Abstract
There is evidence that developmental-like plasticity can be reactivated in the adult visual cortex. Although activity-dependent transcription factors underlying the process of plasticity reactivation are currently unknown, recent studies point towards NPAS4 as a candidate gene for the occurrence of plasticity in the adult visual system. Here, we addressed whether NPAS4 is involved in the reinstatement of plasticity by using the monocular deprivation protocol and long-term fluoxetine treatment as a pharmacological strategy that restores plasticity in adulthood. A combination of molecular assays for gene expression and epigenetic analysis, gene delivery by lentiviral infection, shRNA interference and electrophysiology as a functional read-out, revealed a previously unknown role for the transcription factor NPAS4 in the regulation of adult visual cortical plasticity. We found that NPAS4 overexpression restores ocular dominance plasticity in adult naïve animals whereas NPAS4 down-regulation prevents the plastic outcome caused by fluoxetine in adulthood. Our findings lead the way to the identification of novel therapeutic targets for pathological conditions where reorganization of neuronal networks would be beneficial in adult life.
Key points
Transcription factors at the basis of plasticity in the adult visual system are unknown.
Enhanced levels of NPAS4 transcription factor parallel visual cortical plasticity in adult life.
Overexpression of NPAS4 restores plasticity in the adult visual cortex.
NPAS4 down-regulation prevents the plastic outcome caused by fluoxetine (FLX) in adulthood.
NPAS4 regulates the expression of plasticity genes in the adult visual cortex.
Introduction
Neuronal networks within sensory systems are shaped by experience in a use-dependent manner to optimally represent sensory stimuli (Katz & Shatz, 1996). The ability of environmental inputs to modify brain structure and function has been extensively studied in the visual system. Visual cortex (VC) circuitries are markedly sensitive to experience during the critical period (CP) in early life (Berardi et al. 2000) but a decline of plasticity occurs over postnatal development (Hensch, 2005). Early electrophysiological studies demonstrated that occlusion of one eye (monocular deprivation, MD) during the CP leads to an ocular dominance (OD) shift of visual cortical neurons in favour of the open eye (Wiesel & Hubel, 1963; Hubel & Wiesel, 1970). In addition, the deprived eye becomes amblyopic: its spatial acuity and contrast sensitivity are markedly impaired. As MD does not cause amblyopia in adult life, this early temporal window characterized by an enhanced brain susceptibility to sensory experience is a typical example of a CP.
Among the different factors that regulate the CP for VC plasticity, inhibitory processes seem to play a key role (reviewed in Hensch, 2005; Sale et al. 2010). Visual experience signals the time-course of the CP by promoting the transfer of the protein Otx2 from the retina to the VC, where Otx2 promotes the maturation of parvalbumin-positive GABAergic interneurons (Sugiyama et al. 2008). An initial threshold of inhibition then triggers the CP in which neural networks are highly susceptible to experience (Hensch et al. 1998; Fagiolini & Hensch, 2000), while a second inhibitory threshold signals the end of this phase of enhanced plasticity (Huang et al. 1999). In agreement with this notion, it has been reported that a pharmacological reduction of inhibitory transmission effectively restores OD plasticity in adulthood (Harauzov et al. 2010). Furthermore, experimental protocols such as dark exposure (He et al. 2006, 2007; Montey & Quinlan, 2011), environmental enrichment (Sale et al. 2007; Baroncelli et al. 2010), food restriction (Spolidoro et al. 2011), long-term fluoxetine (FLX) treatment (Maya-Vetencourt et al. 2008, 2011; Chen et al. 2011), and exogenous IGF-1 administration (Maya-Vetencourt et al. 2012), all promote plasticity late in life by reducing the intracortical inhibitory/excitatory (I/E) ratio. This has prompted the search for endogenous factors with the potential to enhance experience-dependent plasticity in adult life by modulating the intracortical I/E balance in the visual system.
In rodents, experimental protocols based upon the enhancement of environmental stimulation levels, genetic manipulations and pharmacological treatments have revealed that the enhanced action of either long-distance neuromodulatory systems (e.g. serotonin and acetylcholine) (Maya-Vetencourt et al. 2008, 2011; Baroncelli et al. 2010; Morishita et al. 2010) or IGF-1 signalling (Maya-Vetencourt et al. 2012) modulates the intracortical I/E balance in favour of excitation (Amar et al. 2010; Moreau et al. 2010), which, in turn, sets in motion cellular and molecular events that eventually mediate the expression of genes associated with structural and functional modifications in the adult visual system (reviewed in Tropea et al. 2009; Maya-Vetencourt & Origlia, 2012). Experience-dependent modifications of chromatin structure that control gene expression are, in fact, recruited as targets of plasticity-associated processes in adult life (Putignano et al. 2007; Maya-Vetencourt et al. 2011; Spolidoro et al. 2011). As yet, however, transcriptional regulatory mechanisms that lie behind the process of plasticity reactivation in the adult visual system remain to be investigated.
There is evidence that the activity-dependent transcription factor NPAS4 plays an important role in brain development and neuronal survival (Flavell & Greenberg, 2008). It has been recently demonstrated that NPAS4 promotes the formation of inhibitory synapses in the developing visual system (Lin et al. 2008), which suggests a role for NPAS4 in controlling the homeostatic I/E balance and therefore in the regulation of visual cortical plasticity. Moreover, NPAS4 interacts with several neuronal activity-regulated gene expression promoters (Kim et al. 2010), and mediates brain-derived neurotrophic factor (BDNF) expression (Ooe et al. 2004; Lin et al. 2008; Pruunsild et al. 2011; Ramamoorthi et al. 2011). As the reinstatement of plasticity in the adult VC is accompanied by enhanced BDNF-trkB signalling (Maya-Vetencourt et al. 2008, 2011; Baroncelli et al. 2010), the possibility arises that NPAS4 may be involved in the process of plasticity reactivation. However, the role of NPAS4 in mediating phenomena of plasticity in the VC is currently unknown. Here, we report that NPAS4 is a key mediator of plasticity in the adult rat visual system.
Methods
Ethical approval
All of the procedures were approved by the Italian ministry of public health and complied with the European Union guidelines and policies (directive86/609/EEC) on animal experimentation. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilise alternatives to in vivo techniques, if available.
Animal treatment and FLX administration
A total of 65 adult Long–Evans hooded rats (Charles Rivers) were used in this study. Animals were group-housed under standard conditions with food and water ad libitum in Plexiglas cages (40 × 30 × 20 cm) and kept in a 12 :12 h light–dark cycle. Adult rats at postnatal day 70 (P70) were systemically treated with FLX (fluoxetine-hydrochloride; 0.2 mg/ml; drinking water) for either 23 days or 28 days. Control animals were housed under the same standard conditions except for chronic treatment with FLX.
Surgical procedures
Two days and 1 week of MD was performed by eyelid suture at the beginning of the last week of chronic FLX, vehicle administration or viral infection (day 21 of treatment). Adult animals (P90), under treatment, were anaesthetized with avertin (i.p. 1 ml/100 g of weight) and mounted on a stereotaxic apparatus to be monocularly deprived. Eyelid closure was inspected daily until complete cicatrization; animals with even minimal spontaneous reopening were excluded. Great care was taken during the first days after MD to prevent inflammation or infection of the deprived eye through topical application of antibiotic and cortisone.
Real-time PCR
After respective treatments, dissection of the binocular region of the primary VC (Oc1b) was carried out and RNA purification performed according to the standard Trizol procedure (Invitrogen). Purified RNA was treated with DNAse (Fermentas) and cDNA was synthesised from 1 μg of RNA (Invitrogen). Real-time PCR was carried out (see Table 1 for primers details) to determine relative enrichment in the samples using the Syber Green method according to the manufacturer's instructions (SYBR Green I master, Light cycler 480, Roche Diagnostics). The comparative Ct method (Livak & Schmittgen, 2001) was used to determine the normalized changes of the target gene relative to a calibrator reference. In particular, for chromatin immunoprecipitation (ChIP) samples, values were normalized on Eef2 Ct whereas for mRNA quantification samples were normalized to GAPDH levels. As calibrator reference we referred to Ct from water-treated animal samples.
Table 1.
Detailed description of the oligos (sense/antisense) used to amplify genes of interest
| Gene | Oligo sense | Oligo antisense |
|---|---|---|
| NPAS4 | CAGGATGACTCACACTGACAGTATTTTTAG | GTGGGAGAAGAGCTATTTATATCACCAG |
| EeF2 | CACTCCCAAGGCAGTTCAAG | TATAGGTCGACGCCGGTTGG |
Chromatin immunoprecipitation assay
After respective treatments, neural tissues (Oc1b) were fixed in 1% PFA for 15 min, rinsed with PBS, and processed for chromatin immunoprecipitation using the ChIP Assay Kit (Upstate cat. 17-295). Acetyl-Histone3 K9 (Millipore 06-599) and 3methyl-Histone3 K27 (Millipore 07-449) were used. See Table 1 for the amplification of gene of interest.
Western blotting
Protein extracts from neural tissues or cultured neurons were loaded on 7.5% acrylamide gel. Proteins were transferred onto PVDF membranes and protein detection performed using a SNAP id system (Millipore) according to manufacturer's instruction. NPAS4 antibody (rabbit 1:2000) was a generous gift from Michael Greenberg.
Vectors and cloning
NPAS4 cds were amplified from rat cDNA using primers (see Table 2) carrying AgeI and XhoI cloning sites and cloned into Syn-LEN vector (custom lentiviral vector with synapsin I promoter). NPAS4-ShRNA and control-ShRNA with a scrambled sequence (Table 2, underlined), were cloned into the PLL 3.7 (http://www.addgene.com).
Table 2.
Detailed description of the oligos (sense/antisense) used to amplify genes of interest
| Gene | Oligo sense | Oligo antisense |
|---|---|---|
| NPAS4 | ATGTACCGATCCACCAAGGG | TCAAAACGTTGGTTCCCCTCC |
| NPAS4-shRNA | GGTTGACCCTGATAATTTA | GGTTCAGCGTCATAATTTA |
In vivo brain infection
The stereotaxic gene delivery in the rat brain was performed as previously described (Cetin et al. 2006). Briefly, in vivo Oc1b infections were carried out in adult animals under isofluorane anaesthesia. Experiments of NPAS4 overexpression were obtained by using the Syn-LEN vector whereas NPAS4 down-regulation was achieved by means of lentiviral delivery of NPAS4-RNA interference (shRNAi). To ensure lentiviral infection in the binocular region of the primary visual cortex, each animal received six different lentiviral injections in Oc1b. Injections were performed at 3, 4 and 5 mm lateral to lambda while at 0.5 mm anterior and 0.5 mm posterior, respectively, to lambda. To ensure appropriate diffusion of lentiviral infection, a volume of 200 nl was infused at 100 nl min−1 flow and at two different depths, 100 μm and 400 μm, per injection site. Each injection, both at superficial and deep layers, was followed by a 2 min pause to allow diffusion to occur. The virus titre used was: 1 × 108 TU ml−1; 1 TU, 1 × 103 lentiviral particles. No damage of Oc1b was observed after infections in any of the experimental groups.
Visual evoked potentials and single units
After respective treatments, adult rats (P100) were anaesthetized with urethane (i.p. 0.7 ml/100 g of weight; 20% solution in saline) by i.p. injection and placed in a stereotaxic frame. Additional doses of urethane were used to keep the anaesthesia level stable throughout the experiment. Body temperature was continuously monitored and maintained at ∼37°C by a thermostated electric blanket during the experiment. An ECG was continuously monitored. A hole was carefully drilled in the skull, corresponding to the binocular portion of the primary visual cortex (Oc1b) contralateral to the deprived eye. After exposure of the brain surface, the dura was removed, and a micropipette (2 MΩ) filled with NaCl (3 m) was inserted into the cortex 5 mm lateral to lambda. Both eyes were fixed and kept open by means of adjustable metal rings surrounding the external portion of the eye bulb. Alterations of binocularity and visual acuity (VA) were measured by using visual evoked potentials (VEPs). During recording through one eye, the other was covered by a black adhesive tape. To record VEPs, the electrode was advanced at a depth of 100 or 400 μm within the cortex (to correspond with the lentiviral infection sites). At these depths, VEPs had their maximal amplitude. Signals were band-pass filtered (0.1–100 Hz), amplified and fed to a computer for analysis, as described previously (Huang et al. 1999). Briefly, at least 128 events were averaged in synchrony with the stimulus contrast reversal. Transient VEPs in response to abrupt contrast reversal (0.5 Hz) were evaluated in the time domain by measuring the peak-to-baseline amplitude and peak latency of the major negative component. Visual stimuli were horizontal sinusoidal gratings of different spatial frequencies and contrast, generated by a VSG2/2 card running custom software and presented on a monitor (20 cm × 22 cm; luminance 15 cd m−2) positioned 20 cm from the rat's eyes and centred on the previously determined receptive fields. Visual acuity (VA) was obtained by extrapolation to zero amplitude of the linear regression through the last four to five data points in a curve where VEP amplitude is plotted against log spatial frequency. Binocularity (OD) was assessed calculating the contralateral to ipsilateral (C/I) VEP ratio, i.e. the ratio of VEP amplitudes recorded by stimulating the eye respectively contralateral and ipsilateral to the visual cortex where recording is performed. To prevent sampling bias, VEPs were recorded at three different penetrations within infected areas in Oc1b and at 100 μm and 400 μm depths for each penetration.
For single units recordings the position of receptive fields were mapped using a hand-held stimulator. Only cells with receptive fields within 20 deg of the vertical meridian were included in the analysis. Spontaneous activity, peak response and receptive field size were determined from peri-stimulus time histograms recorded in response to computer-generated bars, averaged over 10–20 stimulus presentations. Ocular dominance (OD) classes were evaluated according to the method of Wiesel & Hubel (1963). Neurons in OD class 1 were driven only by stimulation of the contralateral eye; neurons in OD classes 2 and 3 were binocular and preferentially driven by the contralateral eye; neurons in OD class 4 were equally driven by the two eyes; neurons in OD classes 5 and 6 were binocular and preferentially driven by the ipsilateral eye; neurons in OD class 7 were driven only by the ipsilateral eye. For each animal, the bias of the OD distribution toward the contralateral eye (contralateral bias index (CBI)) was calculated as follows: CBI =[(N(1) −N(7)) + 2/3(N(2) −N(6)) + 1/3(N(3) −N(5)) +NTot]/2NTot, where N(i) is the number of cells in class i, and NTot is the total number of recorded cells in a specific animal. Additionally, for each cell an OD score was calculated as follows: ([peak(ipsi) − baseline(ipsi)]−[peak(contra) − baseline(contra)])/([peak(ipsi) − baseline(ipsi)]+[peak(contra) − baseline(contra]), where peak is the maximal spike frequency evoked by visual stimulation, ipsi is the ipsilateral eye, baseline is the mean spiking frequency in the absence of stimulation, and contra is the contralateral eye. OD score cumulative distributions were computed for each experimental group.
Immunohistochemistry
Brains were snap-frozen and then sectioned on a cryostat. Slices mounted on slides were fixed for 30 min in 4% paraformaldehyde (PFA) and incubated in blocking solution (3% BSA, Triton X-100, 0.2% Tween-20 in PBS) at room temperature for 4 h. Subsequently, sections were incubated with primary antibody overnight at 4°C and with secondary antibody at room temperature for 2 h, both in blocking solution. Sections were mounted on slides with Gel Mount (Sigma). NPAS4 antibody (rabbit 1:300) was a generous gift from Michael Greenberg.
Results
The process of plasticity reactivation is accompanied by an increased NPAS4 mRNA expression and reduced histone methylation status at the NPAS4 promoter
We initially used real-time PCR to investigate the expression of NPAS4 in the VC of adult rats that were long-term treated with FLX (Fig. 1A, Table 1). We previously demonstrated that 4 weeks (28 days) of FLX administration restores plasticity in the adult VC (Maya-Vetencourt et al. 2008). As we were interested in early molecular mechanisms underlying the process of plasticity reactivation, we analysed gene expression 2 days after the beginning of the last week of pharmacological treatment (23 days of treatment, D23) (Fig. 1A). Interestingly, an increased expression of NPAS4 mRNA was observed in FLX-treated rats (t test, P < 0.05, n= 8) as compared with controls (Fig. 1B). Chromatin immunoprecipitation (ChIP) analysis revealed that a marked decrease (t test, P < 0.05, n= 8) in the tri-methylation of Lys27 of histone H3 at the NPAS4 promoter region (a marker of inactive chromatin) accompanied the enhanced NPAS4 mRNA expression (Fig. 1B, inset 1, Table 1), suggesting a de-repressed state of the gene promoter area. No change in acetylation of Lys9 of H3 (t test, P= 0.1, n= 8) was observed (Fig. 1B, inset 2). Western blot analysis confirmed that NPAS4 protein levels were significantly increased in the VC of FLX-treated rats, as compared with controls, after 2 days of MD or binocular vision during the last week of pharmacological treatment (D23) (Fig. 1C). These findings suggest that the activity-dependent expression of NPAS4 may set in motion early physiological mechanisms that lie behind the process of plasticity reactivation in the adult visual system.
Figure 1. Increased NPAS4 expression parallels the fluoxetine-induced reinstatement of plasticity in the adult visual system.

A, experimental protocol used for long-term FLX administration (28 days). B, long-term treatment with FLX increases NPAS4 mRNA levels in the VC of adult animals (t test, P < 0.05, n= 8). Inset 1: a marked decrease of H3K27 tri-methylation status at the NPAS4 promoter region (t test, P < 0.05, n= 8) was observed in the VC after long-term FLX administration. Inset 2: no change of H3K9 acetylation at the NPAS4 promoter occurred after pharmacological treatment (t test, P= 0.1, n= 8). C, long-term FLX administration increases NPAS4 protein levels (t test, P < 0.05, n= 8) in the VC of adult animals after 2 days of either MD or binocular vision (Bin Vision; D23) during the last week of treatment.
Lentiviral NPAS4 overexpression restores susceptibility to MD in adulthood
To address the functional relevance of NPAS4 in the reinstatement of adult visual cortical plasticity, we next overexpressed it in the VC of naïve animals by in vivo lentiviral infection for 28 days. Viruses were validated in vitro in cultured neurons and in vivo (Supplemental Fig. S1A and B, Table 2). NPAS4 expression was significantly enhanced in Oc1b after in vivo lentiviral infection (Supplemental Fig. S1B). Plasticity was assessed as variations of VC responsiveness in response to 1 week of MD (Fig. 2A). Two different experimental groups were used: adult animals with (i) binocular vision and (ii) monocularly deprived during the last week of infection. Electrophysiological recordings of visual evoked potentials (VEPs) (C/I ratio 0.9 ± 0.07; one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001, n= 8) and single units (CBI: 0.37 ± 0.04; one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001, n= 6, 112 cells) revealed that NPAS4-infected rats displayed a shift of OD in favour of the non-deprived eye in response to MD as compared with experimental controls (Fig. 2B and C). No change of binocularity was observed in NPAS4-infected rats with binocular vision, indicating that NPAS4-overexpression per se does not alter OD properties of VC neurons. We also performed a statistically more robust analysis of OD distributions, by computing a normalized OD score of single units. The cumulative distribution of OD score in NPAS4-infected rats after MD differed (Kolmorogov–Smirnov test, P < 0.05) from that of control groups (Fig. 2D). The electrophysiological analysis performed in animals monocularly deprived during the CP (P28) showed that the degree of plasticity caused by MD in NPAS4-infected rats was similar to that observed during early development (Fig. 2B and C).
Figure 2. NPAS4 overexpression reactivates plasticity in the adult visual system.

A, schematic diagram of the lentiviral infection protocol (28 days) in naïve animals. Adult rats were infected with Syn-NPAS4 in the binocular region of the primary VC contralateral to the deprived eye (see Methods). B and C, functional analysis of plasticity. NPAS4 overexpression in naïve animals restores susceptibility to MD in adulthood as assessed by VEPs (C/I ratio 0.9 ± 0.07; one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001, n= 8) and single units recordings (CBI: 0.37 ± 0.04; one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001, n= 6, 112 cells). No change of binocularity was observed in any of the control experimental groups. The shift of OD caused by NPAS4 overexpression was similar to that observed during the critical period for plasticity at early stages of development (CP (P28)). D, the cumulative distribution of OD score was biased towards the ipsilateral eye in MD NPAS4-infected animals compared with controls (Kolmorogov–Smirnov test, P < 0.05). E, the shift of OD in response to 1 week of MD was due to a decrease in the response stimulation of the deprived eye (t test, P < 0.05, VEP amplitude 0.68 ± 0.12, n= 6 and 0.73 ± 0.03, n= 8 for naïve and scramble, respectively; VEP amplitude 0.34 ± 0.05, n= 8 for NPAS4). VEPs amplitudes at the recording site in the VC contralateral to occlusion were normalized to the sum of the response to stimulation of the contralateral and ipsilateral eye of control animals. F, VA for the deprived eye (0.72 ± 0.02 cycles (c) deg−1) was markedly reduced as compared with the fellow eye (0.99 ± 0.01 cycles deg−1) in NPAS4-overexpressing animals (t test; P < 0.001, n= 6) but not in any of the control experimental groups. Error bars represent SEM. *Statistical significance.
We next examined whether the Npas4-induced reactivation of plasticity involved a weakening of the deprived eye strength or a strengthening of inputs coming from the open eye (Sawtell et al. 2003; Frenkel & Bear, 2004). As seen during early stages of development, the shift of OD after 1 week of MD in NPAS4-infected rats (Fig. 2E) was due to a reduction of the deprived eye strength (t test, P < 0.05, n= 8). A trend towards an increase of the open eye response was also observed. We then investigated whether the enhanced susceptibility to MD was accompanied by modifications of spatial resolution in the same group of NPAS4-overexpressing animals. Visual acuity (VA) for the deprived eye was lower than that of the fellow eye (t test, P < 0.001, n= 6) after MD in NPAS4-infected rats (Fig. 2F). No modification of visual resolution was observed in either NPAS4-infected animals with binocular vision or control scramble-infected rats after MD, indicating that neither lentiviral infection nor NPAS4 overexpression alter normal visual functions in naïve animals. We also analysed response properties of visual cortical neurons in the same experimental group. As expected (Fagiolini et al. 1994), receptive field size of visual cortical neurons in NPAS4-overexpressing animals (n= 112 cells) was larger than that observed in naïve adults or control scramble-infected rats after MD (Fig. 3A) (one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001). Peak discharge after stimulation of the deprived eye in NPAS4-infected animals was lower than that observed in experimental controls (Fig. 3B) (one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001). Spontaneous activity in NPAS4-infected rats did not differ from that of any other experimental group (Fig. 3C).
Figure 3. Response properties of visual cortical neurons following the lentiviral infection approach.

Functional properties of visual cortical neurons were unaltered after either NPAS4 overexpression in naïve animals or NPAS4 down-regulation during FLX treatment. Data are represented as box charts. The central horizontal line represents the median value while the other two horizontal lines are the 25th and 75th percentiles; error bars denote the 5th and 95th percentiles; square symbols indicate the mean value. A, receptive field (RF) size (degrees of visual angle) was higher in NPAS4-overexpressing animals (n= 112 cells) compared with controls (one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001; n= 86 cells for adult MD rats; n= 62 cells for naïve controls). B, peak discharge decreased significantly in NPAS4-overexpressing and FLX-treated animals as compared to controls (one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001). C, spontaneous activity did not change in any of the experimental groups (n= 63 cells for FLX+MD-NPAS4-shRNAi; n= 62 cells for FLX+MD-Scramble). *Statistical significance.
NPAS4 down-regulation prevents the plastic outcome caused by FLX in the adult
We next addressed whether NPAS4 expression underlies the plastic outcome caused by FLX in adulthood. To this end, we inhibited NPAS4 expression in FLX-treated rats by means of a lentivirus delivering NPAS4-shRNAi during pharmacological treatment (Fig. 4A, Supplemental Fig. S1A and C). Adult animals treated with FLX displayed a shift of OD after MD (C/I ratio, 1.0 ± 0.08; one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001, n= 5) whereas no shift was observed in FLX-treated rats infected with NPAS4-shRNAi virus (C/I ratio, 2.4 ± 0.07, n= 6), indicating that NPAS4 expression is necessary for the effects caused by FLX in VC plasticity (Fig. 4B and C). In contrast, binocularity in FLX-treated animals infected with scramble control virus shifted in favour of the open eye following MD (C/I ratio, 1.13 ± 0.06; one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001, n= 6). No change of OD was seen in naive NPAS4-shRNAi-infected rats with binocular vision or after MD, indicating that neither lentiviral infection nor the down-regulation of NPAS4 alter OD properties of VC neurons in naïve animals.
Figure 4. NPAS4 underlies the FLX-induced reactivation of adult visual cortical plasticity.

A, schematic diagram of the lentiviral delivering of NPAS4-shRNAi in rats long-term treated with FLX or vehicle. B, silencing of NPAS4 by RNA interference (NPAS4-shRNAi) during FLX treatment counteracts the process of plasticity reactivation. The shift of OD in FLX-treated rats after MD (C/I ratio, 1.0 ± 0.08; one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001, n= 5) was totally prevented in NPAS4-shRNAi infected animals (C/I ratio, 2.4 ± 0.07, n= 6). Scramble interference did not alter the effects caused by FLX in VC plasticity (C/I ratio, 1.13 ± 0.06). Binocularity in naïve animals with binocular vision (C/I ratio, 2.34 ± 0.15, n= 4) or after 1 week of MD (C/I ratio, 2.24 ± 0.06, n= 6) did not change after silencing of NPAS4 basal levels. C, the cumulative distribution of OD score was biased towards the ipsilateral eye in both adult MD rats treated with FLX and scramble-infected animals after FLX treatment as compared with controls (Kolmorogov–Smirnov test, P < 0.05) but not in any other experimental group (n= 92 cells for FLX+MD-treated rats; n= 63 cells for FLX+MD-NPAS4-shRNAi; n= 42 cells for FLX+MD-Scramble; n= 55 cells for Naïve+MD-NPAS4-shRNAi; n= 70 cells for Naïve+Bin-NPAS4-shRNAi. *Statistical significance.
To assess whether functional properties of cortical neurons were unaltered following NPAS4 down-regulation, we recorded single units (n= 63 cells) in a group of the same FLX-treated NPAS4-shRNAi-infected animals in which plasticity was assessed. Receptive field size and spontaneous activity remained unaltered (one-way ANOVA, P= 0.733) as compared with controls (n= 62 cells for FLX-treated scramble-infected rats; n= 92 cells for FLX-treated animals) (Fig. 3A and C). No change of peak discharge (one-way ANOVA P= 0.597) was retrieved either (Fig. 3B). Instead, peak response in FLX-treated animals after MD was lower (one-way ANOVA, P < 0.0001; post hoc Tukey's test, P < 0.001) than that of FLX-treated NPAS4-shRNAi-infected rats or FLX-treated scramble-infected controls (Fig. 3B).
Discussion
Our findings clearly demonstrate a previously unknown role for the activity-dependent transcription factor NPAS4 in mediating the reinstatement of plasticity in the adult VC. The plastic outcome caused by FLX in adulthood was accompanied by enhanced expression of NPAS4 mRNA and protein, presumably through a mechanism that involves a reduction in the methylation status at the NPAS4 promoter region, which is an epigenetic modification normally associated with increased gene expression. Moreover, our data show that NPAS4 overexpression in the VC of naïve animals restores susceptibility to MD, whereas NPAS4 down-regulation prevents the plastic outcome caused by FLX in the adult.
The process of plasticity reactivation is a multifactorial event that comprises the action of different cellular and molecular mechanisms, working in parallel or in series, the sum of which results in the activation of intracellular signal transduction pathways regulating the expression of plasticity genes in the adult VC (Pizzorusso et al. 2002; He et al. 2006; Putignano et al. 2007; Maya-Vetencourt et al. 2008, 2011, 2012; Baroncelli et al. 2010; Harauzov et al. 2010; Morishita et al. 2010; Silingardi et al. 2010; Cerri et al. 2011). Our present data bring the transcription factor NPAS4 as a new player into this context. There is evidence that the reduction of inhibition caused by FLX in the VC of adult animals after a brief period of MD, leads to an increase in elongations of GABAergic interneuron dendritic branch tips in superficial cortical layers (L 2/3) (Chen et al. 2011). This points toward a compensatory mechanism for the reduction of the inhibitory tone that is likely to involve the formation and/or strengthening of inhibitory synapses onto neighbouring excitatory neurons (Chen et al. 2011) and it has been reported that NPAS4 drives the formation of inhibitory synapses in the visual system (Lin et al. 2008). These observations, together with our finding that NPAS4 underlies the process of plasticity reactivation, suggest that NPAS4 may turn on a transcriptional program that regulates the expression of plasticity genes, while facilitating, in parallel or in series, a functional reorganization of inhibitory circuitries that might contribute to the homeostasis of cortical excitability during this phase of enhanced plasticity. This notion is consistent with two recent observations. First, NPAS4 interacts with several neuronal activity-regulated gene expression enhancers and promoters (Kim et al. 2010). Second, different homeostatic mechanisms assist to maintain neuronal activity within normal levels as modifications of neuronal circuitries occur in the visual system (Mrsic-Flogel et al. 2007; Lyckman et al. 2008). The notion that the NPAS4 transcription factor orchestrates a genetic program that lies behind phenomena of plasticity in the nervous system is further supported by recent findings that NPAS4 is critically involved in processes of memory formation and consolidation in adult life (Ploski et al. 2011; Ramamoorthi et al. 2011).
A role for a combined action of an enhanced serotonergic signalling, reduced inhibitory transmission and increased NPAS4 expression in mediating adult VC plasticity, may be exemplified by a model in which the FLX-induced shift of the intracortical I/E balance in favor of excitation could promote the activity-dependent expression of NPAS4, which, in turn, may drive a genetic program that lies behind the process of plasticity reactivation, and subsequently promote the formation of inhibitory synapses on excitatory neurons as a compensatory mechanism for the reduction of the inhibitory tone caused by FLX treatment (Fig. 5). Another possibility is that independently activated parallel pathways differentially affect the intracortical I/E ratio, thus ensuring a transitory enhancement of plasticity in adult life.
Figure 5. The process of plasticity reactivation caused by FLX in the adult visual system.
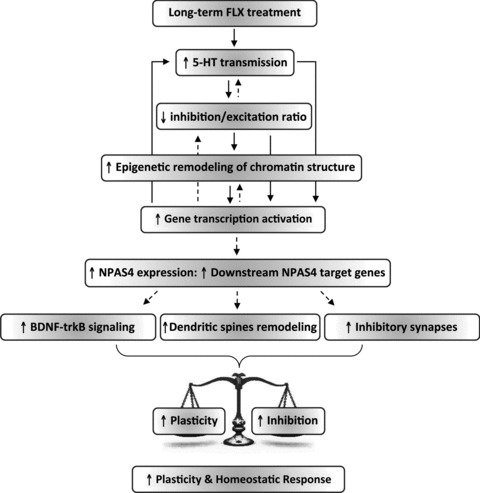
The reinstatement of plasticity caused by FLX in adult life is associated with signal transduction pathways that involve the activation of long-distance serotonergic transmission, a down-regulation of local intracortical inhibitory circuitries and enhanced NPAS4 expression. We propose a model in which the increased serotonergic signalling shifts the inhibitory/excitatory balance in favor of excitation, thus activating intracellular mechanisms that eventually promote epigenetic modifications of chromatin structure that, in turn, allow for the expression of plasticity genes in adult life among which NPAS4 plays a key role. NPAS4 seems to turn on a transcriptional program that underlies structural and functional plasticity while facilitating, in parallel or in series, a reorganization of inhibitory circuitries that might contribute to the homeostasis of cortical excitability by driving inhibition during this phase of enhanced plasticity. The expression of NPAS4 target genes may ultimately set in motion downstream physiological mechanisms that provide a permissive environment for adult visual cortical plasticity (e.g. enhanced BDNF-trkB signalling, removal of extracellular matrix components that are inhibitory for plasticity, enhanced dendritic spines density and remodelling). Accordingly, an increased expression of BDNF and transcription factors involved in the regulation of dendritic spine density and structural plasticity was observed in the VC of NPAS4-infected rats (E.C. L.M. E.T. and J.F.M.V. unpublished data). Continuous arrows represent established interactions between molecular and cellular processes mentioned (boxes). Dashed lines represent interactions that remain to be ascertained.
The observation that NPAS4 underlies the effects caused by FLX in adult VC plasticity immediately suggests that NPAS4-mediated transcriptional mechanisms may also be involved in the reinstatement of plasticity driven by different experimental strategies (e.g. environmental enrichment, dark exposure, food restriction and/or exogenous IGF-1 treatment) in adult life. The identification of NPAS4 downstream target genes that eventually provide a permissive environment for plasticity in the adult visual system is also an important issue that remains to be explored.
The capacity of the brain to change functionally in response to experience is most active during early stages of development but it decreases later in life when major alterations in neuronal network structures no longer take place in response to experience. Hence, the possibility to pharmacologically enhance plasticity as a therapeutic strategy for brain repair would be beneficial in a variety of pathological states where reorganization of neuronal networks is needed in adult life. This could be employed to promote the recovery of sensory functions after long-term sensory deprivation (e.g. amblyopia recovery in adult life) and may facilitate restructuring of mature circuitries impaired by damage or disease. Long-term FLX administration, indeed, enhances the effects of rehabilitation in the recovery from motor deficits after ischaemic stroke in humans (Chollet et al. 2011). In light of these findings, NPAS4-mediated transcriptional mechanisms arise as important therapeutic targets for future drug development.
Acknowledgments
We thank Juha Kuja-Panula and Heikki Rauvala for providing us the lentiviral vector with Syn1 promoter and Michael Greenberg for the generous gift of NPAS4 antibody. This work was supported by grants from Scuola Normale Superiore-Hermo-Pharma research agreement and Progetto di Ricerca di Giovani Ricercatori of the Scuola Normale Superiore to J.F.M.V., Sigrid Juselius Foundation, Centre of Excellence Program in Molecular Neuroscience of Academy of Finland to E.C. J.F.M.V. is supported by a research fellowship provided by the Scuola Normale Superiore, Pisa, Italy.
Glossary
- ChIP
chromatin immunoprecipitation
- CP
critical period
- FLX
fluoxetine
- MD
monocular deprivation
- OD
ocular dominance
- VA
visual acuity
- VC
visual cortex
- VEP
visual evoked potential
Author contributions
E.T., E.C., D.G., P.A., L.M. and J.F.M.V.: designed the general experimental approach. J.F.M.V.: conceived, designed, analysed and interpreted all electrophysiological experiments carried out, performed pharmacological treatments, in vivo brain infection experiments, electrophysiological recordings of VEPs and single units in experiments of NPAS4 over-expression after MD and binocular vision, NPAS4 down-regulation in naïve and FLX-treated animals, adult rats after long-term FLX treatment, control experiments in all groups of electrophysiological recordings. E.T.: MD surgery and pharmacological treatments, Western blot, real-time PCR, immunocitochemistry, histochemistry and ChIP experiments, cloning of NPAS4, silencing and scramble vectors, virus production and in vivo brain infections. L.R.: electrophysiological recordings of VEPs and single units in experiments of NPAS4 over-expression after MD and binocular vision, NPAS4 down-regulation in naïve and FLX-treated animals, respective control experiments and analysis. C.C.: electrophysiological recordings of VEPs and single units in control experiments in young and adult naïve animals with binocular vision and after MD. All authors discussed the results, contributed to the writing of the manuscript, and approved the final version.
Conflict of interest
E.C., L.M. and J.F.M.V. have filed a patent application on the use of antidepressants to treat amblyopia, transferred to Hermo Pharma, Ltd. E.C. is a founder and advisory board member of Hermo Pharma. E.T., D.G., L.R., C.C. and P.A. declare no financial interests.
Supplementary material
Supplemental Figure S1
References
- Amar M, Lucas-Meunier E, Baux G, Fossier P. Blockade of different muscarinic receptor subtypes changes the equilibrium between excitation and inhibition in rat visual cortex. Neuroscience. 2010;169:1610–1620. doi: 10.1016/j.neuroscience.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Baroncelli L, Sale A, Viegi A, Maya Vetencourt JF, De Pasquale R, Baldini S, Maffei L. Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Exp Neurol. 2010;226:100–109. doi: 10.1016/j.expneurol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Cerri C, Fabbri A, Vannini E, Spolidoro M, Costa M, Maffei L, Fiorentini C, Caleo M. Activation of Rho GTPases triggers structural remodeling and functional plasticity in the adult rat visual cortex. J Neurosci. 2011;31:15163–15172. doi: 10.1523/JNEUROSCI.2617-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nat Protoc. 2006;1:3166–3173. doi: 10.1038/nprot.2006.450. [DOI] [PubMed] [Google Scholar]
- Chen JL, Lin WC, Cha JW, So PT, Kubota Y, Nedivi E. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci. 2011;14:587–594. doi: 10.1038/nn.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Ann Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, et al. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci. 2010;30:361–371. doi: 10.1523/JNEUROSCI.2233-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26:2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat Neurosci. 2007;10:1134–1136. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lyckman AW, Horng S, Leamey CA, Tropea D, Watakabe A, Van Wart A, et al. Gene expression patterns in visual cortex during the critical period: synaptic stabilization and reversal by visual deprivation. Proc Natl Acad Sci U S A. 2008;105:9409–9414. doi: 10.1073/pnas.0710172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Baroncelli L, Viegi A, Tiraboschi E, Castren E, Cattaneo A, Maffei L. IGF-1 restores visual cortex plasticity in adult life by reducing local GABA levels. Neural Plasticity. 2012;2012 doi: 10.1155/2012/250421. 250421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Origlia N. Visual cortex plasticity: a complex interplay of genetic and environmental influences. Neural Plasticity. 2012 doi: 10.1155/2012/631965. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Tiraboschi E, Spolidoro M, Castren E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur J Neurosci. 2011;33:49–57. doi: 10.1111/j.1460-9568.2010.07488.x. [DOI] [PubMed] [Google Scholar]
- Montey KL, Quinlan EM. Recovery from chronic monocular deprivation following reactivation of thalamocortical plasticity by dark exposure. Nat Commun. 2011;2:317. doi: 10.1038/ncomms1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau AW, Amar M, Le Roux N, Morel N, Fossier P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cer Cortex. 2010;20:456–467. doi: 10.1093/cercor/bhp114. [DOI] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Ooe N, Saito K, Mikami N, Nakatuka I, Kaneko H. Identification of a novel basic helix-loop-helix-PAS factor, NXF, reveals a Sim2 competitive, positive regulatory role in dendritic-cytoskeleton modulator drebrin gene expression. Mol Cell Biol. 2004;24:608–616. doi: 10.1128/MCB.24.2.608-616.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Monsey MS, Nguyen T, Dileone RJ, Schafe GE. The neuronal PAS domain protein 4 (Npas4) is required for new and reactivated fear memories. PloS One. 2011;6:e23760. doi: 10.1371/journal.pone.0023760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruunsild P, Sepp M, Orav E, Koppel I, Timmusk T. Identification of cis-elements and transcription factors regulating neuronal activity-dependent transcription of human BDNF gene. J Neurosci. 2011;31:3295–3308. doi: 10.1523/JNEUROSCI.4540-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L, Pizzorusso T. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53:747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Ramamoorthi K, Fropf R, Belfort GM, Fitzmaurice HL, McKinney RM, Neve RL, et al. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science. 2011;334:1669–1675. doi: 10.1126/science.1208049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Berardi N, Spolidoro M, Baroncelli L, Maffei L. GABAergic inhibition in visual cortical plasticity. Front Cell Neurosci. 2010;4:10. doi: 10.3389/fncel.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Maya-Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Silingardi D, Scali M, Belluomini G, Pizzorusso T. Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur J Neurosci. 2010;31:2185–2192. doi: 10.1111/j.1460-9568.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- Spolidoro M, Baroncelli L, Putignano E, Maya-Vetencourt JF, Viegi A, Maffei L. Food restriction enhances visual cortex plasticity in adulthood. Nat Commun. 2011;2:320. doi: 10.1038/ncomms1323. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Tropea D, Van Wart A, Sur M. Molecular mechanisms of experience-dependent plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364:341–355. doi: 10.1098/rstb.2008.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


