Abstract
Several laboratories have provided immunohistochemical, molecular biological and electrophysiological evidence that the glutamatergic granule cells of the dentate gyrus can transiently express a GABAergic phenotype during development. Electrophysiological recordings on hippocampal slices obtained during this period have shown that stimulation of the mossy fibres (MFs) provokes simultaneous monosynaptic GABAA and glutamate receptor-mediated responses in their target cells, which have the pharmacological and physiological characteristics of MF neurotransmission. This evidence, although strongly supporting the hypothesis that MFs co-release glutamate and GABA, is indirect, as the extracellular stimulation used in slice experiments could activate fibres other than MFs. In this study, we show that selective stimulation of single, identified MF boutons (MFBs) attached to the apical dendrites of dissociated pyramidal cells of developing rats produced synaptic currents mediated by either glutamate receptors only or by both glutamate and GABAA receptors. By contrast, stimulation of MFBs of adult rats produced exclusively glutamate receptor-mediated responses. All responses evoked by stimulation of MFBs underwent strong frequency-dependent potentiation and were depressed by the activation of presynaptic metabotropic glutamate receptors. On the other hand, synaptic responses evoked by stimulation of interneuronal boutons located on the soma or on the basal dendrites of the same pyramidal cells were exclusively mediated by GABAA receptors, underwent frequency-dependent depression and were unaffected by mGluR agonists. We here demonstrate that the simultaneous glutamatergic and GABAergic responses evoked by MF stimulation in pyramidal cells of CA3 during development have a common origin in the giant MFBs.
Key points
The granule cells and their mossy fibres (MFs) can express, besides glutamate, all the markers of the GABAergic phenotype during development, suggesting that they can co-release glutamate and GABA.
Several groups have presented substantial electrophysiological evidence, albeit indirect, supporting this hypothesis.
We investigated the co-release of these amino acids by recording synaptic responses in mechanically dissociated pyramidal cells to stimulation of single, identified MF boutons attached to their apical dendrite.
In pyramidal cells from developing rats, MF bouton stimulation evoked responses that were mediated by either glutamate receptors (R) only or by both glutamate-R and GABAA-R; in adult rats stimulation of MF boutons produced exclusively glutamate-R-mediated responses. By contrast, responses to stimulation of interneuronal boutons on the same cells were exclusively GABAA-R mediated.
We demonstrate that the pharmacologically isolated GABAergic responses evoked by MF stimulation in CA3 cells in slice preparations may indeed be of MF origin.
Introduction
The pyramidal cells of CA3 of the hippocampus receive glutamatergic signals from the mossy fibres (MFs), the perforant path and collaterals of other pyramidal cells, and GABAergic transmission from diverse interneuronal pools (for a review see Jaffe & Gutiérrez, 2007). However, several laboratories have shown that granule cell activation, besides evoking glutamate receptor-mediated responses, can also evoke monosynaptic GABAA receptor-mediated responses during development (Walker et al. 2001; Gutiérrez et al. 2003; Safiulina et al. 2006; Romo-Parra et al. 2008), suggesting that the MFs co-release glutamate and GABA. This hypothesis is strongly supported by data using immunohistochemical, electron microscopy and molecular biological techniques, which have clearly established that the glutamatergic granule cells can transiently express, in addition to glutamate and the vesicular glutamate transporter (VGlut-1), all the markers of the GABAergic phenotype, glutamic acid decarboxylase (GAD), the vesicular GABA transporter (VGAT-1) and GABA, and that the postsynaptic sites apposed MF terminals have the receptors for both glutamate and GABA (Sloviter et al. 1996; Lamas et al. 2001; Ramírez & Gutiérrez, 2001; Gómez-Lira et al. 2002, 2005; Bergersen et al. 2003; Zander et al. 2010). Despite this evidence, at the functional level, the hypothesis that the MFs release GABA relies on the assumption that the stimulation provided over the hilus or dentate gyrus to evoke neurotransmitter release in slice preparations exclusively activates granule cells and, thus, that the GABAergic responses derive exclusively from the MFs and not from interneurons (Ints). In such experiments, a way to determine that the responses are indeed originated from the MFs is by confirming that they undergo strong frequency potentiation and that they are depressed by activation of metabotropic glutamate receptors (mGluRs). These features have been considered signatures of MF transmission and, therefore, are not present in transmission of interneuronal origin (Kamiya et al. 1996; Salin et al. 1996; Nicoll & Schmitz, 2005). To conclusively demonstrate that MFs co-release GABA, paired recordings of a connected presynaptic granule cell with a pyramidal cell should be conducted. This approach, however, is unlikely to be accomplished in a hippocampal slice preparation given the very scarce connectivity of granule cells with pyramidal cells or interneurons (Acsády et al. 1998).
A way to study responses of indisputable MF origin would be to record synaptic currents/potentials from a pyramidal cell in response to selective stimulation of a single, identified MF bouton (MFB). Moreover, it would be desirable to record synaptic responses to the stimulation of an interneuronal bouton (IntB) to be able to compare neurotransmission derived from the two different sources impinging on the same cell. The isolated cell bouton preparation is a suitable preparation to study evoked neurotransmitter release from single synaptic boutons on identified cells (Vorobjev, 1991; Akaike & Moorhouse, 2003). Moreover, if the synaptic boutons can also be identified, this technique can be a powerful tool to disclose the co-release of neurotransmitters. As the MFBs are known to contain glutamate, GABA, and their respective vesicular transporters (Gómez-Lira et al. 2002; Bergersen et al. 2003; Zander et al. 2010), in this work we were able to label MFBs and, after dissociation of pyramidal cells, MFBs and IntBs adherent to the cell could be identified and directly stimulated to electrophysiologically study their output. We provide a direct demonstration that MFBs of developing rats, but not of adult rats, are able to co-release glutamate and GABA.
Methods
The Ethics Committee for Animal Research of our Institution approved all the experiments. Two-week-old (14–16 days of age) and 23- to 25-day-old Wistar rats were decapitated under pentobarbital anaesthesia (50 mg kg−1, i.p.), their brains were rapidly removed and transversal hippocampal slices of 380 μm were obtained with a Vibroslicer (Leica VT1200) submerged in sucrose-based storage/cutting solution (Bischofberger et al. 2006) containing (in mm): 87 NaCl, 25 NaHCO3, 25 glucose, 75 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2 and 4 MgCl2 equilibrated with 95% O2–5% CO2, at 4°C. The slices were kept in the oxygenated solution at room temperature (20–24°C) for at least an hour before mechanical dissociation.
Labelling of MFBs
We labelled the MFBs with fluorescent dyes using two methods, as previously described (Beltrán et al. 2012). Briefly, taking advantage of the high content of Zn2+ in the MFs and their boutons (Howell et al. 1984), slices were incubated for 3–5 min before, and during the cell dissociation procedure (approximately five additional minutes) in a culture dish, in the presence of a selective fluorescent sensor for Zn2+, Zinpyr-1 (1 μm; Mellitech, Grenoble, France) dissolved in the dissociation solution (see below; Fig. 1A). A second labelling technique consisted of the introduction of a small crystal of rhodamine–dextranamine (Molecular Probes, Eugene, OR, USA) in the granular cell layer of the dentate gyrus with the aid of a microelectrode tip prior to dissociation. The dye is taken up in to the neurons and anterogradly transported along the MF tract (Fig. 1B). For proper dye transport along the axons and to the MFBs, slices were kept in an air–liquid interface chamber for 3–4 h prior to dissociation, constantly perfused with oxygenated storage solution at room temperature.
Figure 1. Dissociated pyramidal cells of CA3 with fluorescent giant MFBs attached to their apical dendrites.

A, prior to dissociation, Zn2+-containing MFBs are labelled with the fluorescent sensor for Zn2+, Zinpyr-1, or (Ba and b) with dextranamine transported from the granule cell layer to the MFs terminals. C, enzyme-free mechanical dissociation is then conducted using a vibrating blunt pipette positioned over CA3 with a micromanipulator. MFBs can be identified attached to the apical dendrites of the dissociated pyramidal cells by their fluorescent label of either Zinpyr-1 (Da and b) or rhodamine-dextranamine (Ea and b). Other non-fluorescent bulks (putative boutons) can also be observed (arrowhead, IntB in Da and b), which can be electrophysiologically verified. F, pyramidal cells and G, interneurons are recognized by their characteristic shape and firing of action potentials on depolarization.
Cell dissociation
For the enzyme-free mechanical dissociation of cells in CA3, the slices were placed on a glass coverslip, treated with poly-l-lysine, in a recording chamber with dissociation solution. The slices were secured with an inert nylon mesh stretched over a C-shaped silver wire. The cells were then dissociated under stereoscopical microscope observation by applying a vibrating fire-polished glass pipette mounted on a micromanipulator to the slice surface. The electronic system that drives an horizontal oscillation of the pipette was set at 20 to 40 Hz and a motion span of 0.5–0.8 mm perpendicular to s. pyramidale, covering from s. radiatum to the alveous in the CA3b-c area, for 3–5 min (Fig. 1C). To construct the blunt tip pipettes, we used capillary glasses (1/0.58 mm OD/ID; WPI, Inc., Sarasota, FL, USA), pulled to obtain microelectrodes and then fire-polished to obtain a blunt tip of around 300 μm in diameter; smaller diameters produce damage to the cells. The processed hippocampal slice was then removed and the mechanically dissociated neurons were left to adhere to the coverslips for at least 15 min (Fig. 1D and E). The recording chamber was then placed under the microscope and perfusion was started with external solution at 0.25 ml min−1 (Beltrán et al. 2012).
Electrophysiological recordings
The dissociated cells were placed under an up-right microscope (Eclipse E600FN; Nikon) equipped with epifluorescence capabilities. The fluorescent MFBs were visualized using 10× and 40× objectives (excitation 450–490 nm, long-pass barrier filter 515 nm for Zynpir-1 detection; excitation 510–560 nm, long-pass barrier filter 590 nm, for rhodamine–dextranamine detection; Fig. 1B, D and E). Before recording, cells were perfused for 10 min at 0.2–0.3 ml min−1 with the recording external solution at room temperature to wash out excess dye. It consisted of (in mm): 150 NaCl, 3 KCl, 1 MgCl2, 2 CaCl2, 10 Hepes and 10 glucose, pH adjusted to 7.4 with Tris-base (Jang et al. 2006). Electrophysiological recordings were performed at 30–32°C with the patch clamp technique in whole-cell mode using borosilicate pipettes pulled to yield 4–6 MΩ (Flaming-Brown P-97 puller; Sutter Instrument Company, Novato, CA, USA) and filled with either of the following intracelular solutions: (a) for recordings in voltage clamp mode, we used a caesium-based intracellular solution consisting of (in mm): 65 CsF, 70 CsMeSO4, 6 CsCl, 6 TEA-Cl, 10 Hepes, 2 EGTA and 4 Mg-ATP, pH adjusted to 7.2. with KOH; (b) for recordings in current clamp mode we used a potassium gluconate intracellular solution consisiting of (in mm): 133 potassium gluconate, 7 KCl, 1 NaCl, 1.5 MgCl2, 4 Mg-ATP, 0.5 Na-GTP, 0.5 EGTA and 10 Hepes, pH adjusted to 7.2 with KOH. Signals were amplified with a Multiclamp 700B amplifier (Molecular Devices, Palo Alto, CA, USA), digitized and sampled at 10 kHz and filtered at 5 kHz (Digidata 1440A; Molecular Devices). Signals were acquired and off-line analysed with the pCLAMP10 program (Molecular Devices). The cells were voltage clamped (Vh) at −90 mV, so given the composition of our intracellular solution (see above), both glutamate receptor- and GABA receptor-mediated currents were inwardly directed. Hyperpolarizing step pulses (5 mV, 25 ms) were used to monitor the access resistance throughout the recordings, which was 18.4 ± 3.2 MΩ, coinciding with previous reports (Harata et al. 1997). The recording was rejected if its access resistance changed by more than 15%.
To evoke synaptic responses in the dissociated neurons, current pulses (50 μs, 1–15 μA) were applied through a theta borosilicate pipette (2/1.4 mm OD/ID; Hilgenberg GmbH, Maisfeld, Germany) filled with external solution (Fig. 2B) placed in close proximity to single, identified MFBs adherent to the apical dendrite of the dissociated cell, or to non-labelled, putative GABAergic boutons on the soma or basal dendrite. Stimulation was provided at a frequency of 0.05 Hz with a stimulator (S48; Grass Instruments, West Warwick, RI, USA) using a stimulus isolation unit (PSIU6, Grass Instruments). A piezoelectric micromanipulator (Burleigh PCS-5000) was used to position the tip of the stimulation pipette directly onto a fluorescent bouton (Fig. 2B). The onset latency of the evoked currents was measured from the beginning of the stimulus artifact to the beginning of the rising phase of the current. The 10 to 90% rise time and decay constants of the currents were obtained with built-in functions of Clampfit 10. Values are given as the mean ± SEM. Means were compared using paired Student's t test. Significance values provided are two-tailed P values.
Figure 2. Spontaneous synaptic events and synaptic responses evoked by stimulation of attached, identified single MF and interneuronal boutons.

A, viable dissociated pyramidal cells presented frequent spontaneous synaptic activity. B, schematic depiction of a pyramidal cell with a fluorescent MFB on the apical dendrite and an IntB on the basal dendrite, which were stimulated with a bipolar theta glass pipette. C, synaptic currents evoked in a pyramidal cell by stimulation of the fluorescent MFB and of an IntB. Both responses occur in an all-or-none fashion. D, intensity dependence of the synaptic responses evoked in pyramidal cells (n= 4) by MFB and IntB stimulation. Notice the all-or-none nature of their appearance and the higher amplitude of the responses to MFB stimulation. E, positioning of the stimulation pipette in the centre of the fluorescent bouton readily evoked a synaptic current (position 1), whereas a shift of the pipette (position 2) failed to evoke responses. F, synaptic currents evoked by stimulation of MFBs presented failures, consistent with previous reports using this preparation. In the cases where two MFBs on the same cell were found, their stimulation provoked a similar failure rate. Interestingly, the failure rate observed on IntB stimulation was lower than in the case of MFBs stimulation (n= 5 MFBs; n= 4 IntBs). G, the onset latency of the responses evoked by direct bouton stimulation was constant. Together, these results demonstrate that the synaptic responses originate from the activation of one bouton only.
The drugs used were diluted in the external solution: the NMDA receptor antagonist (dl)-2-amino-5-phosphonovaleric acid (APV; 30 μm; Tocris Cookson); the non-NMDA receptor antagonist 6-nitro-7-sulfamoyl-benzo (f)quinoxaline-2,3-dione (NBQX; 10 μm; Tocris Cook-son); the GABAA receptor antagonists bicuculline methiodide (20 μm; Sigma); the group III metabotropic glutamate receptor (mGluR) agonist l-(+)-2-amino-4-phosphonobutyric acid (l-AP4; 10 μm; Tocris Cookson); and the group II mGluR agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV; 1 μm; Tocris Cookson).
Results
The exposure of hippocampal slices to Zinpyr-1 or rhodamine–dextranamine prior to cell dissociation allows the identification of the MF tract by either the direct observation of Zn2+-containing elements (Fig. 1A), in the former, or by the transport of the fluorescent dye from the granule cell somata along the MF axons to the terminals, in the latter (Fig. 1B). Dissociation of CA3 pyramidal cells from these treated slices allowed the detection of fluorescent MFBs attached to the proximal part of the apical dendrite (Fig. 1D and E). Also, interneurons could be easily differentiated by their morphological and electrophysiological characteristics (Fig. 1F and G). Viable cells had fast (∼1 ms) action potentials with overshoot and could be recorded for up to 70 min. We recorded from 20 pyramidal cells with fluorescent MFBs attached to the proximal part of the apical dendrite (Fig. 1D and E). They had an input resistance of 1.7 ± 0.5 GΩ, typical firing activity on depolarization with one or two action potentials, which differed from the high-frequency response of the interneurons (Fig. 1G) and presented spontaneous synaptic activity (Fig. 2A).
To analyse evoked synaptic responses, we used a theta pipette to stimulate identified fluorescent MFBs, or boutons of interneuronal origin on the soma or basal dendrite (Fig. 2B; see below). Mossy fibre bouton and IntB stimulation evoked synaptic responses in an all-or-none fashion (Fig. 2C and D). In all experiments, the stimulation pipette was moved away from the centre of the bouton in steps of 0.3 μm until no response was obtained. We determined that shifts of 0.5 μm or more prevented responses to appear (Fig. 2E). A high failure rate of synaptic responses to stimulation of boutons has been described in this preparation (Akaike et al. 2002). We next analysed the rate of success in evoking synaptic responses by stimulation of MFBs and IntBs. Figure 2F depicts this analysis, in which we determined a failure rate of 59.8 ± 2% (n= 6) on MFBs stimulation, and 47.3 ± 5.1% (n= 6) on IntB stimulation (Fig. 2F). Responses to stimulation of MFBs had an onset latency of 0.97 ± 0.03 ms (n= 6; Fig. 2G) and responses to IntB stimulation, 1.0 ± 0.04 ms (n= 6). All this evidence, routinely conducted at the beginning of each experiment, indicated that the responses originated from a single bouton.
Responses of MF origin are known to undergo strong (>250%) frequency potentiation (Salin et al. 1996; Nicoll & Schmitz, 2005). We therefore raised the stimulation frequency from 0.05 to 1 Hz and observed a potentiation of 260.1 ± 45.6%. (P < 0.01; n= 5; Fig. 3Aa and b). This potentiation was accompanied by a decrease in the failure rate to 34.1 ± 0.9% (P < 0.05; n= 5; Fig. 3B). By contrast, raising the frequency of stimulation of IntBs produced a depression of 59.4 ± 12.1% (n= 6; Fig. 3Aa and b) and an increase in the failure rate to 58.2 ± 4.3% (P < 0.05; n= 6; Fig. 3B). A pharmacological characteristic of responses of MF origin is their sensitivity to mGluR activation. We perfused DCG-IV (1 μm), an mGluR agonist known to depress neurotransmission of MF origin (Kamiya et al. 1996), which produced a depression of 54.2 ± 8.5% (P < 0.05; n= 5) of the responses to MFB stimulation, whereas it did not affect the responses to IntB stimulation (Fig. 3Ca). The increase in the ratio of the responses obtained with the paired-pulse protocol (Fig. 3Cb) and the increase of the failure rate of the responses to MFB stimulation (77.3 ± 1.1%, P < 0.05; n= 5; Fig. 3D) confirmed the presynaptic effect of DCG-IV.
Figure 3. The synaptic responses evoked by stimulation of MFBs have the physiological and pharmacological signature of transmission of MF origin.

Aa and b, changing the stimulation frequency of MFBs from 0.05 to 1 Hz produced a robust frequency potentiation (>250%; n= 6; filled circles) and reduction of the failure rate (B; filled bars). By contrast, the same change in stimulation frequency of the IntBs produced a marked depression (60%, n= 6; open circles) and an increase in failure rate (B; open bars). C, perfusion of the mGluR agonist, DCG-IV (1 μm), produced a depression (Ca and b), as well as increase in the failure rate (D; filled bars) of the synaptic responses evoked by MFB stimulation. By contrast responses to IntB stimulation were unaffected (open circles and bars in Cb and D).
After confirming that responses evoked by MFB had the physiological and pharmacological characteristics of transmission of MF origin, we perfused the iGluR antagonists, which either completely blocked (98.5 ± 1.3%, P < 0.01, in 4 out of 14), or reduced (56.3 ± 9.3%, P < 0.05, in 7 out of 14), or did not affect (1.2 ± 3.4%, P < 0.39, 3 out of 14) the responses evoked by MFB stimulation (Fig. 4). The threshold stimulation current needed to evoke the synaptic responses before and during the application of iGluR response was similar and evoked the responses in an all-or-none fashion, confirming the stimulation of a single bouton (Fig. 4A)
Figure 4. Stimulation of MFBs evokes mixed glutamatergic–GABAergic, glutamatergic-only and GABAergic-only currents.
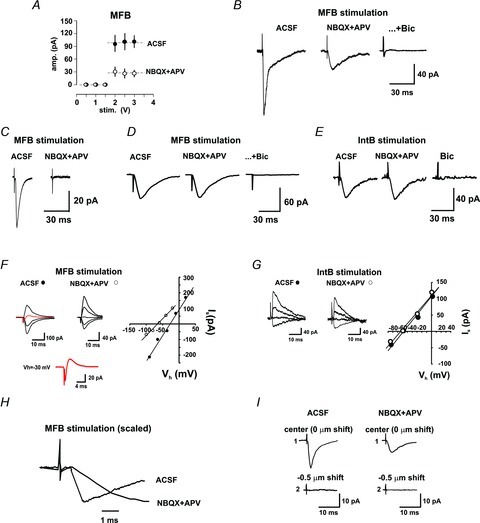
A, the pharmacologically isolated GABAA-R-mediated current was evoked in an all-or-none fashion (open circles) and had a similar intensity threshold to the total current, evoked prior to perfusion of antagonists (filled circles). B, synaptic currents evoked by stimulation of some MFB were partially blocked by the iGluR antagonists NBQX and APV. The GABAA-R antagonist, bicuculline, blocked the remaining current. C, stimulation of some MFBs evoked synaptic currents that were glutamatergic only because they were completely blocked by the iGluR antagonists. D, stimulation of some MFBs evoked synaptic currents that were insensitive to iGluR antagonists, but completely blocked by the GABAA-R antagonist, thus GABAergic only. E, stimulation of all IntBs evoked a synaptic current that was blocked by the GABAA-R antagonist, thus GABAergic only. F, the synaptic current evoked by stimulation of MFBs in normal ACSF reversed at −27 mV and, on blockage of iGluRs, the reversal potential shifted to −60 mV, consistent with currents mediated by GABAA receptors. The trace in red, expanded below, shows a compound inward/outward current evoked at Vh −30 mV. G, by contrast, the synaptic currents evoked by stimulation of IntBs in normal ACSF and during blockage of iGluRs reversed at −64 mV, both consistent with currents mediated by GABAA receptors. H, the synaptic currents evoked by stimulation of the MFBs in the absence (ACSF) and presence of iGluR antagonists (NBQX+APV) had the same onset latency. I, the length of the shift of the stimulation electrode away from the centre of the synaptic bouton, at which responses were no longer evoked, was similar in the absence (ACSF) and presence of iGluR antagonists (NBQX+APV).
The synaptic current resistant to iGluR antagonists was completely blocked by the GABAA receptor antagonist bicuculline (Fig. 4B and D). This indicates that some MFBs release only glutamate, some only GABA and some both amino acids. The mean amplitude of the synaptic current obtained in control conditions was 102.8 ± 24.4 pA (excluding failures, n= 6), while the current insensitive to the iGluR antagonists had a mean amplitude of 24.9 ± 15.2 pA. On the other hand, when MFB-evoked GABAergic-only responses were observed, they had an amplitude of 54.2 ± 12.4 pA. By contrast, stimulation of IntBs evoked an inward current insensitive to iGluR antagonists but blocked by bicuculline, indicating that it was GABAA receptor mediated (Fig. 4E). The I–V relation of the MFB-evoked currents, in the absence and presence of iGluR blockers, is depicted in Fig. 4F, whereby a shift from −27 to −60 mV was observed on GluR blockage. Moreover, stimulation of MFBs at a Vh of around −30 could evoke compound inward/outward currents (Fig. 4F). On the other hand, the reversal potential of the synaptic currents evoked by IntB stimulation was similar in both conditions (P < 0.35; Fig. 4G).
The inversion potential coincided with that expected for responses mediated by GABA receptors, which, with our intracellular solution, was expected to occur at −63 mV. Noteworthy, the onset latency of the synaptic currents evoked by MFB stimulation before and during NBQX–APV perfusion were similar (0.97 ± 0.03 vs. 0.96 ± 0.02 ms; n= 6; Fig. 4H); also, the shifts of the stimulation pipette, after which synaptic responses could no longer be evoked, were similar for both pharmacological conditions (0.53 ± 0.11 vs. 0.52 ± 0.08 μm; n= 6; Fig. 4I). These data further indicate that only one bouton was the origin of both synaptic responses.
The synaptic currents evoked in pyramidal cells by MFB stimulation had a 10–90% rise time of 1.2 ± 0.2 ms (n= 6) and either a fast decay constant (τ= 8.4 ± 2.1 ms, n= 6) which represent glutamatergic-only responses (Fig. 5A), or two decay constants: a fast (τ= 6.9 ± 2 ms; n= 6) and a slow component (τ= 29.7 ± 3.7 ms, n= 6) representing combined glutamatergic and GABAergic responses (Fig. 5A), or only a slow decay constant (τ= 34.6 ± 4.1 ms, n= 6), in which case they also had a slower 10–90% rise time of 3.4 ± 0.4 ms, n= 6), consistent with GABA-R-mediated responses (Fig. 5A and F). For comparison, the GABA-mediated currents evoked by IntB stimulation had a 10–90% rise time of 3.0 ± 0.3 ms (n= 5; Fig. 5D and F). Digital subtraction of the current with slow constant from the current with mixed decay constant yielded a current with a decay constant similar to responses with a fast decay constant, or glutamatergic (τ= 9.3 ± 2.2, n= 5; Fig. 5B) and, therefore, the blockage of iGluR suppressed responses with the fast decay constant. Accordingly, blockage of GABAA-R with bicuculline isolated the currents with a fast decay constant (glutamatergic; Fig. 5C). By contrast, the currents evoked in the presence of iGluR, thus GABAergic, had a decay τ of 32.5 ± 5.7 ms (n= 6), and were similar to those currents with slow decay constant evoked in the absence of iGluR (Fig. 5A, slow). The proportion of the different responses obtained in the different pharmacological conditions is depicted in Fig. 5G. For comparison, we conducted six experiments in cells dissociated from rats 25 days of age, where only glutamate-receptor-mediated responses were observed (Fig. 5E), as previously reported in the literature (Gutiérrez, 2005). On the other hand, spontaneous currents recorded from cells of 15-day-old rats also presented mixed, slow and fast decay constants (Fig. 5Ha), while those recorded from cells of 25-day-old rats had only slow or fast decay constants (Fig. 5Hb).
Figure 5. The synaptic currents evoked by stimulation of MFBs have distinct kinetics.
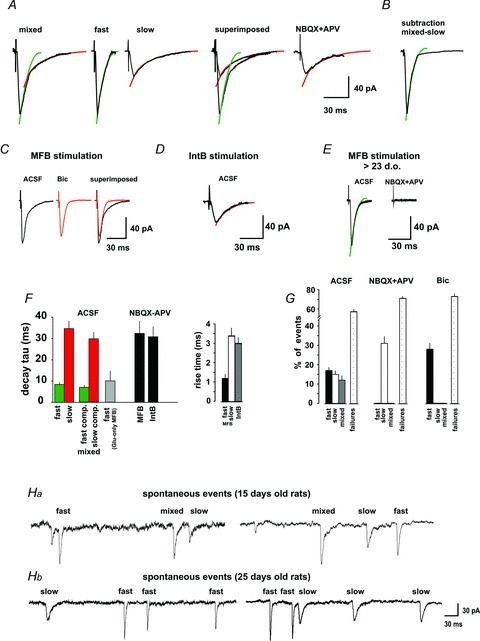
A, the synaptic currents evoked by stimulation of the MFBs had either a fast and a slow decay component (mixed), a fast component (fast), or a slow component (slow). Perfusion of iGluR antagonists isolated the current with the slow component. Subtracting the pharmacologically isolaged GABAergic (slow) component from the mixed response yielded a current with a fast component (B). C, in agreement with this observation, perfusion of bicuculline blocked the slow component and isolated a current with a fast component. D, the synaptic current evoked by IntBs stimulation under normal ACSF had a slow decay, similar to that obtained by MFB stimulation in the presence of iGluR antagonists. E, stimulation of MFBs of preparations older than 23 days of age evoked synaptic currents with a fast decay constant that were blocked by iGluR antagonists. F, values of the decay constant and rise time of each synaptic current evoked by MFB (n= 6) and IntB (n= 6). G, percentage of each type of current (mixed, slow – GABAergic, or fast – glutamatergic) evoked and failures on MFB and IntB stimulation in the different pharmacological conditions. Notice that iGluR blockage prevented currents with a fast decay constant and blockage of GABAA-R prevented currents with a slow decay constant, while failure rates were not modified. Ha, spontaneous currents in 15-day-old rats could have either a fast and a slow decay component (mixed), a fast component (fast), or a slow component (slow), as the evoked ones, depicted in panel A. Hb, in 25-day-old rats, the spontaneous currents had either a slow or a fast decay component.
As expected from responses of MF origin, the pharmacologically isolated GABA-R-mediated currents evoked by MFB stimulation underwent strong frequency potentiation (213.9 ± 34%, P < 0.01; n= 5; Fig. 6A) and the failure rate decreased to 49 ± 8% (P < 0.05; Fig. 6B). By contrast, the responses evoked by IntB stimulation underwent frequency-dependent inhibition (66.1 ± 11.9%, P < 0.05; n= 5; Fig. 6A) and the failure rate increased from 45 ± 5.4 to 55 ± 3.9% (P < 0.05; Fig. 6B). On the other hand, perfusion of l-AP4 (10 μm) depressed the MFB GABA-R-mediated responses by 79.8 ± 6.8% (P < 0.01; n= 6), while those evoked by stimulation of interneuronal boutons were not affected (Fig. 6C and D). The effect of mGluR activation on MFB responses was corroborated to be presynaptic by the increased ratio of the responses in the paired-pulse protocol (Fig. 6D) and because of the increase in the failure rate to 92 ± 8% (P < 0.01; Fig. 6E).
Figure 6. The pharmacologically isolated GABAergic synaptic currents evoked by stimulation of MFBs have the signature of transmission of MF origin.
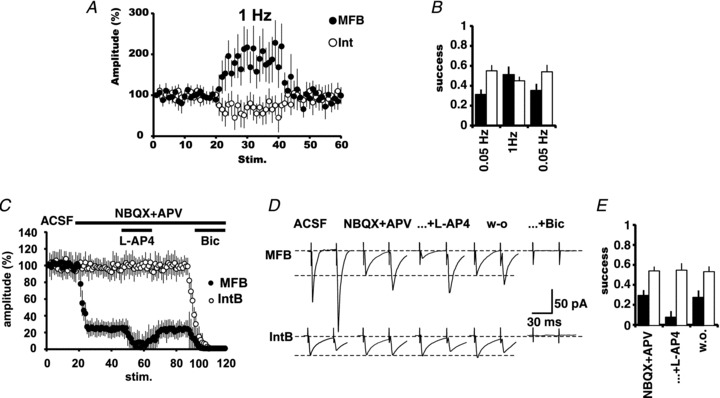
A, changing the stimulation frequency from 0.05 to 1 Hz produced a strong potentiation (>200%) of the GABAergic synaptic responses (filled circles) and reduction of the failure rate (B; filled bars; n= 6). By contrast stimulation of IntBs (A, open circles) produced frequency-dependent depression and increase of the failure rate (B; open bars; n= 6). C, group synaptic responses to MFB (filled circles; n= 6) and IntB stimulation (open circles; n= 6). Responses to MFB stimulation were partially blocked by iGluR blockers. The remaining responses were strongly and reversibly depressed by the activation of mGluRs with l-AP4, and completely blocked by bicuculline. Responses to IntB were not affected by the perfusion of iGluR blockers, but completely blocked by bicuculline. D, the effect of l-AP4 on the pharmacologically isolated GABAergic current evoked by MFB stimulation is presynaptic, as evidenced by the paired-pulse protocol. By contrast, GABAergic responses evoked by IntB were unaffected. E, further evidence for the presynaptic action of l-AP4 is the increase in the failure rate of MFB responses. Responses to IntB were unaffected.
Discussion
Our data show that electrical stimulation restricted to MFBs (Beltrán et al. 2012) produces either glutamatergic-only, GABAergic only, or mixed glutamatergic–GABAergic responses in CA3 pyramidal cells in 15- to 16-day-old rats, while it produces glutamatergic-only responses in >23-day-old rats. This evidence coincides with reports using bulk and minimal stimulation of the MFs (Walker et al. 2001; Gutiérrez et al. 2003; Safiulina et al. 2006) and provides direct evidence that the giant MFBs co-release glutamate and GABA. By using Zn2+ labelling or dye transport along the MFs, followed by mechanical dissociation of the pyramidal cells, we could identify and directly stimulate single giant MFBs attached to the apical dendrite of the recorded pyramidal cell. Moreover, we could compare the MFB-evoked responses to those evoked by activation of boutons of interneuronal origin attached to the soma or basal dendrite of the same cells. The synaptic currents evoked by stimulation of identified MFBs had the signature of neurotransmission of MF origin, as shown in hippocampal slices, namely, frequency potentiation and depression by mGluR agonists (Kamiya et al. 1996; Salin et al. 1996; Nicoll & Schmitz, 2005).
The dissociation technique that we used (Vorobjev, 1991; Akaike & Moorhouse, 2003), combined with fluorescent labelling of the MFBs, ensures high specificity of the responses that we obtained. Indeed, the boutons that we identified as MFBs and that were stimulated were all localized in the proximal third region of the apical dendrite of the pyramidal cells. Further, our experimental design ensures that the responses that we evoked originated from single MFBs because: (a) shifting the stimulation pipette from the centre of the targeted fluorescent MFB avoided synaptic responses; the responses (b) were evoked in an all-or-none fashion, (c) had the same stimulation threshold, (d) had a constant latency and, finally, (e) had the signature of MF neurotransmission as they underwent frequency potentiation and were inhibited by mGluR activation. By contrast, the stimulation of IntBs evoked responses that underwent frequency depression, and that were insensitive to mGluR activation. Noteworthy, it has been reported that even high-frequency stimulation of severed MFs causes potentiation, indicating that the soma is not needed for potentiation to be expressed in the MF synapse (Calixto et al. 2003).
Mossy fibre bouton-evoked synaptic currents were characterized by distinctive decay kinetics. Indeed, glutamatergic-only responses had a single decay constant, while mixed, glutamatergic–GABAergic responses had two decay constants. Further, evoking synaptic responses while holding the membrane potential at a value between the estimated reversal potentials for pure glutamatergic responses and pure GABAergic responses produced a biphasic inward-outward current, consistent with co-release of these amino acids. Perfusing iGluR antagonists that blocked the current with the fast decay component could isolate the slow, GABA-R-mediated component, which, in turn, was blocked by the GABAA receptor antagonist. Conversely, blockage of the current with the slow decay component with bicuculline isolated the current with the fast decay component. Moreover, both the glutamate-R-mediated and the GABA-R-mediated responses obtained by stimulation of a single MFB had the same latency. On the other hand, analysis of spontaneous currents showed that they also had either a single, fast (or slow) decay constant, or two decay constants. Currents with a slow decay constant may arise from IntBs or from MFBs, but fast and mixed decay constants may only arise from MFBs, as shown above. Indeed, these responses are evoked by stimulation of single MF boutons, which were not only identified by their Zn2+ content but they were restricted to the proximal third region of the apical dendrite of the pyramidal cells. Noteworthy, the differential distribution of excitatory inputs from the perforant path and MFs allows us to further discriminate the type of bouton that we stimulated (for a review see Jaffe & Gutiérrez, 2007). On the other hand, the inhibitory inputs that we stimulated were on the soma or the proximal part of the basal dendrite, as distal dendritic terminals are lost by the dissociation procedure.
Altogether, our evidence confirms that MFBs are able to co-release glutamate and GABA and, in agreement with data provided by Walker et al. (2001), support the idea that these amino acids are released from distinct populations of vesicles because independent glutamatergic, GABAergic and mixed, glutamatergic–GABAergic events can be observed.
Several authors have reported that minimal stimulation of the MFs provokes monosynaptic GABAergic-only responses during the first week of life in rodents (Safiulina et al. 2006), mixed glutamatergic and GABAergic responses in young, developing rodents (Walker et al. 2001; Bergersen et al. 2003; Gutiérrez et al. 2003; Safiulina et al. 2006) and glutamatergic-only responses also in developing rodents (Uchigashima et al. 2007). By contrast, MF stimulation after the third week of age no longer evokes GABAergic responses (Gutiérrez et al. 2003; Romo-Parra et al. 2008). In accordance, we show here that stimulation of MFBs in preparations of >23 days of age evoked a current with a fast decay component only, which was blocked by iGluR antagonists. Indeed, the likelihood of finding monosynaptic GABAergic responses on MF stimulation decreases with age until, after 3 weeks of age, they can no longer be evoked (Gutiérrez et al. 2003; Romo-Parra et al. 2008; this work). Developmental regulation of neurotransmitter phenotype in neurons bearing a dual phenotoype has already been documented. GABA has depolarizing actions during the first week of life. Because of this, it has been proposed that the GABAergic input onto CA3 cells, thought to be originated in hilar and CA3 interneurons, exerts a trophic effect during development (Ben-Ari et al. 1994, 1997). Therefore, the granule cells could normally express their GABAergic phenotype during development, when GABA is needed to exert the trophic input to pyramidal cells adding to the hilar and CA3 interneurons GABAergic input. This depolarizing effect can even be exerted for a longer period in the dendritic compartment until full maturation of the pyramidal cells (Romo-Parra et al. 2008). After completion of development, the expression of the GABAergic phenotype could be downregulated to establish the adult interneuron-mediated disynaptic inhibition onto CA3 targets. In the auditory system the release of GABA and glycine along with the VGlut3-dependent co-release of glutamate is necessary for adequate refinement of the circuit (Noh et al. 2010). VGlut2 expression and glutamate release is developmentally regulated in dopaminergic neurons (El Mestikawy et al. 2011). In the hippocampal granule cells, we have shown that VGlut1 and GAD67 are developmentally regulated (Lamas et al. 2001; Gutiérrez et al. 2003; Gómez-Lira et al. 2005) and, during this period, their stimulation provokes monosynaptic GABA-R-mediated responses in their target cells (Walker et al. 2001; Gutiérrez et al. 2003; Kasyanov et al. 2004; Safiulina et al. 2006). Moreover, it can produce collateral inhibition by activating GABAA receptors in other MFs (Ruiz et al. 2003; Treviño & Gutiérrez, 2005). It has been suggested that GABA released from the MFs may contribute to the refinement of neuronal connectivity during development in a very site-specific manner (Kasyanov et al. 2004; Romo-Parra et al. 2008).
Acknowledgments
Work in partial fulfillment of the requirements for the Degree of Doctor of Science by J.Q.B. at the Centro de Investigación y Estudios Avanzados. We thank Dr N. Akaike and Dr M.-C. Shin for their help in establishing the nerve-bouton technique, Dr M. Treviño and Dr Y. Yaari for helpful discussions and Dr David Elias and José A. Pérez-Guzmán for the construction of the cell dissociation device. This study was supported by grants to R.G. (45754 and I020/193/10 FON.INST.-29-10) and J.Q.B. by a PhD scholarship, both from Consejo Nacional de Ciencia y Tecnología.
Glossary
- GAD
glutamic acid decarboxylase
- iGluR
ionotropic glutamate receptor
- IntB
interneuronal bouton
- MF
mossy fibre
- MFB
mossy fibre bouton
- mGluR
metabotropic glutamate receptor
Author contributions
R.G. conceived and designed the experiments, J.Q.B. conducted the experiments, J.Q.B. and R.G. analysed the data. R.G. wrote the paper. Both authors approved the final version.
References
- Acsády L, Kamondi A, Sik A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N, Moorhouse AJ. Techniques: applications of the nerve-bouton preparation in neuropharmacology. Trends Pharmacol Sci. 2003;24:44–47. doi: 10.1016/s0165-6147(02)00010-x. [DOI] [PubMed] [Google Scholar]
- Akaike N, Murakami M, Katsurabayashi S, Jin Y-H, Imazawa T. Focal stimulation of single GABAergic presynaptic boutons on the rat hippocampal neuron. Neurosci Res. 2002;42:187–195. doi: 10.1016/s0168-0102(01)00320-0. [DOI] [PubMed] [Google Scholar]
- Beltrán JQ, Reyes S, Pérez-Guzmán JA, Elías-Viñas D, Gutiérrez R. Dissociation of CA3 pyramidal cells with attached, functional, identified mossy fiber and interneuronal boutons for studying glutamatergic and GABAergic synaptic transmission. J Neurosci Methods. 2012;208:155–160. doi: 10.1016/j.jneumeth.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘ménage a trois’. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tseeb V, Raggozzino D, Khazipov R, Gaiarsa JL. γ-Aminobutyric acid (GABA): a fast excitatory transmitter, which may regulate the development of hippocampal neurones in early postnatal life. Prog Brain Res. 1994;102:261–273. doi: 10.1016/S0079-6123(08)60545-2. [DOI] [PubMed] [Google Scholar]
- Bergersen L, Ruiz A, Bjaalie JG, Kullmann DM, Gundersen V. GABA and GABAA receptors at hippocampal mossy fibre synapses. Eur J Neurosci. 2003;18:931–941. doi: 10.1046/j.1460-9568.2003.02828.x. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Li L, Geiger JR, Jonas P. Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat Protoc. 2006;1:2075–2081. doi: 10.1038/nprot.2006.312. [DOI] [PubMed] [Google Scholar]
- Calixto E, Thiels E, Klann E, Barrionuevo G. Early maintenance of hippocampal mossy fiber–long-term potentiation depends on protein and RNA synthesis and presynaptic granule cell integrity. J Neurosci. 2003;23:4842–4849. doi: 10.1523/JNEUROSCI.23-12-04842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mestikawy S, Wallén-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- Gómez-Lira G, Lamas M, Romo-Parra H, Gutiérrez R. Programmed and induced phenotype of the hippocampal granule cells. J Neurosci. 2005;25:6939–6946. doi: 10.1523/JNEUROSCI.1674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Lira G, Trillo E, Ramírez M, Asai M, Sitges M, Gutiérrez R. The expression of GABA in mossy fiber synaptosomes coincides with the seizure-induced expression of GABAergic transmission in the mossy fiber synapse. Exp Neurol. 2002;177:276–283. doi: 10.1006/exnr.2002.7986. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. The dual glutamatergic-GABAergic phenotype of the hippocampal granule cells. Trends Neurosci. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Romo-Parra H, Maqueda J, Vivar C, Ramírez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J Neurosci. 2003;23:5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harata N, Wu J, Ishibashi H, Ono K, Akaike N. Rundown of GABAA responses under experimental ischemia in acutely dissociated CA1 pyramidal neurons of the rat. J Physiol. 1997;500:673–688. doi: 10.1113/jphysiol.1997.sp022052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Gutiérrez R. Mossy fiber synaptic transmission: communication from the dentate gyrus to area CA3. Prog Brain Res. 2007;163:109–132. doi: 10.1016/S0079-6123(07)63006-4. [DOI] [PubMed] [Google Scholar]
- Jang I-S, Nakamura M, Ito Y, Akaike N. Presynaptic GABAA receptors facilitate spontaneous glutamate release from presynaptic terminals on mechanically dissociated rat CA3 pyramidal neurons. Neuroscience. 2006;138:25–35. doi: 10.1016/j.neuroscience.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol. 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101:3967–3972. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas M, Gómez-Lira G, Gutiérrez R. Vesicular GABA transporter mRNA expression in the dentate gyrus and in mossy fiber synaptosomes. Mol Brain Res. 2001;93:209–214. doi: 10.1016/s0169-328x(01)00202-9. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci. 2010;13:232–238. doi: 10.1038/nn.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez M, Gutiérrez R. Activity-dependent expression of GAD67 in the granule cells of the rat hippocampus. Brain Res. 2001;917:139–146. doi: 10.1016/s0006-8993(01)02794-9. [DOI] [PubMed] [Google Scholar]
- Romo-Parra H, Treviño M, Heinemann U, Gutiérrez R. GABA actions in hippocampal area CA3 during postnatal development: differential shift from depolarizing to hyperpolarizing in somatic and dendritic compartments. J Neurophysiol. 2008;99:1523–1534. doi: 10.1152/jn.01074.2007. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAA receptors at hippocampal mossy fibres. Neuron. 2003;39:961–973. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safiulina VF, Fattorini G, Conti F, Cherubini E. GABAergic signaling at mossy fiber synapses in neonatal rat hippocampus. J Neurosci. 2006;26:597–608. doi: 10.1523/JNEUROSCI.4493-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;23:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Treviño M, Gutiérrez R. The GABAaergic projection of the dentate gyrus to hippocampal area CA3 of the rat: pre- and postsynaptic actions after seizures. J Physiol. 2005;567:939–949. doi: 10.1113/jphysiol.2005.092064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M, Fukaya M, Watanabe M, Kamiya H. Evidence against GABA release from glutamatergic mossy fiber terminals in the developing hippocampus. J Neurosci. 2007;27:8088–8100. doi: 10.1523/JNEUROSCI.0702-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev V. Vibrodissociation of sliced mammalian nervous tissue. J Neurosci Methods. 1991;38:145–150. doi: 10.1016/0165-0270(91)90164-u. [DOI] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron. 2001;29:703–715. doi: 10.1016/s0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Zander JF, Münster-Wandowski A, Brunk I, Pahner I, Gómez-Lira G, Heinemann U, Gutiérrez R, Laube G, Ahnert-Hilger G. Synaptic and vesicular co-existence of VGLUT and VGAT in selected excitatory and inhibitory synapses. J Neurosci. 2010;30:7634–7645. doi: 10.1523/JNEUROSCI.0141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


