Abstract
A cholinergic synaptic input has been highlighted in recent years as an important modulator of motoneuron excitability. In the present study, it was examined whether, as in other regions, the cholinergic inputs on spinal motoneurons modulate the glutamatergic synaptic activity, evoked by electrical stimulation at the dorsolateral funiculus. The present results show that the pharmacological stimulation of muscarinic receptors produces a selective depression of AMPA-mediated glutamatergic activity in thoracic and lumbar spinal motoneurons. This effect does not involve a change in transmitter release and occurs both on synaptic currents and on AMPA responses evoked by photolysis of MNI-glutamate. Thus, it is concluded that muscarinic modulation occurs at the postsynaptic level. The results suggest that cholinergic inputs contacting motoneurons, which are known to increase motoneuron excitability, also directly interfere with incoming synaptic inputs by changing their amplitudes.
Key points
The muscarinic receptors increase the excitability in spinal cord motoneurons.
In the present study, I report the muscarinic modulation of postsynaptic AMPA receptors at glutamatergic synapses on spinal cord motoneurons.
The muscarinic modulation acts specifically on synapses originated at the dorsolateral funiculus, which contains supraspinal and spinal intersegmental influences.
The present results suggest that the modulatory actions of muscarinic receptors reduce the synaptic weight of excitatory inputs originating at intersegmental and/or supraspinal levels but not at the intrasegmental level.
The muscarinic receptor activity might amplify the differences between synaptic weights of inputs with different origins, potentially contributing to the diversity of motor outputs implicating the same muscular groups, as well as to the refinement of locomotor behaviour at early postnatal stages.
Introduction
Spinal motoneurons convey central nervous system commands to control muscular contraction. Motoneuron activity integrates synaptic inputs from numerous supraspinal and intraspinal origins and modulatory influences exerted at presynaptic and postsynaptic levels (Eccles et al. 1961; Rudomin, 1990; Betley et al. 2009). Glutamatergic afferents represent the main excitatory input onto motoneurons, with axonal projections originating at the proprioceptive sensory terminals (Jahr & Yoshioka, 1986; Ragnarson et al. 2003), spinal interneurons (Bannatyne et al. 2003; Kullander et al. 2003; O’Donovan et al. 2010), descending propiospinal afferents (see Rekling et al. 2000) as well as a small proportion of axons from the dorsolateral portion of the corticospinal tract in rodents (Bareyre et al. 2005; Betley et al. 2009).
Previous studies have also shown prominent cholinergic inputs on motoneurons, mainly represented by large C terminals contacting the soma and proximal dendrites and originating from interneurons located near the central canal (Miles et al. 2007). In addition, cholinergic inputs onto motoneurons also come from neighbouring motoneuron colaterals (Cullheim et al. 1977; Mentis et al. 2005). Cholinoceptive receptors were long ago described in motoneurons (Zieglgansberger & Bayerl, 1976; Evans, 1978). The activity of cholinergic afferents, acting on muscarinic receptors, enhances the motoneuron firing, through modulation of postsynaptic K+ channels (Alaburda et al. 2002; Miles et al. 2007; Chevallier et al. 2008). Muscarinic receptors also produce a rise in intracellular Ca2+ concentration that is responsible for the facilitation of plateau potentials (Delgado-Lezama et al. 1997; Mejia-Gervacio et al. 2004). Since in other systems muscarinic receptors modulate glutamatergic inputs (Dutar et Nicoll, 1988; Higley et al. 2009), I decided to explore whether the cholinergic inputs modulate glutamatergic synapses onto motoneurons when glutamatergic activity is evoked by stimulation of the spinal cord dorsolateral funiculus.
Methods
Animals and slice preparation
C57Bl6 (Janvier) or ChAT_GFP mice (kindly provided by J. Engelhardt and H. Monyer; Von Engelhardt et al. 2007) at postnatal days 6 to 11 were used. Thoracic and lumbar transverse spinal cord slices were obtained as described elsewhere (Lamotte d’Incamps & Ascher, 2008). Briefly, mice were anaesthetized with 0.1% pentobarbital (20 mm) before intracardiac perfusion with cold solution 1. After decapitation, the spinal cord was dissected out in cold solution 1, before inclusion in 2% agar at 37°C. The agar block was chilled to 4°C in bubbled solution 2 and 400 μm transverse slices were prepared using a vibratome (Leica S100V). The slices were incubated at 34°C for 30 min before the recording session, in solution 3. Solutions 1–3 are listed in Table 1. All experimental procedures were performed in accordance with the Charter of Fundamental Rights of the European Union (2000/C 364/01), the European Communities Council Directive of 24 November 1986 (86/609/EEC) and European Union guidelines.
Table 1.
Composition of dissection and recording solutions
| mM | Solution 1 | Solution 2 | Solution 3 | HBS |
|---|---|---|---|---|
| NaCl | 130 | — | 130 | 135 |
| Potassiu gluconate | — | 130 | — | — |
| KCl | 2.5 | 15 | 2.5 | 4 |
| NaH2PO4 | 1.3 | — | 1.3 | — |
| EGTA | — | 0.5 | — | — |
| Hepes | — | 18 | — | 10 |
| Glucose | 25 | 25 | 25 | 25 |
| Sucrose | 230 | — | — | — |
| NaHCO3 | 26 | — | 26 | 2 |
| Ascorbic acid | 0.395 | — | 0.395 | — |
| CaCl2 | 0.8 | — | 2 | 2 |
| MgCl2 | 8 | — | 1 | 2 |
| Kynurenic acid | 1 | 1 | — | — |
| Sodium pyruvate | 2 | 2 | 2 | — |
| KOH | — | 8.7 | — | — |
Solutions 1 to 3 were bubbled with 95% O2–5% CO2. pH was adjusted to 7.4 in all the solutions.
For recording, the slices were continuously perfused with solution 3 at room temperature. The cells were visualized using an upright Axioscope (Zeiss), equipped with a 40× water-immersion objective and an EMCCD camera (Luca, Andor). A double optical path allowed the alignment of illumination with either a 405 nm laser diode (Deepstar, Omicron) using a dichroic reflector 425 DCXR (Chroma) or with a blue LED (Cairn Research) and excitation filters for 470 nm epifluorescence (Chroma). Optical adjustments were performed to obtain a ∼1 μm 405 nm spot at the objective focus (Trigo et al. 2009).
Electrophysiological data
Motoneurons were large green fluorescent protein positive (GFP+) cells (soma >20 μm) located in the lateral ventral horn, when ChAT_GFP mice were used (Fig. 1A). In wild type (WT) animals, motoneurons were recognized as large ventral horn neurons firing a single action potential, with a short latency (1.06 ± 0.22 ms), after electrical stimulation of the ventral roots. For stimulation, the ventral roots were aspirated into glass electrodes containing recording solution and connected to a constant current stimulator (Digitimer DS3). Intensities between 10 and 100 V (100 μs) were adjusted to obtain stable responses.
Figure 1. Muscarinic modulation of AMPA synaptic activity in spinal cord motoneurons.
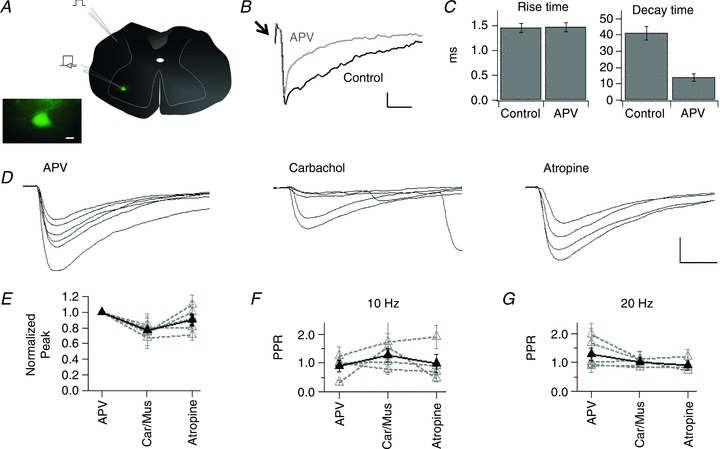
A, schema representing the location of the stimulation electrode in the dorsolateral funiculus and the recording electrode in the ventral horn motoneurons. Inset, photomicrograph showing a large GFP+ neuron, located in the spinal cord ventral horn. B, traces showing evoked synaptic currents in control and after the addition of APV to the perfusion bath. Arrow indicates the stimulus artifact. C, mean ± SEM rise and decay time of EPSCs in control and in APV, for the cell in B. D, traces of evoked AMPA EPSCs from a motoneuron recorded in APV and after subsequent addition of carbachol and atropine. E, normalized average peak for AMPA responses in APV, after the addition of either carbachol or muscarine (Car/Mus) and after atropine. For this and the rest of the figures grey discontinuous traces represent the mean ± SEM for individual cells and the thick black line represents the group values. F, mean ± SEM PPR for cells recorded in the same experimental conditions described for E and stimulated at 10 Hz and for G at 20 Hz. Calibration bars: 10 μm in A (inset),10 pA and 20 ms in B, 200 pA and 5 ms in D.
Motoneurons were recorded using the whole-cell patch-clamp configuration, with an EPC-10 amplifier (Heka_Electronik) and Patchmaster software. The intracellular solutions were either a Cs+-based solution, containing (in mm) caesium gluconate 125, QX-314 5, EGTA 10, Hepes 10, MgATP 4, NaGTP 0.3 and CaCl2 1, pH 7.4 or a K+-based solution containing potassium gluconate 120, KCl 5, QX-314 5, EGTA 10, Hepes 10, MgATP 4, NaGTP 0.3 and CaCl2 1, pH 7.4. Alexa Fluor 488 (5 μm) was added to the solution when recording from WT animals. The pipette resistances varied between 4 and 6 MΩ. The cells were held at −75 or −45 mV (after junction potential correction, −15 mV for both solutions). The input resistance was calculated from the steady current produced by 60 ms steps −20 and −10 mV more hyperpolarized than the holding potential. Cells showing series resistance variations above 25% were withdrawn from the analysis.
Evoked EPSCs
For stimulation of evoked synaptic activity the tip of a theta-glass pipette, backfilled with recording solution, was placed at the dorsolateral funiculus (Fig. 1A). An isolated pulse stimulator (AM systems 2100) was used to apply single pulses or 3-pulse trains at 10 or 20 Hz, every 5 s. The stimulation was established using the minimal intensity producing reproducible responses. The synaptic responses’ peak amplitude, rise time (20–80% peak) and decay time were determined using NeuroMatic in Igor (Wavemetrics). The paired-pulse ratio (PPR) was calculated from the individual peak amplitudes of the second/first responses in each trial. Test drugs were added to the perfusion bath and the effects were evaluated 10 min after the arrival of the drugs to the chamber.
MNI-glutamate photorelease
The slices were incubated for at least 1 h in 13 μm MNI-glutamate diluted in Hepes-buffered solution (HBS; Table 1). Recordings were carried out in similar conditions. After establishing a whole-cell recording, a 405 nm laser spot was focused onto the motoneuron's somatic or proximal dendritic membrane. Usually one to three different locations were tested to obtain stable inward currents. At this point, neither the objective focus nor the laser duration or intensity were further modified during the experiment. For pharmacological experiments, concentrated stock solutions of the test drugs were added directly to the recording chamber, which contained a known and constant liquid volume. The solution in the chamber was carefully mixed using a pipette. Test recordings started 10 min after addition of the drugs.
Statistical analysis
Results are expressed as means ± SEM. Experiments involving the comparison of a control and experimental groups were analysed using a Student's t test. In other cases, an ANOVA comparison was followed by Tukey post hoc test. Differences were considered significant for values of P < 0.05.
Drugs
Glycinergic and GABAA receptors were pharmacologically blocked during the recordings using a mix of strychnine (3 μm) and SR95531 (10 μm). Mecamylamine (50 μm), carbamylcholine chloride (carbachol, 10 μm), muscarine (10 μm), atropine (1 μm), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo(f)quinoxaline-7-sulfonamide (NBQX, 2 μm), d-2-amino-5-phosphonovaleric acid (d-APV, 50 μm) and (+)-α-methyl-4-carboxyphenylglycine (MCPG, 10 μm) were used as indicated. SR95531, d-APV, MNI-glutamate and QX-314 were from Tocris. The rest of the drugs were from Sigma-Aldrich.
Results
The whole-cell patch-clamp configuration was used to record synaptically evoked activity in motoneurons held at −45 or −75 mV. Synaptic currents were evoked using electrical stimulation of the dorsolateral funiculus (Fig. 1A). The responses were inward currents whose amplitudes diminished as the holding potential approached 0 mV, resulting in an average reversal potential of −2.2 ± 2.42 mV, calculated by linear extrapolation. The evoked responses were identified as glutamatergic since they were reversibly blocked by the application of NBQX (2 μm) and APV (50 μm), suggesting mixed AMPA and NMDA components. Mixed AMPA and NMDA currents, reversing near 0 mV have been reported in spinal cord motoneurons at early postnatal ages (Konnerth et al. 1990; Han et al. 2007).
The effects of muscarinic agonists were studied on each isolated component and thus experiments were performed in the presence of either APV or NBQX. The addition of APV accelerated, in a non-significant manner, the kinetics of the evoked responses compared with those recorded in control conditions. The rise time decreased from 3.85 ± 0.45 to 1.94 ± 0.36 ms (n= 5 cells; P= 0.33) and the decay time from 21.54 ± 4.48 to 11.74 ± 3.33 ms (P= 0.17; Fig. 1B and C). Overall the kinetics recorded in APV were faster than the responses recorded in slices bathed in NBQX (rise time 8.88 ± 3.86 ms, decay time 17.18 ± 9.93 ms; n= 4 cells; Fig. 2A and B).
Figure 2. NMDA responses are not modulated by muscarinic activity.
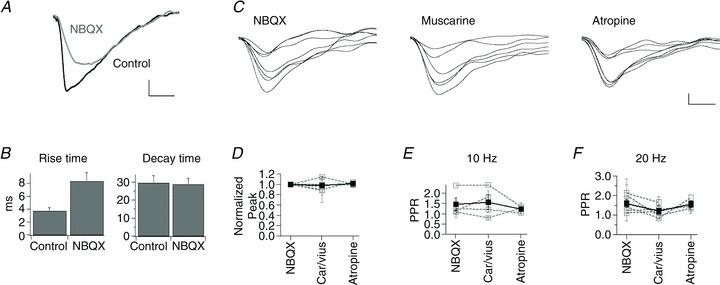
A, traces of evoked synaptic activity recorded in control conditions and in NBQX. B, mean ± SEM rise and decay time of evoked synaptic activity for the cell in A in control and in NBQX. C, traces depicting NMDA-synaptic responses in NBQX and the consecutive addition of muscarine and atropine. D, mean ± SEM normalized peak values for cells recorded in NBQX, after the addition of either carbachol or muscarine (Car/Mus), and subsequent addition of atropine. E, mean ± SEM PPR in the conditions described in D for stimulation frequencies at 10 Hz and in F at 20 Hz. Calibration bars: 10 pA and 20 ms in A and 2 pA and 20 ms in C.
AMPA- but not NMDA-evoked activity is reduced by activation of muscarinic receptors
The effects of muscarinic receptor activation on evoked AMPA-mediated synaptic currents (EPSCs) were investigated in cells held at either −75 or −45 mV (n= 2 and 3 cells, respectively), recorded in the presence of APV. The peak values of single EPSCs were compared in control, in the presence of the muscarinic agonists, and after antagonizing muscarinic receptors. The experiments using carbachol were performed in the presence of mecamylamine to exclude the participation of nicotinic receptors (Lamotte d’Incamps & Ascher, 2008). The activation of muscarinic receptors significantly decreased the peak AMPA responses to 0.76 ± 0.02 (n= 6 cells; P= 6.8 × 10−4) of the normalized control response (Fig. 1D and E). The amplitude returned to control levels after application of the muscarinic antagonist atropine (0.90 ± 0.06; n= 5 cells, P= 0.17).
The effects of muscarinic agents were also evaluated on isolated NMDA currents in cells held at −45 mV and recorded in NBQX. In contrast to the effects on AMPA responses, the amplitude of the evoked EPSCs in NBQX was not altered by muscarinic agonists or antagonists. The peak amplitude values, with respect to the normalized control responses, corresponded to 0.97 ± 0.04 (5 cells; P= 0.80) in muscarinic agonists and 1.01 ± 0.01 (3 cells; P= 0.92) in atropine (Fig. 2C and D).
In order to understand whether the observed decrease of AMPA-mediated EPSCs was selective for the glutamatergic input stimulated at the dorsolateral funiculus, the spontaneous glutamatergic synaptic currents were analysed in control and after muscarinic receptor pharmacological activation. Spontaneous synaptic currents were recorded at frequencies between 0.5 and 19.8 events s−1. The addition of muscarinic agonists did not induce significant differences on the amplitude or the frequency of spontaneous EPSCs (P= 0.52 and P= 0.45; n= 5).
To evaluate whether the observed results could be attributed to changes in input resistance, the population values of this parameter, in the different pharmacological conditions, were compared. The muscarinic agonists induced a small but significant increase in the input resistance when the cells were recorded using K+-based intracellular solution (84.98 ± 22.58 vs. 78.86 ± 21.19 MΩ in control conditions; n= 12, P= 0.03). In contrast, the same comparison showed no change in cells recorded with Cs+-based solution (86.50 ± 16.84 vs. 81.17 ± 15.02 MΩ in control conditions; n= 14, P= 0.39). Previous reports have shown varied effects of muscarinic agents on lumbar and hypoglossal motoneurons ranging from slight increases (Miles et al. 2007) to the absence of net effects on input resistance (Bellingham & Berger, 1996). The differences observed in previous studies correspond to the use of different intracellular solutions, as in the present experiments. Nevertheless, neither the slight increase nor the absence of changes on input resistance explain the consistent and selective reduction of AMPA glutamatergic responses caused by muscarinic agonists.
The PPR of glutamatergic synaptic currents is not affected by activation of muscarinic receptors
To explore whether the observed modulation was due to presynaptic effects, the PPR of evoked synaptic responses at 10 and 20 Hz was determined. PPR values remained unchanged after the application of muscarine or atropine, regardless of the frequency of stimulation (Fig. 1F and G). The PPR at 10 Hz for isolated AMPA responses in control conditions was 0.89 ± 0.19 (n= 4), 1.28 ± 0.22 (n= 4, P= 0.47) in muscarine and 0.98 ± 0.31 (n= 4, P= 0.94) in atropine. PPR values at 20 Hz were 1.28 ± 0.22 in control (n= 5), 0.99 ± 0.05 (n= 5, P= 0.37) in muscarine and 0.90 ± 0.15 (n= 3, P= 0.28) in atropine.
The PPR for NMDA responses was similarly unaffected by the pharmacological manipulation of muscarinic receptors. PPR values at 10 Hz in control (1.47 ± 0.30; n= 4) were not different from those in muscarine (1.55 ± 0.34, n= 4, P= 0.97) or atropine (1.22 ± 0.09, n= 3, P= 0.83) at 10 Hz (Fig. 2E). Analogously, the values at 20 Hz were 1.57 ± 0.20, 1.19 ± 0.14 (n= 5, P= 0.28) and 1.55 ± 0.16 (n= 3, P= 0.99), respectively (Fig. 2F).
Muscarinic agonists affect AMPA receptors activity
Due to the lack of effect on PPR, I examined the modulation of postsynaptic glutamatergic activity. The cholinergic modulation of currents evoked at the somatic and proximal dendritic sites by photorelease of MNI-glutamate was analysed. The photolysis of MNI-glutamate produced inward currents, which remained stable in magnitude and kinetics during at least 30 min when tested in constant illumination and pharmacological conditions.
MNI-glutamate-induced AMPA responses were recorded in five cells held at −75 mV and recorded in APV. The addition of either muscarine or carbachol to the bath induced a significant decrease of the responses to 0.78 ± 0.02 (n= 5, P= 1.17 × 10−4) of the normalized peak amplitude, recorded in control conditions. The decrease induced by muscarine was reversed by atropine (0.92 ± 0.04 Fig. 3A and B; n= 4, P= 0.09). In contrast, the addition of muscarine did not affect the MNI-glutamate-induced NMDA responses in cells recorded in NBQX and held at −45 mV. In these conditions, values remained 1.04 ± 0.02 after either muscarine or carbachol (Fig. 3C and D; n= 5, P= 0.16).
Figure 3. Muscarinic modulation of MNI-glutamate photolysis responses in spinal cord motoneurons.
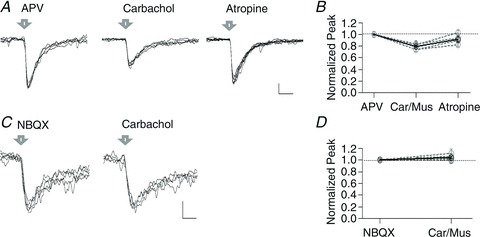
A, example traces of AMPA responses evoked by laser photolysis (arrow) of MNI-glutamate in APV and after the addition of carbachol and atropine to the recording chamber. B, normalized average peak of MNI-glutamate evoked responses in APV, after the addition of either carbachol or muscarine (Car/Mus), and the subsequent addition of atropine. C, example traces of NMDA responses evoked by laser photolysis of MNI-glutamate recorded in NBQX and after addition of carbachol to the bath. D, normalized average peak of MNI-glutamate evoked responses in NBQX and after carbachol or muscarine (Car/Mus). Calibration bars: 10 pA and 30 ms in A, 5 pA and 100 ms in C.
The evoked EPSCs were entirely abolished by pharmacological blockade of AMPA and NMDA receptors. Nonetheless, the possibility remains that direct photorelease of MNI-glutamate could potentially activate metabotropic glutamatergic receptors in motoneurons. The participation of metabotropic glutamate receptors can be ruled out because when MCPG was added to the bath solution, in three independent experiments, the activation of muscarinic receptors continued to reduce AMPA responses (0.73 ± 0.06; P= 0.02).
Overall, the MNI-glutamate photolysis results support the hypothesis that muscarinic receptor activation affects glutamatergic synaptic function through a postsynaptic mechanism.
Discussion
The present results show that muscarinic receptors can modulate glutamatergic synaptic inputs from the dorsolateral funiculus to spinal motoneurons. The modulation affected AMPA-mediated currents but left NMDA responses unchanged. A series of observations strongly support a postsynaptic action of muscarinic receptors on AMPA receptors, as opposed to on glutamate release from presynaptic terminals. First, the PPR of evoked synaptic responses was not modified by any of the muscarinic agents tested. Second, muscarinic receptor activation directly reduced the peak amplitude of AMPA responses induced by MNI-glutamate photorelease. Finally, the muscarinic action did not change either the synaptically evoked or the photolysis-evoked NMDA responses, indicating a lack of muscarinic modulation of NMDA synaptic activity.
The present results complement reports concerning various effects of postsynaptic muscarinic receptors on spinal cord motoneuron excitability, regarding the regulation of their firing frequency (Alaburda et al. 2002; Chevallier et al. 2006; Miles et al. 2007; Nieto-Gonzalez et al. 2009; Zagoraiou et al. 2009) and firing pattern (Delgado-Lezama et al. 1997; Mejia-Gervacio et al. 2004). Several of the experiments from the previous studies were performed in the presence of antagonists for AMPA and NMDA receptors and have not addressed glutamatergic activity. Thus, the modulatory influence of the muscarinic system onto the glutamatergic synaptic activity in spinal cord motorneurons remained largely unstudied, despite the fact that important interactions between these two neurotransmitter systems are firmly documented in other central nervous system regions (see below).
Previous studies have established a potent muscarinic modulation of glutamatergic synaptic function at the presynaptic level in the striatum (Higley et al. 2009), and in other central nervous regions (Li et al. 2002; Guo et al. 2010), including the lateral tegmental input onto hypoglossal motoneurons (Bellingham & Berger, 1996). Moreover, a form of EPSC depression induced by muscarinic receptors has been attributed to AMPA receptor internalization in the hippocampus (Volk et al. 2007; Dickinson et al. 2009).
The analysis of the mechanism responsible for postsynaptic muscarinic modulation of AMPA receptors in motoneurons was not conducted here, but the results permit some possible mechanisms to be ruled out. First, the photolysis experiments using MCPG rule out a role of mGluRs in the modulation of AMPA activity. Muscarinic receptor families activate two principal intracellular cascades. These cascades depend on the activation of phospholipase C and lead to intracellular Ca2+ release and on adenylate cyclase inhibition. Since the current pharmacology for muscarinic receptors is non-specific, it will be difficult to determine the intracellular pathway involved in muscarinic modulation of glutamatergic activity onto motoneurons. Nonetheless, the high buffer capacity of the intracellular solutions used makes a mechanism dependent on the intracellular release of Ca2+ unlikely. Finally, the results exclude the possibility that the reduction of glutamatergic activity would be due to the slight changes (∼6 MΩ) in input resistance, observed in cells recorded with K+ solution. The expected effect of increased input resistance would be a concomitant increase of the recorded conductance, associated with improved space clamp. This is not observed for either the AMPA or at NMDA responses. Furthermore, a significant reduction of the peak AMPA EPSC was observed without changes in input resistance, in cells recorded using Cs+ solution.
Previous studies have shown muscarinic-induced changes in K+ conductance associated with slight increases in input resistance of motoneurons (Lape & Nistri, 2000; Alaburda et al. 2002; Miles et al. 2007). The modulation of K+ conductance is associated with increased excitability in motoneurons, resulting in a robust augmentation of the motor output (Miles et al. 2007; Bertrand & Cazalets, 2011). The results described here indicate that it is likely that increased excitability occurs concomitantly but independently of changes induced in somatic AMPA receptor activity. In this context, the present results point to a mechanism that would specifically decrease synaptic weight of glutamatergic inputs originating at the dorsolateral funiculus, in a manner independent of the regulation of the general excitability of motoneurons and to the integration of other synaptic inputs.
The dorsolateral funiculus contains mainly rubrospinal fibres (Liang et al. 2012), and a discrete component from the dorsolateral corticospinal tract (Bareyre et al. 2005). Therefore, modulatory actions of muscarinic receptors probably reduce the synaptic weight of excitatory inputs originating at intersegmental and/or supraspinal levels but not at the intrasegmental level (Bertrand & Cazalets, 2011). The work by Zagoraiou et al. (2009) has shown that cholinergic inputs from C boutons are phase locked to intrasegmental motoneuron activity, and recent anatomical data showed that partition cholinergic interneurons contact corresponding motor pools bilaterally (Stepien et al. 2010). Thus, cholinergic interneurons are ideally placed to amplify differences between synaptic weights of inputs originated at the local vs. extrasegmental origins in specific motor pools, probably contributing to the diversity of motor outputs implicating the same muscular groups, as well as to the refinement of locomotor behaviour at early postnatal stages.
The present results show an absence of muscarinic modulation of NMDA-evoked synaptic activity, confirmed by synaptic activation as well as by direct activation of NMDA receptors with somatic MNI-glutamate uncaging, suggesting that muscarinic modulation specifically affects AMPARs. Nevertheless, since the C boutons contact mainly somatic and proximal dendritic locations, the possibility exists that the lack of modulation of synaptic NMDA activity results from a remote dendritic location of these receptors. However, previous studies in neonatal rats have provided indirect but convincing evidence concerning the co-localization of functional NMDA and AMPA receptors on the spinal cord motoneurons (Konnerth et al. 1990).
In summary, the present study shows that AMPA receptors are strongly modulated by cholinergic inputs, in spinal motoneurons. Functional and morphological changes in both cholinergic and glutamatergic afferents contacting motoneurons have been documented after spinal cord injury (Kitzman, 2006; Chevallier et al. 2008) and a gradual loss of cholinergic inputs on motoneurons has been correlated with the development of spasticity (Kitzman, 2006). These results suggest that the glutamatergic and muscarinic systems, being highly sensitive to spinal cord insults, are probably involved in consequent locomotor deficits, thus accentuating the importance of fully understanding how muscarinic modulation affects the integration of different sensorimotor information to control specific locomotor behaviours.
Acknowledgments
I wish to thank Alain Marty and Isabel Llano for support to develop the present experiments as well as for helpful discussion throughout the development of the project. I am grateful to Boris Lamotte and Phillipe Ascher for advice concerning the spinal cord preparation. I thank Genevieve Rougon, Boris Lamotte, Phillipe Ascher, Brandon Stell and Alain Marty for helpful discussions on the manuscript.
Glossary
- ChAT
choline acetyltransferase
- GFP
green fluorescent protein
- HBS
Hepes-buffered solution
- MNI-glutamate
4-methoxy-7-nitroindolinyl-l-glutamate
- PPR
paired-pulse ratio
- WT
wildtype
Author's present address
S. Mejia-Gervacio: Developmental Biology Institute of Marseille, 163 av. de Luminy, Campus de Luminy - case 907, 13288 Marseille, Cedex 9, France.
References
- Alaburda A, Perrier J, Hounsgaard J. An M-like outward current regulates the excitability of spinal motoneurones in the adult turtle. J Physiol. 2002;540:875–881. doi: 10.1113/jphysiol.2001.015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneuron by muscarinic M2 receptors. J Neurophysiol. 1996;76:3758–3770. doi: 10.1152/jn.1996.76.6.3758. [DOI] [PubMed] [Google Scholar]
- Bertrand SS, Cazalets JR. Cholinergic partition cells and lamina x neurons induce a muscarinic-dependent short-term potentiation of commissural glutamatergic inputs in lumbar motoneurons. Front Neural Circuits. 2011;5:15. doi: 10.3389/fncir.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Wright CVE, Kawaguchi Y, Erdélyi F, Szabó G, Jessell TM, Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell. 2009;139:161–174. doi: 10.1016/j.cell.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier S, Nagy F, Cabelguen J. Cholinergic control of excitability of spinal motoneurones in the salamander. J Physiol. 2006;570:525–540. doi: 10.1113/jphysiol.2005.098970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier S, Nagy F, Cabelguen J. Muscarinic control of the excitability of hindlimb motoneurons in chronic spinal-transected salamanders. Eur J Neurosci. 2008;28:2243–2253. doi: 10.1111/j.1460-9568.2008.06506.x. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Kellerth J, Conradi S. Evidence for direct synaptic interconnections between cat spinal α-motoneurons via the recurrent axon collaterals: a morphological study using intracellular injection of horseradish peroxidase. Brain Res. 1977;132:1–10. doi: 10.1016/0006-8993(77)90702-8. [DOI] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Nedergaard S, Svirskis G, Hounsgaard J. Metabotropic synaptic regulation of intrinsic response properties of turtle spinal motoneurones. J Physiol. 1997;504:97–102. doi: 10.1111/j.1469-7793.1997.097bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson BA, Jo J, Seok H, Son GH, Whitcomb DJ, Davies CH, Sheng M, Collingridge GL, Cho K. A novel mechanism of hippocampal LTD involving muscarinic receptor-triggered interactions between AMPARs, GRIP and liprin-alpha. Mol Brain. 2009;17:18. doi: 10.1186/1756-6606-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J Neurosci. 1988;8:4214–4224. doi: 10.1523/JNEUROSCI.08-11-04214.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Magni F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol. 1961;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RH. Cholinoceptive properties of motoneurons of the immature rat spinal cord maintained in vitro. Neuropharmacology. 1978;17:277–279. doi: 10.1016/0028-3908(78)90112-0. [DOI] [PubMed] [Google Scholar]
- Guo J, Sorenson EM, Chiappinelli VA. Cholinergic modulation of non-N-methyl-D-aspartic acid glutamatergic transmission in the chick ventral lateral geniculate nucleus. Neuroscience. 2010;166:604–614. doi: 10.1016/j.neuroscience.2009.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Nakanishi ST, Tran MA, Whelan PJ. Dopaminergic modulation of spinal neuronal excitability. J Neurosci. 2007;27:13192–13204. doi: 10.1523/JNEUROSCI.1279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Soler-Llavina GJ, Sabatini BL. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat Neurosci. 2009;12:1121–1128. doi: 10.1038/nn.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Yoshioka K. Ia afferent excitation of motoneurones in the in vitro new-born rat spinal cord is selectively antagonized by kynurenate. J Physiol. 1986;370:515–530. doi: 10.1113/jphysiol.1986.sp015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman P. Changes in vesicular glutamate transporter 2, vesicular GABA transporter and vesicular acetylcholine transporter labeling of sacrocaudal motoneurons in the spastic rat. Exp Neurol. 2006;197:407–419. doi: 10.1016/j.expneurol.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Keller BU, Lev-Tov A. Patch clamp analysis of excitatory synapses in mammalian spinal cord slices. Pflügers Archiv. 1990;417:285–290. doi: 10.1007/BF00370994. [DOI] [PubMed] [Google Scholar]
- Kullander K, Butt SJB, Lebret JM, Lundfald L, Restrepo CE, Rydström A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- Lamotte d’Incamps B, Ascher P. Four excitatory postsynaptic ionotropic receptors coactivated at the motoneuron-Renshaw cell synapse. J Neurosci. 2008;28:14121–14131. doi: 10.1523/JNEUROSCI.3311-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Nistri A. Current and voltage clamp studies of the spike medium afterhyperpolarization of hypoglossal motoneurons in a rat brain stem slice preparation. J Neurophysiol. 2000;83:2987–2995. doi: 10.1152/jn.2000.83.5.2987. [DOI] [PubMed] [Google Scholar]
- Li D, Chen S, Pan Y, Levey AI, Pan H. Role of presynaptic muscarinic and GABAB receptors in spinal glutamate release and cholinergic analgesia in rats. J Physiol. 2002;543:807–818. doi: 10.1113/jphysiol.2002.020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Paxinos G, Watson C. The red nucleus and the rubrospinal projection in the mouse. Brain Struct Funct. 2012;217:221–232. doi: 10.1007/s00429-011-0348-3. [DOI] [PubMed] [Google Scholar]
- Mejia-Gervacio S, Hounsgaard J, Diaz-Muñoz M. Roles of ryanodine and inositol triphosphate receptors in regulation of plateau potentials in turtle spinal motoneurons. Neuroscience. 2004;123:123–130. doi: 10.1016/j.neuroscience.2003.08.049. [DOI] [PubMed] [Google Scholar]
- Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O’Donovan MJ. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci U S A. 2005;102:7344–7349. doi: 10.1073/pnas.0502788102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Gonzalez JL, Carrascal L, Nunez-Abades P, Torres B. Muscarinic modulation of recruitment threshold and firing rate in rat oculomotor nucleus motoneurons. J Neurophysiol. 2009;101:100–111. doi: 10.1152/jn.90239.2008. [DOI] [PubMed] [Google Scholar]
- O’Donovan MJ, Bonnot A, Mentis GZ, Chub N, Pujala A, Alvarez FJ. Mechanisms of excitation of spinal networks by stimulation of the ventral roots. Ann N Y Acad Sci. 2010;1198:63–71. doi: 10.1111/j.1749-6632.2010.05535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnarson B, Ornung G, Grant G, Ottersen OP, Ulfhake B. Glutamate and AMPA receptor immunoreactivity in Ia synapses with motoneurons and neurons of the central cervical nucleus. Exp Brain Res. 2003;149:447–457. doi: 10.1007/s00221-003-1388-6. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P. Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends Neurosci. 1990;13:499–505. doi: 10.1016/0166-2236(90)90084-n. [DOI] [PubMed] [Google Scholar]
- Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron. 2010;68:456–472. doi: 10.1016/j.neuron.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Trigo FF, Corrie JET, Ogden D. Laser photolysis of caged compounds at 405 nm: photochemical advantages, localisation, phototoxicity and methods for calibration. J Neurosci Methods. 2009;180:9–21. doi: 10.1016/j.jneumeth.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 2007;27:11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27:5633–5642. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieglgänsberger W, Bayerl H. The mechanism of inhibition of neuronal activity by opiates in the spinal cord of cat. Brain Res. 1976;115:111–128. doi: 10.1016/0006-8993(76)90826-x. [DOI] [PubMed] [Google Scholar]


