Abstract
High body temperatures are generally associated with somnolence, lethargy, hypophagia and anhedonia. Orexin neurons have been suggested to play a role in such sickness behaviours due to their known functions in appetite, behavioural and autonomic activation. Furthermore, the activity of orexin neurons is inhibited by lipopolysaccharide that induces fever. However, the cellular mechanism(s) underlying this suppression of orexin neurons was unknown. We used patch-clamp recordings in acute rat brain slices to demonstrate that orexin neurons, including those projecting to the wake-promoting locus coeruleus, are inhibited by increasing the ambient temperature by a 2–4°C increment between 26 and 40°C. This effect was not mediated by conventional thermosensing mechanisms but instead involved the activation of ATP-sensitive potassium (KATP) channels. Since KATP channels can also sense energy substrate levels and cellular metabolism, our results suggest that orexin neurons can integrate the state of energy balance and body temperature, and adjust their output accordingly. Thus, the thermosensitivity of orexin neurons may be an important part of maintaining energy homeostasis during hyperthermia and fever.
Key points
High body temperature accompanies sleepiness, and loss of appetite and motivation.
Suppression of orexin neurons has been suggested to be involved in such behavioural responses.
In this study we determined that orexin neurons are inhibited by an increase in temperature itself.
The effect of warming is mediated by a novel temperature sensing mechanism involving ATP-sensitive potassium (KATP) channels.
As KATP channels are also sensitive to energy balance, our results suggest that orexin neurons play an important role in regulating energy balance by inducing sickness behaviour when body temperature is elevated.
Introduction
It is well known that a body temperature increase (during fever or in a warm environment) is accompanied by fatigue, behavioural inactivity and anorexia. Previous studies have demonstrated that lipopolysaccharide (LPS), the gram-negative bacteria-derived pyrogen that mimics infection, reduces c-Fos expression in orexin (hypocretin) neurons, suggesting that these neurons are suppressed during fever (Becskei et al. 2008; Park et al. 2008; Gaykema & Goehler, 2009). Suppression of orexin neurons during elevated body temperatures is suited to contribute to associated sickness behaviours given the diversity of physiological functions mediated by these neurons, including the induction and maintenance of wakefulness (Saper et al. 2005), locomotor activity (Kotz et al. 2006), sympathetic activation (Ferguson & Samson, 2003), thermogenesis (Yoshimichi et al. 2001) and behavioural responses to natural rewards and drugs of abuse (Harris et al. 2005). Thus, suppression of the orexin system can be expected to induce somnolence, inactivity, loss of appetite, anhedonia and reduced sympathetic outflow, which may be beneficial as a means to conserve energy during infection and/or as a negative feedback mechanism to keep body temperature in control. Indeed, orexin neurons have been suggested to mediate the suppression of behavioural activity (Grossberg et al. 2011) and palatable food intake induced by LPS (Park et al. 2008). It is currently unknown how orexin neurons are inhibited during LPS challenge, although it does not seem to involve direct action of pro-inflammatory mediators (Grossberg et al. 2011).
There are neurons that are capable of directly sensing ambient temperature, in particular within the hypothalamus. These so-called thermosensitive neurons are defined as either warm or cold sensitive by their excitatory or inhibitory response to an increase in temperature, respectively, and play critical roles in various physiological functions including the central regulation of body temperature. Given that orexin neurons are inhibited by the pyrogen LPS, it is plausible that orexin neurons are inhibited by an increase in brain temperature per se. To test this hypothesis, we conducted an in vitro electrophysiological study. Here we show that orexin neurons are indeed inhibited by elevated temperatures by utilizing a novel mechanism of temperature sensing that is KATP channel-dependent.
Methods
All experiments were performed following the Canadian Council on Animal Care guidelines and as approved by Memorial University Institutional Animal Care Committee. Male Sprague–Dawley rats (60–70 g) were obtained from the breeding colony at Memorial University.
Electrophysiology
Rats were deeply anaesthetized with halothane or isoflurane and decapitated, and brains were quickly removed. For the majority of experiments, 250 μm coronal hypothalamic slices were sectioned in ice-cold artificial cerebrospinal fluid (ACSF) composed of (in mm): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 18 NaHCO3, 2.5 glucose, 2 CaCl2, pH 7.30–7.35. Following dissection, slices were incubated in ACSF at 32–35°C for 30–45 min, then at room temperature until recording. In one series of experiments, brain slices were sectioned in Ca2+-free ACSF (CaCl2 replaced by MgCl2) and maintained at 37°C throughout the day including the dissection. Following dissection, these slices were initially kept in the Ca2+-free ACSF, and CaCl2 replaced MgCl2 at 0.5 mm increments over the next hour to reach a standard ACSF composition. All ACSF was continuously bubbled with O2 (95%)–CO2 (5%).
Patch-clamp recordings were performed on brain slices perfused with ACSF at 1.5–2 ml min−1 under an infrared-differential interference contrast/fluorescence microscope (DM LFSA, Leica Microsystems) using a Multiclamp 700B amplifier and pCLAMP 9.2 software (Molecular Devices, Sunnyvale, CA, USA). The conventional whole cell internal solution contained (mm): 120 potassium gluconate, 1 NaCl, 1 MgCl2, 1 CaCl2, 10 Hepes, 10 EGTA, 3 K2ATP, corrected to pH 7.30–7.35 with KOH. For loading cells with high ATP, K2ATP in internal solution was increased to 13 mm, and a minimum of 20 min following break-in was allowed for sufficient diffusion of ATP into the cell under investigation. For the perforated patch internal solution, amphotericin was dissolved in DMSO (60 mg ml−1 final) and added to (in mm): 110 KCl, 5 MgCl2, 40 Hepes, 10 EGTA, pH 7.30–7.35 with KOH. Cell-attached voltage-clamp recordings used the conventional whole cell internal solution. Electrodes had a tip resistance of 3–8 MΩ when filled by these internal solutions.
Upon achieving whole-cell access with a series/access resistance of 5–20 MΩ, a series of hyperpolarizing and depolarizing current steps were applied (−200 to +200 pA in 100 pA increments, 300–600 ms). Orexin neurons were identified based on previously described electrophysiological characteristics (Eggermann et al. 2003), namely the presence of Ih, rebound depolarization, minimal spike adaptation and spontaneous firing (Fig. 2Aa). In our hands, these characteristics have been highly reliable for identifying orexin neurons (over 90% successes in correlating with post hoc immunohistochemical phenotyping) (Parsons & Hirasawa, 2010). Non-orexin neurons (neurochemical phenotype unknown) examined in this study were found in the same region as orexin neurons (perifornical and lateral hypothalamic area), had relatively hyperpolarized resting membrane potential (−60.2 ± 2.0 mV, n= 16) and prominent fast and slow afterhyperpolarizing potentials (Fig. 2Ba) unlike those of orexin or melanin concentrating hormone neurons (Eggermann et al. 2003). These characteristics were used to define this group of neurons. Following all cell-attached experiments, the membrane was ruptured to enter whole-cell mode and the orexin phenotype was confirmed by their electrophysiological characteristics.
Figure 2. Thermosensing in the lateral hypothalamus/perifornical area is intrinsic and unique to orexin neurons.
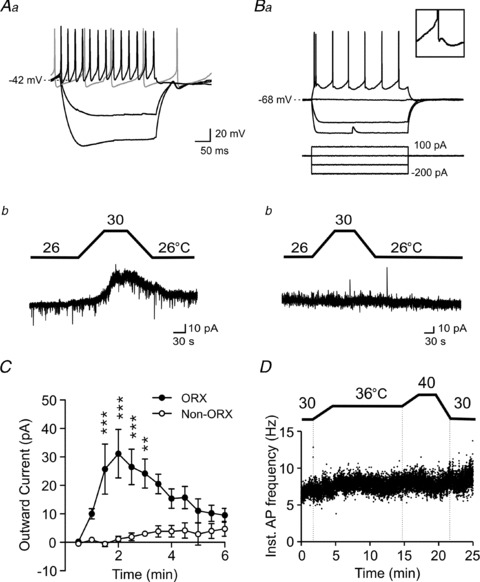
Aa, orexin neurons display Ih (sag upon hyperpolarization), rebound depolarization following hyperpolarization, minimal spike adaptation and spontaneous activity. The grey trace shows spontaneous activity during no current injection. Ab, a rise in temperature induces a reversible outward current in orexin neurons. Ba, top, characteristics of a typical non-orexin neuron examined in the lateral hypothalamus/perifornical area. Scale is the same as in Aa. Inset shows the characteristic afterhyperpolarization seen in this cell type. Bottom, the current clamp protocol used in Aa and Ba. Bb, temperature change does not affect these non-orexin neurons. C, time–effect plot of warming on orexin (n= 5) and non-orexin neurons (n= 4). Warming started at time zero and lasted for 3–4 min. The response is specific to orexin neurons. **P < 0.01, ***P < 0.001, two-way ANOVA with Bonferroni test. D, a representative cell attached patch experiment showing no apparent temperature effect on the firing activity of non-orexin neurons.
The bath temperature was controlled using an in-line heater (TC-324B, Warner Instruments, Hamden, CT, USA, or HW-30, Dagan Corp., Minneapolis, MN, USA). The thermoprobe was placed in close proximity to the slice in the recording chamber. Temperatures were increased at a rate of approximately 1–2°C min-1 and maintained at the peak test temperature for 1–10 min before reversing back to the starting temperature. There was no measurable change in the pH of ACSF within the temperature range used. Whole-cell voltage clamp experiments were performed at a holding potential of −50 mV with the exception of voltage ramps (−140 to −20 mV in 600 ms).
Tetrodotoxin (TTX) was from Alomone Labs (Jerusalem, Israel), tolbutamide and picrotoxin were obtained from Sigma-Aldrich, and DNQX and d-AP5 were from Ascent Scientific (abcamBiochemicals, Cambridge, MA, USA).
Tracing
Rats were anaesthetized with isoflurane (4% induction, 2% maintenance) and placed in a stereotaxic frame. A small cut was made in the scalp, a hole was drilled into the skull and 300 nl of green retrobeads (LumaFluor Inc., Durham, NC, USA) were injected unilaterally into the LC using a Hamilton syringe. The coordinates were modified from the rat brain atlas by Paxinos and Watson (2005) to adjust to young rats: 8.9 mm posterior and 1.3 mm lateral from the bregma, and 0.9–1.0 mm dorsal from the interaural line. Incisions were sutured and animals were allowed 5–7 days recovery to ensure sufficient transport of the retrograde tracer. Rats were then anaesthetised with halothane and 250 μm thick hypothalamic slices were obtained in ice-cold ACSF as described above. The brainstem was frozen immediately and sectioned to determine the injection site. When the retrobead injection was confirmed to be largely confined to the LC, perforated patch clamp recordings were performed using hypothalamic slices under the fluorescence/DIC optics. Only cells that were fluorescent and displayed the typical electrophysiological characteristics of orexin neurons were studied.
Statistical analysis
Action potential frequency, membrane potential and holding current were measured using Mini Analysis 6.0 (Synaptosoft, Inc., Decatur, GA, USA) and Clampfit 9.2 (Molecular Devices). Instantaneous action potential frequency was calculated using the time interval between two consecutive action potentials (1 s/interval = frequency in Hz). All data are expressed as means ± SEM. Statistical tests used included one- or two-way ANOVA with Bonferroni post hoc test for multiple-group comparisons and Student's paired or unpaired t test for two-group comparisons. A value of P < 0.05 was considered significant.
Results
Orexin neurons are thermosensitive
We first examined the effect of increasing the ambient temperature on the firing frequency of orexin neurons using cell-attached recordings. We found that an increase from 36 to 40°C significantly inhibited action potential firing, which reversed within several minutes of returning to 36°C (Fig. 1A–C). In some cases, kainate (0.5–1 μm) was added to ACSF to drive spontaneous activity as it was difficult to detect spontaneously active cells using cell-attached patch at this temperature range. The temperature effect was apparent regardless of the presence of kainate. We also used brain slices that were obtained under a ‘physiological’ dissection protocol in which the brain tissues were never exposed to temperatures below 37°C (see Methods). A majority of orexin neurons in these preparations were sensitive to an elevation from 37 to 39°C (Fig. 1C). Whole-cell conventional patch clamp recordings were also performed to test an increase from 26 or 28 to 30°C. This also resulted in a significant inhibition of orexin neuron firing (Fig. 1C).
Figure 1. Orexin neurons are temperature sensitive.
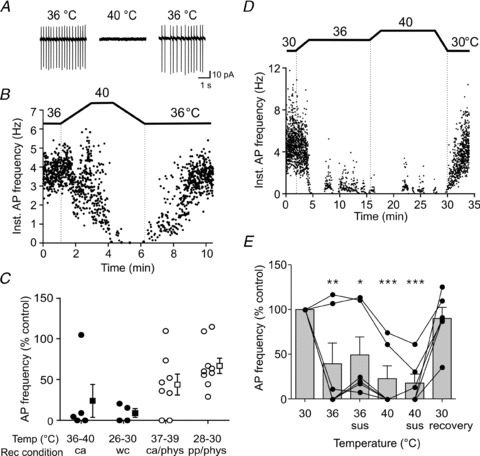
A and B, representative cell attached patch clamp traces (A) and time–effect plot of instantaneous action potential frequency (Inst. AP frequency) (B), showing that raising the temperature reversibly suppresses the activity of orexin neurons. C, summary data showing the effect of warming on the AP frequency shown by the percentage of control levels, tested by various basal/peak temperature combinations and recording conditions as indicated. ca: cell-attached, wc: whole cell, pp: perforated patch recording. Filled circles represent individual cells from hypothalamic slices sectioned in ice-cold ACSF, and open circles represent individual cells from slices prepared in physiological condition (indicated as ‘phys’ on the x-axis), i.e. always maintained at 37°C and never exposed to ice-cold temperatures. Filled and open squares with error bars indicate the mean and SEM for each group. Regardless of the baseline/peak temperatures during recordings, recording methods or the temperature during slice dissection, a rise in temperature significantly suppresses the firing of a majority of orexin neurons (P < 0.05 for all conditions, paired t test). D and E, a representative experiment (D) and grouped data (E, n= 6) demonstrating that the inhibitory effect of warming does not desensitize by prolonged clamping of the bath temperature at certain levels. Individual responses are shown to highlight an apparent cell-to-cell variability in the threshold of the temperature effect (connected circles represent repeated measures from individual cells). Sus: AP frequency was measured after the temperature was sustained at the indicated level for 10 min. *P < 0.05, **P < 0.01, ***P < 0.005 vs. 30°C, one-way ANOVA with Bonferroni post hoc test.
To determine whether the effect was long lasting, the bath temperature was clamped at 36°C and at 40°C for 10 min each while the firing frequency was monitored using cell attached mode (Fig. 1D and E). Compared to the baseline of 30°C, raising the temperature to 36°C strongly decreased the firing frequency of 4 out of 6 orexin neurons tested (to 2.9 ± 0.03% of control, Fig. 1E). These cells remained suppressed over the next 10 min period although 3 of 4 cells showed a partial recovery. During the next 10 min at 40°C, the firing activity remained suppressed. The remaining 2 of 6 cells were not suppressed but instead mildly excited at 36°C (Fig. 1E), while both were inhibited at 40°C and did not show desensitization at this temperature. These data suggest that each cell may have its own intrinsic window of temperature sensitivity and that there may be inhibitory as well as excitatory effects of warming with distinct temperature threshold, at least in some cells. Upon returning to 30°C, all cells resumed firing, showing their viability and the reversible nature of the temperature response. Overall in six cells, the effect of increasing the temperature from 30°C to 36°C or 40°C was statistically significant (Fig. 1E, P < 0.0001, one-way repeated measures ANOVA). Therefore, at least for the majority of orexin neurons, warming induces an inhibitory effect with minimal desensitization, at least within the 10–20 min time period investigated in the present study.
Since slice preparations and subsequent whole-cell recordings were generally more stable at subphysiological temperatures, these were used for most of the remaining study to investigate the mechanism underlying thermosensing by orexin neurons. In voltage clamp mode, we observed a clear temperature-induced outward current in orexin neurons in the presence of TTX (1 μm; Fig. 2Aa, Ab and C), suggesting that this is an intrinsic property of these neurons. In contrast, neighbouring non-orexin neurons with distinct electrophysiological characteristics did not respond to the same manipulation (Fig. 2Ba, Bb, C and D).
KATP channels mediate orexin neuron thermosensitivity
To determine the underlying mechanism for the inhibition of orexin neurons, we examined the voltage–current relationship during warming. Membrane potential was ramped from −140 to −20 mV and the resulting membrane currents were monitored in the presence of TTX at 26°C and 30°C. Subtraction of the current responses revealed a warming-induced inwardly rectifying current that reversed at −79.8 ± 2.0 mV (n= 5, Fig. 3A). These characteristics were suggestive of KATP channels, which we have previously shown in orexin neurons (Parsons & Hirasawa, 2010).
Figure 3. KATP channels mediate orexin neuron thermosensitivity.
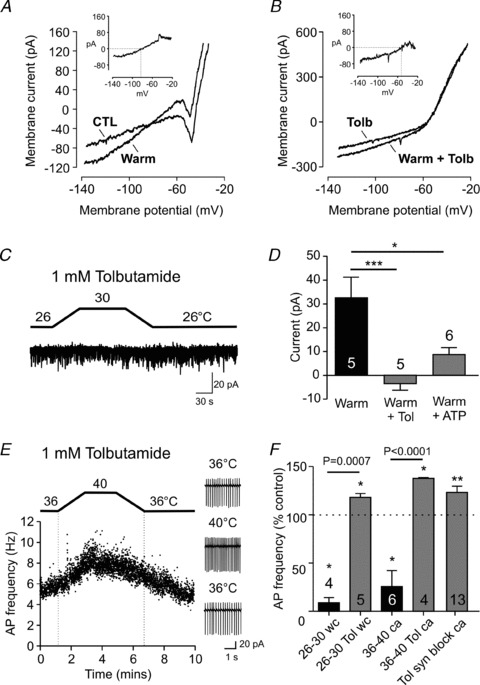
A, representative current responses to voltage ramps (−140 to −20 mV) recorded in the presence of TTX at baseline (CTL) and 4°C warmer conditions. Inset, a warming-induced current trace generated by subtracting CTL from Warm. B, the same experimental protocol as (A) except performed in the presence of tolbutamide, the KATP channel blocker. The reversal potential is more depolarized compared to the control condition shown in A. C, tolbutamide prevents the warming effect seen at the holding potential of −50 mV. D, summary graph showing that tolbutamide significantly blocks the warming-induced outward current (Warm + Tol). High ATP concentration (13 mm) in the intracellular solution also significantly attenuates the response to warming (Warm + ATP). *P < 0.05, ***P < 0.005, unpaired t test. E and F, tolbutamide unmasks an excitatory effect at physiological (36–40°C) and subphysiological (26–30°C) temperatures, which persists even when glutamatergic and GABAergic transmission is blocked by DNQX, d-AP5 and picrotoxin (syn block). wc: whole cell, ca: cell attached recording. *P < 0.05, **P < 0.01 vs. respective baseline (paired t test). P values indicated above the horizontal bars correspond to statistical results between groups (unpaired t test). For D and F, numbers in bars represent the number of cells examined in each group.
To test this idea, the KATP channel blocker tolbutamide (1 mm) was used. After a 5 min pretreatment with tolbutamide, the warming-induced current was abolished at the holding potential of −50 mV (Fig. 3C and D). We also tested the effect of loading the postsynaptic cell with a high concentration of ATP (13 mm ATP in the pipette instead of 3 mm), which significantly attenuated the warming-activated outward current (Fig. 3D), further supporting the role of KATP channels. In addition, tolbutamide unmasked a warming-induced excitation in cell attached recordings (Fig. 3E and F). This excitation appears to be a direct effect, as it persisted in the presence of antagonists for glutamate (DNQX 10 μm, d-AP5 50 μm) and GABAA receptors (picrotoxin 50 μm) (Fig. 3F), and tolbutamide uncovered a warm-induced current with a reversal potential of −49.7 ± 4.4 mV (P < 0.0005 vs. control, n= 5, Fig. 3B), which may represent a non-selective cation current. These results suggest that warming has both inhibitory and excitatory effects in orexin neurons, but the KATP current is the dominant one in our recording conditions, resulting in inhibition.
Orexinergic projection to the locus coeruleus is thermosensitive
Orexin neurons send a dense projection to the locus coeruleus (LC) where they activate LC neurons to increase arousal (Hagan et al. 1999). Suppression of this pathway may contribute to sleepiness and lethargy at high body temperatures. Thus, we asked whether orexin neurons projecting to the LC are temperature sensitive. To do so, we injected a fluorescent tracer into the right LC to retrogradely label neurons that send projections to this area. In animals with the injection site largely confined to the LC, fluorescent cells were observed bilaterally within the orexin-containing regions, the lateral hypothalamus and perifornical area, consistent with the finding that the orexin innervation of the LC is bilateral and originates from both of these hypothalamic areas (Espana et al. 2005; Gonzalez et al. 2012). Few, if any, fluorescent cells were found in the same hypothalamic regions in animals whose tracer injection landed outside the LC. We found that all LC-projecting orexin neurons tested decreased their firing activity when temperature was increased (Fig. 4). Thus, excitatory orexinergic projections to the wake-promoting LC are also thermosensitive.
Figure 4. Orexin neurons projecting to the locus coeruleus are thermosensitive.
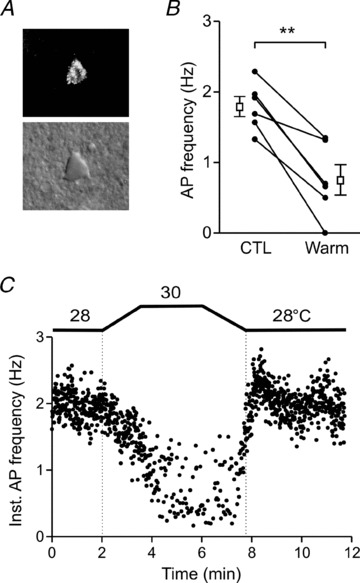
A, a representative fluorescence (top) and associated differential interference contrast (bottom) image of an orexin neuron retrogradely labelled from the LC. B, action potential frequency in control (CTL) and 2°C warmer condition (Warm). Circles connected by lines correspond to data from individual cells. Open squares and error bars indicate the mean and SEM for each condition. **P < 0.01 (paired t test). C, a representative plot of the action potential frequency of an LC-projecting orexin neuron, responding to 2°C increase in bath temperature.
Discussion
The present study demonstrates a unique thermosensitive property of orexin neurons mediated by KATP channels. The most obvious difference compared to previously described thermosensitive neurons is that orexin neurons are inhibited by an increase in temperature (cold-sensitive) whereas the majority of thermosensitive neurons are excited (warm-sensitive). Thermosensitive neurons are known to exist most notably within the preoptic area of the hypothalamus where approximately 30% of neurons are warm sensitive while less than 5% are cold sensitive (Dean & Boulant, 1989). This trend appears to hold true for adjacent diencephalic regions (Dean & Boulant, 1989).
The mechanisms underlying warm-sensitivity include a faster inactivation of A-currents (Griffin et al. 1996), modulation of afterhyperpolarization (Burgoon & Boulant, 2001) and an activation of several ion channels such as non-selective cation channels (Hori et al. 1999), transient receptor potential vanilloid channels (Sharif-Naeini et al. 2008) and TTX-sensitive Na+ channels (Kiyohara et al. 1990). One of these, likely to be the non-selective cation channels, may be involved in warm-induced excitation of orexin neurons that was unmasked by the KATP channel blocker. Cold sensitivity in other central neurons has been mainly attributed to a synaptically mediated inhibition by neighbouring warm-sensitive neurons (Boulant, 2000). In addition, two-pore domain potassium channels TRAK-1, TRAAK and TREK-2 become activated with increasing temperatures in cerebellar granule neurons and dorsal root ganglion neurons (Kang et al. 2005). To the best of our knowledge, the present study is the first to describe KATP channels as a mediator of neuronal thermosensitivity.
Our study indicates that orexin neurons can directly sense temperature changes, since the temperature-induced KATP current persisted in the presence of TTX and was abolished by postsynaptic loading of a high concentration of ATP. The precise mechanism by which temperature changes are transduced to KATP currents remains unknown and is of interest for future study. One possibility is via an increased ATP hydrolysis due to an accelerated metabolic rate at higher temperature (Gillooly et al. 2001). This can lead to a reduction in ATP or pH, which would activate KATP channels (Xu et al. 2001; Nichols, 2006). Alternatively, reactive oxygen species may activate KATP channels (Avshalumov et al. 2005), whose levels of production by mitochondria are influenced at febrile temperatures in isolated mitochondria (Zukiene et al. 2010). It seems unlikely that the presence of KATP channels is the sole determinant of thermosensitivity, because cold-sensitive neurons are uncommon despite the wide expression of KATP channels in the brain. For example, the supraoptic nucleus contains warm-sensitive neurons (Sharif-Naeini et al. 2008) while expressing a high density of KATP channels (Thomzig et al. 2005). This is somewhat akin to the fact that KATP channel-mediated glucosensing ability is only seen in specialized neurons (Levin et al. 2004). It may be that the relative proximity of the channels to the microdomains of modulatory factors is an important determinant.
It appears that the majority of orexin neurons are temperature sensitive and responsive to a wide range of temperatures including both a subphysiological and physiological range. Although slight desensitization was sometimes apparent, we never observed full recovery of basal firing rate when the temperature was elevated and sustained. It should be noted, however, that whole-cell patch clamp recordings of orexin neurons have been conducted at various bath temperatures, as high as 37°C (Schone et al. 2011), in which spontaneous orexin neuron activity appears to be preserved. This may be an indication that at least some degree of desensitization occurs over longer periods. On the other hand, using the cell-attached patch, we generally had difficulty finding spontaneously active neurons within the LH/PFA at temperatures of 36°C or higher and required bath application of kainite to induce firing activity. Thus, the basal excitability of orexin neurons may also be influenced by recording methods as has been shown previously (Liu et al. 2011).
We also found that the threshold for warming-induced inhibition varied among neurons, as an increase within the subphysiological range (<36°C) was sufficient to inhibit some cells while others required an elevation to higher temperature (>36°C) before a response was observed. These differences were not correlated with electrophysiological features that distinguish subpopulations of orexin neurons into D- or H-type based on the presence or absence of firing immediately upon relief from hyperpolarizing current steps (Schone et al. 2011). Interestingly, in two cells that were inhibited only at higher temperature (36–40°C), a modest excitatory response was seen at lower temperature (30–36°C) (Fig. 1E). Therefore, in a given orexin neuron the inhibitory and excitatory effects may not necessarily have the same temperature threshold, and the ambient temperature and other factors (such as neurotransmitter and nutrient environment) would be likely to determine which effect would outweigh the other.
During infection, fever and sickness behaviours are thought to aid in suppressing multiplication of pathogens and conserving energy for immune responses. Cytokines, which can increase the excitability of neurons mainly by altering synaptic transmission (Galic et al. 2012), play a critical role in the initiation of sickness behaviours (Kent et al. 1996; Pecchi et al. 2009). However, orexin neurons do not seem to receive direct proinflammatory signals (Grossberg et al. 2011). This and the present study are consistent with the idea that direct thermosensing is the main cellular mechanism for LPS-induced suppression of orexin neurons. This mechanism may be involved in behavioural responses to fever or hyperthermia that can be activated in parallel to cytokine signalling (Grossberg et al. 2011). Since orexin A stimulates LC neurons to induce arousal (Hagan et al. 1999), a decrease in the excitatory orexinergic signalling to the LC, as we have shown, provides a putative mechanism that contributes to somnolence and lethargy during elevated body temperature. In addition, orexins mediate thermogenesis (Yoshimichi et al. 2001) and food intake (Sakurai et al. 1998), which is also thermogenic (diet-induced thermogenesis), suggesting that the warming-inhibited property of orexin neurons may also serve as a negative feedback within the thermoregulatory system.
As KATP channels also act as sensors for cellular metabolic state and extracellular fuel availability (Parsons & Hirasawa, 2010; Nichols, 2006), our findings indicate that orexin neurons are responsible for integrating the state of energy balance and body temperature and for optimizing sickness behaviours and resource allocation accordingly. This is critical as there would be different levels of necessity to conserve or dissipate energy depending on the energy status of an organism. In all, the thermosensitivity of orexin neurons may constitute an important component of the physiological mechanism for maintaining energy homeostasis when body temperature is elevated.
Acknowledgments
This work was funded by the Canadian Institutes of Health Research. M.P.P was a recipient of the Heart of Stroke Foundation of Canada Fellowship and N.B.-W. holds a Heart and Stroke Foundation of Newfoundland and Labrador Scholarship.
Glossary
- d-AP5
d-2-amino-5-phosphonopentanoic acid
- CTL
control
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- KATP channel
ATP-sensitive potassium channel
- LC
locus coeruleus
- LPS
lipopolysaccharide
- TTX
tetrodotoxin
Author contributions
M.P.P. and M.H. contributed to the conception and design of the experiments, collection, analysis and interpretation of the data and drafting/revising of the manuscript. N.B.-W. and V.L. contributed to the collection and analysis of the data and revising the manuscript for important intellectual content. All authors approved the final version.
References
- Avshalumov MV, Chen BT, Koos T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci. 2005;25:4222–4231. doi: 10.1523/JNEUROSCI.4701-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becskei C, Riediger T, Hernadfalvy N, Arsenijevic D, Lutz TA, Langhans W. Inhibitory effects of lipopolysaccharide on hypothalamic nuclei implicated in the control of food intake. Brain Behav Immun. 2008;22:56–64. doi: 10.1016/j.bbi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31:S157–S161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- Burgoon PW, Boulant JA. Temperature-sensitive properties of rat suprachiasmatic nucleus neurons. Am J Physiol Regul Integr Comp Physiol. 2001;281:R706–R715. doi: 10.1152/ajpregu.2001.281.3.R706. [DOI] [PubMed] [Google Scholar]
- Dean JB, Boulant JA. In vitro localization of thermosensitive neurons in the rat diencephalon. Am J Physiol Regul Integr Comp Physiol. 1989;257:R57–R64. doi: 10.1152/ajpregu.1989.257.1.R57. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Bayer L, Serafin M, Saint-Mleux B, Bernheim L, Machard D, Jones BE, Muhlethaler M. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci. 2003;23:1557–1562. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol. 2005;481:160–178. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Samson WK. The orexin/hypocretin system: a critical regulator of neuroendocrine and autonomic function. Front Neuroendocrinol. 2003;24:141–150. doi: 10.1016/s0091-3022(03)00028-1. [DOI] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front Neuroendocrinol. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RP, Goehler LE. Lipopolysaccharide challenge-induced suppression of Fos in hypothalamic orexin neurons: their potential role in sickness behavior. Brain Behav Immun. 2009;23:926–930. doi: 10.1016/j.bbi.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Convergent inputs from electrically and topographically distinct orexin cells to locus coeruleus and ventral tegmental area. Eur J Neurosci. 2012;35:1426–1432. doi: 10.1111/j.1460-9568.2012.08057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JD, Kaple ML, Chow AR, Boulant JA. Cellular mechanisms for neuronal thermosensitivity in the rat hypothalamus. J Physiol. 1996;492:231–242. doi: 10.1113/jphysiol.1996.sp021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg AJ, Zhu X, Leinninger GM, Levasseur PR, Braun TP, Myers MG, Jr, Marks DL. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J Neurosci. 2011;31:11376–11386. doi: 10.1523/JNEUROSCI.2311-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hori A, Minato K, Kobayashi S. Warming-activated channels of warm-sensitive neurons in rat hypothalamic slices. Neurosci Lett. 1999;275:93–96. doi: 10.1016/s0304-3940(99)00732-6. [DOI] [PubMed] [Google Scholar]
- Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bret-Dibat JL, Kelley KW, Dantzer R. Mechanisms of sickness-induced decreases in food- motivated behavior. Neurosci Biobehav Rev. 1996;20:171–175. doi: 10.1016/0149-7634(95)00037-f. [DOI] [PubMed] [Google Scholar]
- Kiyohara T, Hirata M, Hori T, Akaike N. Hypothalamic warm-sensitive neurons possess a tetrodotoxin-sensitive sodium channel with a high Q10. Neurosci Res. 1990;8:48–53. doi: 10.1016/0168-0102(90)90056-k. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, Levine JA. Orexin a mediation of time spent moving in rats: Neural mechanisms. Neuroscience. 2006;142:29–36. doi: 10.1016/j.neuroscience.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- Liu ZW, Gan G, Suyama S, Gao XB. Intracellular energy status regulates activity in hypocretin/orexin neurones: a link between energy and behavioural states. J Physiol. 2011;589:4157–4166. doi: 10.1113/jphysiol.2011.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Park SM, Gaykema RP, Goehler LE. How does immune challenge inhibit ingestion of palatable food? Evidence that systemic lipopolysaccharide treatment modulates key nodal points of feeding neurocircuitry. Brain Behav Immun. 2008;22:1160–1172. doi: 10.1016/j.bbi.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP, Hirasawa M. ATP-sensitive potassium channel-mediated lactate effect on orexin neurons: implications for brain energetics during arousal. J Neurosci. 2010;30:8061–8070. doi: 10.1523/JNEUROSCI.5741-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fifth edition. Elsevier Academic Press; 2005. [Google Scholar]
- Pecchi E, Dallaporta M, Jean A, Thirion S, Troadec JD. Prostaglandins and sickness behavior: old story, new insights. Physiol Behav. 2009;97:279–292. doi: 10.1016/j.physbeh.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Schone C, Venner A, Knowles D, Karnani MM, Burdakov D. Dichotomous cellular properties of mouse orexin/hypocretin neurons. J Physiol. 2011;589:2767–2779. doi: 10.1113/jphysiol.2011.208637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif-Naeini R, Ciura S, Bourque CW. TRPV1 gene required for thermosensory transduction and anticipatory secretion from vasopressin neurons during hyperthermia. Neuron. 2008;58:179–185. doi: 10.1016/j.neuron.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Thomzig A, Laube G, Pruss H, Veh RW. Pore-forming subunits of K-ATP channels, Kir6.1 and Kir6.2, display prominent differences in regional and cellular distribution in the rat brain. J Comp Neurol. 2005;484:313–330. doi: 10.1002/cne.20469. [DOI] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Wu J, Giwa LR, Abdulkadir L, Sharma P, Jiang C. Direct activation of cloned KATP channels by intracellular acidosis. J Biol Chem. 2001;276:12898–12902. doi: 10.1074/jbc.M009631200. [DOI] [PubMed] [Google Scholar]
- Yoshimichi G, Yoshimatsu H, Masaki T, Sakata T. Orexin-A regulates body temperature in coordination with arousal status. Exp Biol Med (Maywood) 2001;226:468–476. doi: 10.1177/153537020122600513. [DOI] [PubMed] [Google Scholar]
- Zukiene R, Nauciene Z, Ciapaite J, Mildaziene V. Acute temperature resistance threshold in heart mitochondria: Febrile temperature activates function but exceeding it collapses the membrane barrier. Int J Hyperthermia. 2010;26:56–66. doi: 10.3109/02656730903262140. [DOI] [PubMed] [Google Scholar]


