N, Moriwaki A, Hori Y. Co-localization of c-fos protein and protein kinase C gamma in the rat brain following anodal polarization. Indian J Physiol Pharmacol. 1995b;39:209–215. [PubMed] [Google Scholar]
- Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. Effects of serotonin on the induction of long-term depression in the rat visual cortex. Korean J Physiol Pharmacol. 2010;14:337–343. doi: 10.4196/kjpp.2010.14.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yamazaki Y, Nakauchi S, Ito K, Sumikawa K. Nicotine facilitates long-term potentiation induction in oriens-lacunosum moleculare cells via Ca2+ entry through non-α7 nicotinic acetylcholine receptors. Eur J Neurosci. 2010;31:463–476. doi: 10.1111/j.1460-9568.2009.07058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Magee JC, Colbert CM, Cristie BR. Active properties of neuronal dendrites. Annu Rev Neurosci. 1996;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- Jung P, Ziemann U. Homeostatic and nonhomeostatic modulation of learning in human motor cortex. J Neurosci. 2009;29:5597–5604. doi: 10.1523/JNEUROSCI.0222-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Clark GD, Bazan NG, Zorumski CF. Platelet-activating factor as a potential retrograde messenger in CA1 hippocampal long-term potentiation. Nature. 1994;367:175–179. doi: 10.1038/367175a0. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Izumi Y, Zorumski CF. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol. 1997;77:3013–3020. doi: 10.1152/jn.1997.77.6.3013. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, Brunelin J, Moller HJ, Reiser M, Padberg F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J Neurosci. 2011;31:15284–15293. doi: 10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The 5-hydroxytryptamine4 receptor exhibits frequency-dependent properties in synaptic plasticity and behavioural metaplasticity in the hippocampal CA1 region in vivo. Cereb Cortex. 2005;15:1037–1043. doi: 10.1093/cercor/bhh204. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. β-Adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb Cortex. 2008;18:1326–1334. doi: 10.1093/cercor/bhm164. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Homosynaptic long-term depression in the visual cortex. J Neurosci. 1994;14:3404–3412. doi: 10.1523/JNEUROSCI.14-05-03404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore A, Joseph T, Velayudhan B, Popa T, Meunier S. Early, severe and bilateral loss of LTP and LTD-like plasticity in motor cortex (M1) in de novo Parkinson's disease. Clin Neurophysiol. 2012;123:822–828. doi: 10.1016/j.clinph.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Koganemaru S, Mima T, Nakatsuka M, Ueki Y, Fukuyama H, Domen K. Human motor associative plasticity induced by paired bihemispheric stimulation. J Physiol. 2009;587:4629–4644. doi: 10.1113/jphysiol.2009.174342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 1997;101:299–304. doi: 10.1016/s0165-3806(97)00083-7. [DOI] [PubMed] [Google Scholar]
- Kojima T, Matsumoto M, Togashi H, Tachibana K, Kemmotsu O, Yoshioka M. Fluvoxamine suppresses the long-term potentiation in the hippocampal CA1 field of anaesthetized rats: an effect mediated via 5-HT1A receptors. Brain Res. 2003;959:165–168. doi: 10.1016/s0006-8993(02)03756-3. [DOI] [PubMed] [Google Scholar]
- Korchounov A, Ziemann U. Neuromodulatory neurotransmitters influence LTP-like plasticity in human cortex: a pharmaco-TMS study. Neuropsychopharmacology. 2011;36:1894–1902. doi: 10.1038/npp.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Gold PE. Epinephrine converts long-term potentiation from transient to durable form in awake rats. Hippocampus. 2008;18:81–91. doi: 10.1002/hipo.20372. [DOI] [PubMed] [Google Scholar]
- Kuo M-F, Grosch J, Fregni F, Paulus W, Nitsche MA. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neurosci. 2007;27:14442–14447. doi: 10.1523/JNEUROSCI.4104-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M-F, Paulus W, Nitsche MA. Boosting focally-induced brain plasticity by dopamine. Cereb Cortex. 2008;18:648–651. doi: 10.1093/cercor/bhm098. [DOI] [PubMed] [Google Scholar]
- Lang N, Nitsche M, Paulus W, Rothwell J, Lemon R. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res. 2004;156:439–443. doi: 10.1007/s00221-003-1800-2. [DOI] [PubMed] [Google Scholar]
- Lang N, Nitsche MA, Dileone M, Mazzone P, De Andres-Ares J, Diaz-Jara L, Paulus W, Di Lazzaro V, Oliviero A. Transcranial direct current stimulation effects on I-wave activity in humans. J Neurophysiol. 2011;105:2802–2810. doi: 10.1152/jn.00617.2010. [DOI] [PubMed] [Google Scholar]
- Lemon N, Aydin-Abidin S, Funke K, Manahan-Vaughan D. Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on β-adrenergic receptor activation. Cereb Cortex. 2009;19:2827–2837. doi: 10.1093/cercor/bhp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche M, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Lin YW, Yang HW, Min MY, Chiu TH. Inhibition of associative long-term depression by activation of β-adrenergic receptors in rat hippocampal CA1 synapses. J Biomed Sci. 2008;15:123–131. doi: 10.1007/s11373-007-9205-z. [DOI] [PubMed] [Google Scholar]
- Lisman JE. The pre/post LTP debate. Neuron. 2009;63:281–284. doi: 10.1016/j.neuron.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Litvak V, Zeller D, Oostenveld R, Maris E, Cohen A, Schramm A, Gentner R, Zaaroor M, Pratt H, Classen J. LTP-like changes induced by paired associative stimulation of the primary somatosensory cortex in humans: source analysis and associated changes in behaviour. Eur J Neurosci. 2007;25:2862–2874. doi: 10.1111/j.1460-9568.2007.05531.x. [DOI] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Dunwiddie T, Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977;266:737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res. 2007;180:181–186. doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kaneko S, Satoh M. Bidirectional modulation of long-term potentiation by carbachol via M1 and M2 muscarinic receptors in guinea pig hippocampal mossy fibre-CA3 synapses. Brain Res. 1993;619:324–330. doi: 10.1016/0006-8993(93)91628-6. [DOI] [PubMed] [Google Scholar]
- Maffei A. The many forms and functions of long term plasticity at GABAergic synapses. Neural Plast. 2011;2011:254724. doi: 10.1155/2011/254724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R, Bear M. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Zucker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A. Regulation of depotentiation and long-term potentiation in the dentate gyrus of freely moving rats by dopamine D2-like receptors. Cereb Cortex. 2003;13:123–135. doi: 10.1093/cercor/13.2.123. [DOI] [PubMed] [Google Scholar]
- Markram H, Gerstner W, Sjostrom PJ. A history of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2011;3:4. doi: 10.3389/fnsyn.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo A, Bai J, Caboche J, Vanhoutte P, Otani S. Cellular mechanisms of long-term depression induced by noradrenaline in rat prefrontal neurons. Neuroscience. 2010;169:74–86. doi: 10.1016/j.neuroscience.2010.04.046. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Nitsche M, Tsuji S, Rothwell J. Effect of transcranial DC sensorimotor cortex stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol. 2004;115:456–460. doi: 10.1016/s1388-2457(03)00362-6. [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, El Mansari M, Espana A, Sanchez C, Haddjeri N. Allosteric modulation of the effects of the 5-HT reuptake inhibitor escitalopram on the rat hippocampal synaptic plasticity. Neurosci Lett. 2006;395:23–27. doi: 10.1016/j.neulet.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Mondaca M, Hernandez A, Perez H, Valladares L, Sierralta W, Fernandez V, Soto-Moyano R. α2-adrenoceptor modulation of long-term potentiation elicited in vivo in rat occipital cortex. Brain Res. 2004;1021:292–296. doi: 10.1016/j.brainres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo M-F, Thirugnanasambandam N, Liebetanz D, Paulus W, Nitsche MA. Dose-dependent inverted U-shaped effect of dopamine (D2-like) receptor activation on focal and nonfocal plasticity in humans. J Neurosci. 2009;29:6124–6131. doi: 10.1523/JNEUROSCI.0728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Silva K, Liebetanz D, Grundey J, Paulus W, Nitsche MA. Dosage-dependent non-linear effect of l-dopa on human motor cortex plasticity. J Physiol. 2010;588:3415–3424. doi: 10.1113/jphysiol.2010.190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Silva K, Ruge D, Teo JT, Paulus W, Rothwell JC, Nitsche MA. D2 receptor block abolishes theta burst stimulation-induced neuroplasticity in the human motor cortex. Neuropsychopharmacology. 2011;36:2097–2102. doi: 10.1038/npp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson's disease and levodopa-induced dyskinesias. Brain. 2006;129:1059–1069. doi: 10.1093/brain/awl031. [DOI] [PubMed] [Google Scholar]
- Mori K, Togashi H, Kojima T, Matsumoto M, Ohashi S, Ueno K, Yoshioka M. Different effects of anxiolytic agents, diazepam and 5-HT1A agonist tandospirone, on hippocampal long-term potentiation in vivo. Pharmacol Biochem Behav. 2001;69:367–372. doi: 10.1016/s0091-3057(01)00546-9. [DOI] [PubMed] [Google Scholar]
- Morrell F, Naitoh P. Effect of cortical polarization on a conditioned avoidance response. Exp Neurol. 1962;6:507–523. [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O’Carroll C. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus F, Ziemann U, Classen J. Plasticity resembling spike-timing dependent synaptic plasticity: the evidence in human cortex. Front Synaptic Neurosci. 2010;2:34. doi: 10.3389/fnsyn.2010.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, Sjostrom P, Turrigiano G. Rate and timing in cortical synaptic plasticity. Philos Trans R Soc Lond B Biol Sci. 2002;357:1851–1857. doi: 10.1098/rstb.2002.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003a;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb Cortex. 2004a;14:1240–1245. doi: 10.1093/cercor/bhh085. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology. 2004b;29:1573–1578. doi: 10.1038/sj.npp.1300517. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci. 2006;23:1651–1657. doi: 10.1111/j.1460-9568.2006.04676.x. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Liebetanz D, Schlitterlau A, Henschke U, Fricke K, Frommann K, Lang N, Henning S, Paulus W, Tergau F. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci. 2004c;19:2720–2726. doi: 10.1111/j.0953-816X.2004.03398.x. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Nitsche M, Klein C, Tergau F, Rothwell J, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003b;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003c;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Seeber A, Frommann K, Klein C, Rochford C, Nitsche M, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Kuo M-F, Grosch J, Bergner C, Monte-Silva K, Paulus W. D1-receptor impact on neuroplasticity in humans. J Neurosci. 2009a;29:2648–2653. doi: 10.1523/JNEUROSCI.5366-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Kuo M-F, Karrasch R, Wächter B, Liebetanz D, Paulus W. Serotonin affects transcranial direct current-induced neuroplasticity in humans. Biol Psychiatry. 2009b;66:503–508. doi: 10.1016/j.biopsych.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Normann C, Clark K. Selective modulation of Ca2+ influx pathways by 5-HT regulates synaptic long-term plasticity in the hippocampus. Brain Res. 2005;1037:187–193. doi: 10.1016/j.brainres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- O’Dell TJ, Kandel ER, Grant SG. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991;353:558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Matsumoto M, Otani H, Mori K, Togashi H, Ueno K, Kaku A, Yoshioka M. Changes in synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway induced by repeated treatments with fluvoxamine. Brain Res. 2002;949:131–138. doi: 10.1016/s0006-8993(02)02973-6. [DOI] [PubMed] [Google Scholar]
- Otani S, Blond O, Desce J, Crepel F. Dopamine facilitates long-term depression of glutamatergic transmission in rat prefrontal cortex. Neuroscience. 1998;85:669–676. doi: 10.1016/s0306-4522(97)00677-5. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci. 1996;16:7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T, Fitzgerald PB, Brown TL, de Castella A, Daskalakis ZJ, Kulkarni J. Repetitive transcranial magnetic stimulation reveals abnormal plastic response to premotor cortex stimulation in schizophrenia. Biol Psychiatry. 2004;56:628–633. doi: 10.1016/j.biopsych.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz RD, Tardito S, Atzori M, Tseng KY. Glutamatergic dysfunction in schizophrenia: from basic neuroscience to clinical psychopharmacology. Eur Neuropsychopharmacol. 2008;18:773–786. doi: 10.1016/j.euroneuro.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor F, Pinto-Hamuy T, Kupferman I. Cortical stimulation during learning in rabbits. Neuropsychologia. 1964;2:305–310. [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the “long” in long-term potentiation. Trends Neurosci. 2007;30:167–175. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy S, Beck H, Yaari Y. Plasticity of voltage-gated ion channels in pyramidal cell dendrites. Curr Opin Neurobiol. 2010;20:503–509. doi: 10.1016/j.conb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Arico I, Mastroeni C, Morgante F, Liotta G, Girlanda P, Silvestri R, Quartarone A. Dopamine agonists restore cortical plasticity in patients with idiopathic restless legs syndrome. Mov Disord. 2009a;24:710–715. doi: 10.1002/mds.22436. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Siebner HS, Morgante F, Mastroeni C, Girlanda P, Quartarone A. Paired associative stimulation of left and right human motor cortex shapes interhemispheric motor inhibition based on a Hebbian mechanism. Cereb Cortex. 2009b;19:907–915. doi: 10.1093/cercor/bhn144. [DOI] [PubMed] [Google Scholar]
- Rosen SC, Stamm JS. Transcortical polarization: facilitation of delayed response performance by monkeys. Exp Neurol. 1972;35:282–289. doi: 10.1016/0014-4886(72)90154-9. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotem A, Moses E. Magnetic stimulation of one-dimensional neuronal cultures. Biophys J. 2008;94:5065–5078. doi: 10.1529/biophysj.107.125708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkegel H, Sommer M, Paulus W. Breaks during 5 Hz rTMS are essential for facilitatory after effects. Clin Neurophysiol. 2010;121:426–430. doi: 10.1016/j.clinph.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Ryan B, Musazzi L, Mallei A, Tardito D, Gruber SH, El Khoury A, Anwyl R, Racagni G, Mathe AA, Rowan MJ, Popoli M. Remodelling by early-life stress of NMDA receptor-dependent synaptic plasticity in a gene-environment rat model of depression. Int J Neuropsychopharmacol. 2009;12:553–559. doi: 10.1017/S1461145708009607. [DOI] [PubMed] [Google Scholar]
- Sanberg CD, Jones FL, Do VH, Dieguez D, Jr, Derrick BE. 5-HT1a receptor antagonists block perforant path-dentate LTP induced in novel, but not familiar, environments. Learn Mem. 2006;13:52–62. doi: 10.1101/lm.126306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada S, Yamamoto C, Ohno-Shosaku T. Long-term potentiation and depression in the dentate gyrus, and effects of nicotine. Neurosci Res. 1994;20:323–329. doi: 10.1016/0168-0102(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Scheiderer CL, Smith CC, McCutchen E, McCoy PA, Thacker EE, Kolasa K, Dobrunz LE, McMahon LL. Coactivation of M1 muscarinic and α1 adrenergic receptors stimulates extracellular signal-regulated protein kinase and induces long-term depression at CA3–CA1 synapses in rat hippocampus. J Neurosci. 2008;28:5350–5358. doi: 10.1523/JNEUROSCI.5058-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Seamans J, Yang C. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Segal M. Dendritic spines and long-term plasticity. Nat Rev. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci. 2005;25:11194–11200. doi: 10.1523/JNEUROSCI.2338-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, Mochizuki H, Bohning DE, Boorman ED, Groppa S, Miniussi C, Pascual-Leone A, Huber R, Taylor PC, Ilmoniemi RJ, De Gennaro L, Strafella AP, Kähkönen S, Klöppel S, Frisoni GB, George MS, Hallett M, Brandt SA, Rushworth MF, Ziemann U, Rothwell JC, Ward N, Cohen LG, Baudewig J, Paus T, Ugawa Y, Rossini PM. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2:58–80. doi: 10.1016/j.brs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Rancz EA, Roth A, Hausser M. Dendritic excitability and synaptic plasticity. Physiol Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Murphy KP. Bi-directional changes in synaptic plasticity induced at corticostriatal synapses in vitro. Exp Brain Res. 2000;135:497–503. doi: 10.1007/s002210000523. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H. What are we measuring with GABA magnetic resonance spectroscopy. Commun Integr Biol. 2011;4:573–575. doi: 10.4161/cib.4.5.16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009a;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, Bestmann S. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009b;101:2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Otaky N. Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Res. 1994;643:10–16. doi: 10.1016/0006-8993(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen L, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stephani C, Nitsche MA, Sommer M, Paulus W. Impairment of motor cortex plasticity in Parkinson's disease, as revealed by theta-burst-transcranial magnetic stimulation and transcranial random noise stimulation. Parkinsonism Relat Disord. 2011;17:297–298. doi: 10.1016/j.parkreldis.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Strafella A, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- Sugisaki E, Fukushima Y, Tsukada M, Aihara T. Cholinergic modulation on spike timing-dependent plasticity in hippocampal CA1 network. Neuroscience. 2011;192:91–101. doi: 10.1016/j.neuroscience.2011.06.064. [DOI] [PubMed] [Google Scholar]
- Suppa A, Iezzi E, Conte A, Belvisi D, Marsili L, Modugno N, Fabbrini G, Berardelli A. Dopamine influences primary motor cortex plasticity and dorsal premotor-to-motor connectivity in Parkinson's disease. Cereb Cortex. 2010;20:2224–2233. doi: 10.1093/cercor/bhp288. [DOI] [PubMed] [Google Scholar]
- Suppa A, Marsili L, Belvisi D, Conte A, Iezzi E, Modugno N, Fabbrini G, Berardelli A. Lack of LTP-like plasticity in primary motor cortex in Parkinson's disease. Exp Neurol. 2011;227:296–301. doi: 10.1016/j.expneurol.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Takamatsu I, Iwase A, Ozaki M, Kazama T, Wada K, Sekiguchi M. Dexmedetomidine reduces long-term potentiation in mouse hippocampus. Anesthesiology. 2008;108:94–102. doi: 10.1097/01.anes.0000296076.04510.e1. [DOI] [PubMed] [Google Scholar]
- Tenorio G, Connor SA, Guevremont D, Abraham WC, Williams J, O’Dell TJ, Nguyen PV. ‘Silent’ priming of translation-dependent LTP by β-adrenergic receptors involves phosphorylation and recruitment of AMPA receptors. Learn Mem. 2010;17:627–638. doi: 10.1101/lm.1974510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo JT, Swayne OB, Rothwell JC. Further evidence for NMDA-dependence of the after-effects of human theta burst stimulation. Clin Neurophysiol. 2007;118:1649–1651. doi: 10.1016/j.clinph.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Testa-Silva G, Verhoog MB, Goriounova NA, Loebel A, Hjorth J, Baayen JC, de Kock CP, Mansvelder HD. Human synapses show a wide temporal window for spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010;2:12. doi: 10.3389/fnsyn.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirugnanasambandam N, Grundey J, Adam K, Drees A, Skwirba AC, Lang N, Paulus W, Nitsche MA. Nicotinergic impact on focal and non-focal neuroplasticity induced by non-invasive brain stimulation in non-smoking humans. Neuropsychopharmacology. 2011;36:879–886. doi: 10.1038/npp.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010;22:219–232. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully K, Li Y, Tsvetkov E, Bolshakov VY. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci U S A. 2007;104:14146–14150. doi: 10.1073/pnas.0704621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Mima T, Kotb MA, Sawada H, Saiki H, Ikeda A, Begum T, Reza F, Nagamine T, Fukuyama H. Altered plasticity of the human motor cortex in Parkinson's disease. Ann Neurol. 2006;59:60–71. doi: 10.1002/ana.20692. [DOI] [PubMed] [Google Scholar]
- Wang ZM, Qi YJ, Wu PY, Zhu Y, Dong YL, Cheng ZX, Zhu YH, Dong Y, Ma L, Zheng P. Neuroactive steroid pregnenolone sulphate inhibits long-term potentiation via activation of α2-adrenoreceptors at excitatory synapses in rat medial prefrontal cortex. Int J Neuropsychopharmacol. 2008;11:611–624. doi: 10.1017/S1461145707008334. [DOI] [PubMed] [Google Scholar]
- Wankerl K, Weise D, Gentner R, Rumpf JJ, Classen J. L-type voltage-gated Ca2+ channels: a single molecular switch for long-term potentiation/long-term depression-like plasticity and activity-dependent metaplasticity in humans. J Neurosci. 2010;30:6197–6204. doi: 10.1523/JNEUROSCI.4673-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Ahlijanian MK, Catterall WA. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990;347:281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Large long-lasting potentiation in the dentate gyrus in vitro during blockade of inhibition. Brain Res. 1983;275:153–158. doi: 10.1016/0006-8993(83)90428-6. [DOI] [PubMed] [Google Scholar]
- Wojtowicz AM, Fidzinski P, Heinemann U, Behr J. Beta-adrenergic receptor activation induces long-lasting potentiation in burst-spiking but not regular-spiking cells at CA1-subiculum synapses. Neuroscience. 2010;171:367–372. doi: 10.1016/j.neuroscience.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen L, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, Kunesch E, Benecke R, Reiners K, Classen J. Timing-dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–1052. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Overcoming obstacles to developing new analgesics. Nat Med. 2010;16:1241–1247. doi: 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- Xu TX, Yao WD. D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proc Natl Acad Sci U S A. 2010;107:16366–16371. doi: 10.1073/pnas.1004108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir O, Gunraj C, Ni Z, Mazzella F, Chen R. Effects of theta burst stimulation on motor cortex excitability in Parkinson's disease. Clin Neurophysiol. 2012;123:815–821. doi: 10.1016/j.clinph.2011.07.051. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Lau PM, Bi GQ. Gain in sensitivity and loss in temporal contrast of STDP by dopaminergic modulation at hippocampal synapses. Proc Natl Acad Sci U S A. 2009;106:13028–13033. doi: 10.1073/pnas.0900546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. TMS induced plasticity in human cortex. Rev Neurosci. 2004;15:253–266. doi: 10.1515/revneuro.2004.15.4.253. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Ilic TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, Siebner HR, Classen J, Cohen LG, Rothwell JC. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008;1:164–182. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]


