Abstract
Light shifts the timing of the circadian clock according to a phase response curve (PRC). To date, all human light PRCs have been to long durations of bright white light. However, melanopsin, the primary photopigment for the circadian system, is most sensitive to short wavelength blue light. Therefore, to optimise light treatment it is important to generate a blue light PRC. We used a small, commercially available blue LED light box, screen size 11.2 × 6.6 cm at ∼50 cm, ∼200 μW cm−2, ∼185 lux. Subjects participated in two 5 day laboratory sessions 1 week apart. Each session consisted of circadian phase assessments to obtain melatonin profiles before and after 3 days of free-running through an ultradian light–dark cycle (2.5 h wake in dim light, 1.5 h sleep in the dark), forced desynchrony protocol. During one session subjects received intermittent blue light (three 30 min pulses over 2 h) once a day for the 3 days of free-running, and in the other session (control) they remained in dim room light, counterbalanced. The time of blue light was varied among subjects to cover the entire 24 h day. For each individual, the phase shift to blue light was corrected for the free-run determined during the control session. The blue light PRC had a broad advance region starting in the morning and extending through the afternoon. The delay region started a few hours before bedtime and extended through the night. This is the first PRC to be constructed to blue light and to a stimulus that could be used in the real world.
Key points
Misalignment between the internal circadian clock driving daily rhythms in physiology and behaviour, such as sleepiness, performance and metabolism, and the sleep–wake schedule, as occurs in jet lag and night shift work, can have profound, harmful consequences for health, performance and safety.
Light applied at specific times of day can be used to shift the timing of the clock and reduce this circadian misalignment.
We show for the first time that a small, commercially available, portable blue light device is capable of shifting the clock when it is administered daily over a 2 h window (90 min blue light as 30 min pulses with 15 min breaks).
The direction and amount that the clock is shifted depends on the time of day that the light is administered.
The results of this work provide a practical, effective light treatment that can be used in the real world.
Introduction
The ability of light to phase shift the circadian clock can be exploited to treat situations of circadian misalignment in which the clock is inappropriately aligned with the desired sleep—wake schedule, such as jet lag, night and early morning shift work, the delayed sleep propensity of extreme night owls (evening types), and the delayed sleep phase disorder (DSPD). Phase response curves (PRCs) to light describe the relationship between the timing of a light stimulus and the direction and size of the phase shift induced. To date, several full and partial PRCs have been generated to bright white, polychromatic light using either a single pulse (Honma et al. 1987; Minors et al. 1991; Dawson et al. 1993; Van Cauter et al. 1994; Khalsa et al. 2003) or multiple pulse (Czeisler et al. 1989; Minors et al. 1991; Khalsa et al. 2003; Revell & Eastman, 2005; Kripke et al. 2007) protocols.
However, the primary photopigment for non-visual responses to light has now been identified as blue light sensitive melanopsin which is expressed in the intrinsically photosensitive retinal ganglion cells (ipRGCs), rather than the rods and cones (Gooley et al. 2001; Berson et al. 2002). Rodent melanopsin appears to be maximally sensitive to ∼480 nm light (Hattar et al. 2003; Panda et al. 2005; Qiu et al. 2005). In humans, a number of non-visual light responses exhibit short wavelength sensitivity (Lockley et al. 2003; Cajochen et al. 2005; Revell et al. 2005; Lockley et al. 2006; Revell et al. 2006a; Ackermann et al. 2009; Sletten et al. 2009) with action spectra for melatonin suppression exhibiting maximal sensitivity ∼460 nm (Brainard et al. 2001; Thapan et al. 2001). Although blue light is highly efficacious, there are now a number of lines of evidence indicating that the rod and cone photopigments can also contribute to non-visual responses under specific circumstances (Dkhissi-Benyahya et al. 2007; Altimus et al. 2010; Lall et al. 2010). More specifically, in humans a comparison of 460 and 555 nm light over a range of irradiances for melatonin suppression and phase shift responses suggested that at lower irradiances the M-/L-cones may dominate non-visual photoreception (Gooley et al. 2010). Being able to identify the relative contribution of rods, cones and melanopsin to non-visual light responses will be key to being able to predict responses to specific lighting scenarios.
The identification of melanopsin led lighting manufacturers to develop ‘blue light’ products or ‘blue-enriched’ white lights. At the high intensities typically used in light treatment paradigms the blue-enriched lights perform similarly to white light, presumably because the system is saturated (Smith & Eastman, 2009; Smith et al. 2009; Terman, 2009). However, if melanopsin is being specifically targeted then it should be possible to reduce the irradiance and duration of the light whilst still inducing an effect. Previous studies assessing the phase shifting capacity of blue light have indeed demonstrated the efficacy of low irradiances but this is primarily utilising long duration light pulses administered via specialised spheres to dark adapted subjects with pharmacologically dilated pupils (Lockley et al. 2003; Sletten et al. 2009). Such artificial scenarios have limited direct application.
The goLITE is a commercially available, small blue light device of LEDs. Its irradiance is much lower than that currently recommended for light treatment, which could improve compliance and tolerability. The size and portability of the device mean that it can be easily transported and utilised in a variety of settings making this device representative of the type that will likely be used in real life. It was important to determine if such a small, nearly monochromatic, blue light box could actually phase shift the circadian rhythms of humans with freely moving pupils whilst using a practical administration paradigm and light intensity. We also wanted to identify the optimal time to achieve phase advances and delays. The generation of a PRC to a blue light device will be a crucial tool to researchers and for those prescribing light treatments with other blue light devices besides the goLITE.
Previously, we have generated PRCs to 0.5 and 3.0 mg exogenous melatonin (Burgess et al. 2008, 2010) and a partial PRC to bright white light (Revell & Eastman, 2005) using subjects that were free-running through an ultradian light–dark (LD) cycle. In the current study we have used the same protocol to generate a PRC to the blue goLITE. These studies will allow the phase shifting effects of blue light and melatonin to be directly compared within the same protocol which will be crucial for establishing the relative phase shifting efficacy of these two stimuli which are both utilised in the treatment of circadian misalignment.
Methods
Ethical approval
The study was approved by the Rush University Medical Centre Institutional Review Board and conformed to the standards set by the Declaration of Helsinki. Written informed consent was obtained from all participants prior to their participation. Participants were reimbursed for their participation.
Subjects
This study took place in the Biological Rhythms Research Laboratory in Chicago from 2008 to 2011. A total of 54 healthy young subjects were enrolled in the study, 42 completed the study, and 37 had sufficient data to be included in the final analysis (22 M, 15 F; mean age ± SD = 25.8 ± 5.4 years; range 18–41; mean body mass index ± SD = 24.2 ± 3.1 kg m−2). Eligibility for the study was assessed via a telephone and in-person interview, self-reported medical history, a series of self-completed questionnaires as previously detailed (Burgess et al. 2008) and the Ishihara Colour Blindness test. There were four moderate evening types, 25 neither types and eight moderate morning types (Horne & Ostberg, 1976). Habitual caffeine (≤300 mg per day) and alcohol (≤2 drinks per day) consumption did not exceed study criteria and subjects were non-smokers and tested negative for common drugs of abuse. Subjects were free from prescription medication except for four females who reported taking oral contraceptive pills. Subjects had not worked night shifts or crossed more than two time zones in the month preceding the study.
Protocol
The protocol (Fig. 1) has been described in detail previously (Burgess et al. 2008). Briefly, subjects were required to maintain a fixed sleep schedule similar to their habitual sleep times for 7 days prior to being admitted to a 5 day residential laboratory session which included three 24 h days of a 4 h ultradian LD cycle and pre-stimulus (baseline) and post-stimulus (final) phase assessment sessions. During the ultradian LD cycles, subjects slept on cots arranged around the edges of a large room. During wake episodes they sat on their cots or at a large table near the centre of the room, and food and drink were available ad libitum. The room was illuminated by three fluorescent (4100 K) ceiling fixtures controlled by a dimmer switch locked to the lowest position. During the wake periods the light level experienced by the subjects was 36.5 ± 13.0 lux (mean ± SD). The circadian phase assessments took place in an adjoining room where subjects sat in comfortable recliners in dim light <5 lux. The subjects were continuously observed by research assistants who ensured that the subjects remained awake throughout the session, collected saliva samples from the subjects every 30 min using Salivettes (Sarstedt, Newton, NC, USA), and provided the subjects with snacks and beverages. No food or drink was permitted in the 10 min before each saliva sample. Upon discharge, subjects maintained their fixed sleep schedule for a further 9 days before being admitted to the second 5 day laboratory session.
Figure 1. Protocol diagram of the five day laboratory session.
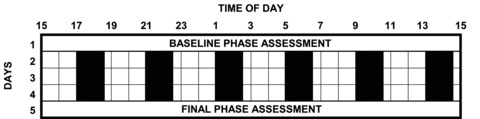
During the circadian phase assessments (days 1 and 5), subjects provided saliva samples every 30 min in dim light (<5 lux). There were 3 days (days 2 to 4) of ultradian light–dark cycles. Subjects had a sleep opportunity during each 1.5 h dark episode (black bars), and were required to remain awake for each 2.5 h dim light episode (∼35 lux). The blue light stimulus (three 30 min pulses) was administered once a day for 3 days at the same clock time during one of the six wake, dim light episodes. Subjects were run in groups of two to four people. The timing of the light stimulus varied between groups in order to span the entire 24 h day. There were two 5 day laboratory sessions separated by about a week. The light stimulus was only administered during one of these labaratory sessions; during the other control laboratory session subjects remained in dim room lighting throughout each wake episode (counterbalanced). Figure reproduced from Burgess et al. 2008.
Blue light was administered during one of the laboratory sessions, counterbalanced; 16 subjects received blue light in the first laboratory session and 21 received blue light in the second session. Each study group (maximum n= 4) had a pre-determined light stimulus administration time. There were six possible clock times for the light stimulus, because there were six wake episodes per 24 h. Subjects were enrolled in the first available group. Thus, the assignment of subjects to a particular time slot was random. As the light exposure was administered at specific clock times, this was not the same circadian phase in each subject, but this just meant that the stimuli were administered across a broader range of circadian phases.
Saliva samples from the circadian phase assessments were immediately centrifuged and frozen for later analysis by Pharmasan Laboratories (Osceola, WI, USA). Samples from each subject were assayed together to minimize assay variability. The sensitivity of the assay was 0.7 pg ml−1 and the intra- and interassay variability were 12.1% and 13.2%, respectively. The salivary melatonin profiles from the baseline and final phase assessments sessions were compared to derive the phase shift that had occurred during the session.
Blue light stimulus
The light stimulus was provided by the goLITE BLU (14 × 14 × 2.5 cm, Apollo Light Systems Inc, American Fork, UT, USA; currently Philips CL, Drachten, the Netherlands) which contains a small panel of 60 LEDs (11.2 × 6.6 cm) with a spectral power output in the short wavelength, blue region of the visible light spectrum. The goLITE emits light between ∼450 and 500 nm with a λmax∼467 nm and half-maximal bandwidth ∼22 nm. Philips Lighting recommends that the light should be placed approximately 20–30 inches (50–70 cm) from the eyes in such a way that the light bathes the face from the side.
Subjects were required to sit round a table coated with white reflective material in an appropriate position relative to their individual goLITE. The goLITE (set to the 100% level) was positioned according to the manufacturer's recommendations at a 20 deg angle and a distance of 51 cm (20 inches). When the light was turned on, the subjects’ faces were bathed in blue light; the subjects were instructed not to look directly at the light. During the light exposure periods and the corresponding times in the control session, the subjects read quietly.
An intermittent light stimulus was used as this represented a practical paradigm that could be incorporated into everyday life around specific tasks. The overhead lights remained on during the blue light administration as this is how such light boxes would be used in the real world, i.e. with background lighting. Within the assigned 2.5 h wake episode, the blue light stimulus began 15 min after the subjects were woken up. The subjects were exposed to a total of 90 min of blue light administered as three 30 min pulses interspersed with 15 min of dim room light. Thus, the light train spanned a total of 2 h. Individual light intensities at eye level were measured at 1, 5, 15 and 25 min into each 30 min light pulse with an IL-1400 Radiometer/Photometer (International Light Technologies, detector head SEL033, Input Optic W no. 10878 and Filter F no. 25809). If the irradiance was out of range (<200 or >230 μW cm−2), subjects were asked to adjust their position accordingly. The irradiance was 213.3 ± 10.0 μW cm−2 (mean ± SD) which is equivalent to 5.0 × 1014 photons cm−2 s−1 and 184.8 ± 3.5 lux. In addition, melanopic lux (m-lux), which describes melanopsin photoreception, was calculated according to the methods of Enezi et al. (2011), as 7856 m-lux. During the corresponding clock times in the control sessions the subjects sat at the same table and remained in dim room lighting of 32.2 ± 8.9 lux (mean ± SD).
Data analysis
The melatonin profiles were smoothed with a locally weighted least squares (LOWESS) curve using the ‘fine’ setting in GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA). Each profile was then normalized by taking the maximum and minimum of the fitted curve and assigning them the values of 100% and 0%, respectively. A threshold of 25% was used to determine the time that the ascending limb of the smoothed curve crossed this threshold, and this was designated as the dim light melatonin onset (DLMO).
To be able to accurately determine the phase shifts to the blue light stimulus, it was essential to be able to eliminate the impact of the ultradian protocol (the free run). As such, the phase shift to the stimulus was corrected for the shift due to the free run on an individual basis. The phase shift in each session was the change in phase of the DLMO from the baseline to the final phase assessment. Subsequently, the phase shift in the room light (control) session was subtracted from the phase shift in the blue light session to provide the net phase shift due to blue light alone. This net phase shift was plotted on the y-axis and the start of the light stimulus relative to the baseline DLMO was plotted on the x-axis.
Results
Figures 2 and 3 show example melatonin profiles from two different subjects to demonstrate how the net phase shift was calculated, and the necessity for the control session in determining the magnitude and direction of the phase shift. Figure 2 shows the raw data from a subject who delayed during both lab sessions. The large phase delay observed during the blue light session (bottom) was corrected by subtracting out the smaller phase delay due to free-running during the control session (top) in order to plot the net phase delay on the PRC. Figure 3 shows the normalized, fitted curves from a subject whose net phase shift was an advance. The DLMO delayed less during the blue light session (bottom) than during the free-run session (top). Thus, the blue light reduced the magnitude of the delay due to the free-run; hence, the circadian clock was advanced by the blue light stimulus.
Figure 2. Raw melatonin profiles from one subject in the control and ‘blue light’ laboratory sessions.
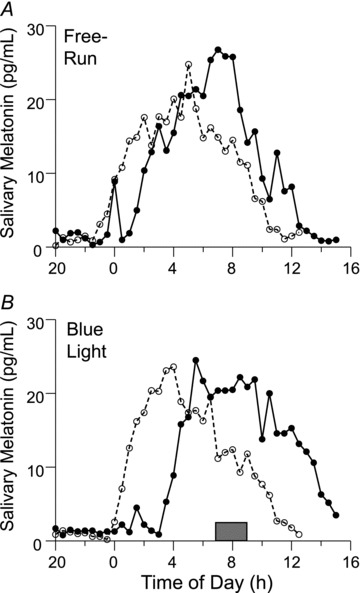
The profiles with the dashed lines and open circles are from the baseline phase assessments and the profiles with the continuous lines and filled circles are from the final phase assessments. A, control lab session without blue light. The profile delayed ∼2 h due to the free-run through the ultradian LD cycle. B, lab session in which intermittent blue light was applied once a day during the 3 days of ultradian LD cycles. The shaded rectangle over the x-axis shows the time of the blue light stimulus, from the start of the first 30 min pulse to the end of the third 30 min pulse. The profile delayed ∼3 h. Thus, the net phase shift due to the blue light alone, after subtracting out the delay from the free-run, was a delay of ∼1 h.
Figure 3. Fitted and normalized curves to the melatonin profiles of one subject.
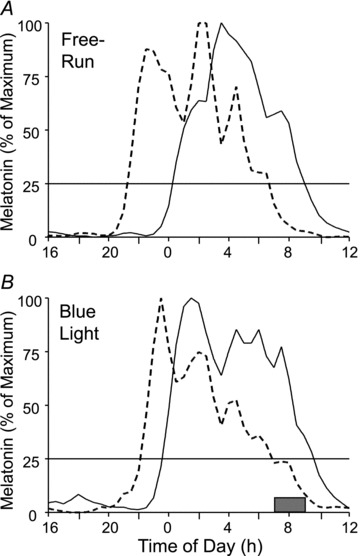
The profiles with the dashed lines are from the baseline phase assessments and the profiles with the continuous lines are from the final phase assessments. The horizontal lines at 25% are the thresholds for determining the dim light melatonin onset (DLMO) of each curve. A, lab session without blue light. The DLMO delayed 3.0 h due to the free-run through the ultradian LD cycle. B, lab session in which intermittent blue light was applied once a day during the 3 days of ultradian LD cycles. The shaded rectangle over the x-axis shows the time of the blue light stimulus. The DLMO delayed 1.5 h. Thus, the net phase shift due to the blue light alone, after subtracting out the delay from the free-run, was an advance of 1.5 h.
Figure 4 shows the net phase shifts of all the subjects. The data were then binned and double plotted (Fig. 5) and the familiar light PRC shape is seen. Delays predominate in the evening from around the time of the DLMO through the time when subjects would ordinarily be asleep. Advances predominate during the rest of the day, especially during the time corresponding to usual wake up time in the morning through the afternoon. There is no pronounced dead zone. This binned PRC shows slightly larger advances than delays. The individual phase shifts (Fig. 4) show maximum phase shifts of ∼2 h, but when binned the average phase shifts are less than 1 h.
Figure 4. Individual net phase shifts of the dim light melatonin onset (DLMO) in response to an intermittent blue light stimulus, on a background of dim white light from ceiling fluorescent fixtures, administered daily at a fixed clock time for 3 days.
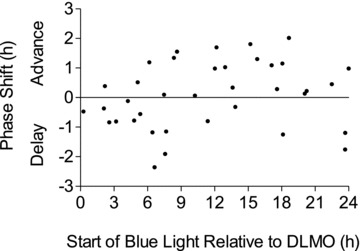
The net phase shifts were calculated as the phase shifts to blue light corrected for the individual's free-run which was determined during the 5 day control session during which no blue light stimuli were applied. The x-axis indicates the time that the intermittent blue light train started relative to the baseline DLMO.
Figure 5. Phase response curve to an intermittent blue light stimulus.
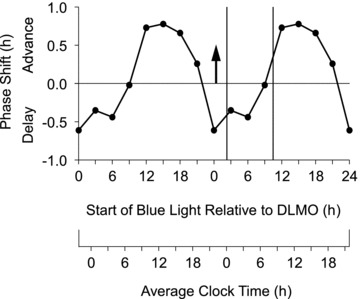
The stimulus consisted of 90 min of narrow bandwidth blue light (λmax 467 nm) administered as three 30 min pulses (∼185 lux) separated by 15 min of dim room light (∼30 lux). The x-axis shows the time of the start of the first light pulse relative to the baseline DLMO. The arrow at zero on the x-axis represents the DLMO. The vertical lines enclose the average baseline sleep schedule prior to the laboratory sessions. This PRC was generated from the individual data points in Fig. 4 which were averaged into 3 h bins and double plotted. The points are plotted according to the midpoint of the bin, e.g. the bin at 3 h after the DLMO contains data from 1.5 h to 4.5 h after the DLMO. The average clock time axis shows clock time for the average subject whose DLMO was at ∼22.00 h and whose typical sleep time was from about 00.00 to 08.00 h. To apply this PRC to individuals with earlier sleep schedules, move the clock time axis to the right, for later schedules move the axis to the left.
The mean ± SD free-running period during the control session was 24.38 ± 0.25 h and ranged from 23.8 to 24.8 h.
Discussion
The PRC generated during the current study is the first light PRC to be constructed using blue light and an administration paradigm that could be practically used in real-world settings, i.e. an intermittent pattern containing 1.5 h of light from a single light box. Thus, our blue light PRC could be a key tool in the treatment of circadian misalignment. Our binned blue light PRC (Fig. 5) shows that the optimal time to achieve a phase delay is before bedtime and during the time that people are usually asleep. Since this small blue light box produced delays at these times, the high nocturnal usage of light emitting devices including laptops, tablets and televisions could potentially influence circadian phase as well as sleep quality and timing. Our blue light PRC also shows that the optimal time to achieve phase advances occurs when light starts in the morning or afternoon. The most important difference from previous white light PRCs is that the advance region extends much later in the day, suggesting that blue light can have a phase advancing effect even when used in the afternoon. This difference between our blue light PRC and previous white light PRCs could conceivably be due to the different methodologies used to generate the PRCs, rather than to the wavelength of the light sources. To be absolutely sure we would need to generate a white light PRC using our ultradian LD cycle, forced desynchrony method.
In previous PRCs to white light the light stimulus has frequently been provided by multiple light boxes, and even entire ceilings or walls covered with light fixtures (Czeisler et al. 1989; Kripke et al. 2007). Whilst this will likely ensure uniform retinal illumination and receipt of sufficient bright light to generate a response, this is not a practical scenario. We chose the goLITE for our PRC because it is a commercially available device that is popular due to its low cost, its small size, and the fact it is easy to transport and fit into a home or work environment. One potential disadvantage of using any small light box is that there is the possibility for the light box or the individual to move such that the full irradiance of light is no longer completely illuminating the eyes. In our laboratory sessions subjects were continually supervised and the light intensity was monitored frequently. If the light intensity was insufficient, then the subjects were requested to move back into the correct position to ensure that the required irradiance of light was delivered to the eyes. To avoid this problem larger light boxes are often recommended for home use (Eastman, 2011). Nevertheless, this small blue LED device is popular with consumers and patients due to its convenience and portability; thus it was important to assess its phase shifting capacity.
Ours is the first light PRC to utilise a practical duration, pattern and irradiance of light. Previous PRCs have utilised long duration light pulses of from 3 to 6.7 h (Honma et al. 1987; Czeisler et al. 1989; Minors et al. 1991; Dawson et al. 1993; Van Cauter et al. 1994; Khalsa et al. 2003; Kripke et al. 2007); whilst such stimuli are useful for answering theoretical questions they could not be practically implemented in the majority of real-world scenarios. Our blue light stimulus was a total of 1.5 h administered over 2 h as an intermittent light pattern on a background of room lighting similar to the way it would be used out of the laboratory.
How does our blue light PRC compare to the white light PRCs? We previously generated a partial PRC to white light with a similar ultradian protocol, but with only seven subjects (seven data points) (Revell & Eastman, 2005). In our blue light PRC the advance and delay regions are much broader, the maximum advances are later in the day, and the phase delays start much earlier (compare Fig. 1 in Revell & Eastman, 2005 with the current Fig. 5). These difference could be attributable to wavelength (primarily blue vs. white), or other differences between the light stimuli (continuous vs. intermittent), or because we had so few points in our white light PRC. The light box set-up was very different for these two PRCs. For white light there were four large fluorescent light boxes (screen size 56 × 32 cm, 5095 K, SunRay, Sun Box Co., Gaithersburg, MD, USA) set up on stands around a large round table pointing inwards so that each subject was illuminated by all four light boxes. The intensity ranged from about 3000 to 6000 lux depending on the direction of gaze, and averaged ∼1200 μW cm−2 and ∼3.1 × 1015 photons cm−2 s−1. In comparison, for the blue light PRC each subject was only exposed to light from one small goLITE (screen size 11.2 × 6.6 cm) and with background white ceiling lighting they were exposed to ∼185 lux, ∼200 μW cm−2 and 5 × 1014 photons cm−2 s−1. The white light boxes were on for two continuous hours, whereas the goLITE was on for a total of 1.5 h spread over 2 h. Despite these differences (a greater intensity, duration and size of the white light boxes), the goLITE produced a few individual phase shifts as large as the maximum phase shifts from the white light set-up. For a rigorous comparison we would need a white light PRC that was generated with more than seven subjects. It would be even more useful to compare the goLITE PRC to a PRC generated with a single large white light box, i.e. the way it would be used in the real world.
Another relevant three-pulse white light PRC was generated by Kripke et al. (2007) with subjects free-running through a 90 min ultradian LD cycle for about 5 days. Bright white light (3000 lux for 3 continuous hours) was applied for 3 days. One of the phase markers, the onset of urinary 6-sulphatoxymelatonin (aMT6s, a melatonin metabolite), can be compared to the DLMO, and showed the phase change from before to after the 3 days with bright light. The binned PRC for their young subjects (18–31 years, similar in age to the blue light PRC subjects) shows maximum advances when the 3 h light exposure was centred at 08.00 h, 10.00 h and noon, and maximum delays with the light centred at midnight, 02.00, 04.00 and 06.00 h (see their Fig. 5, bottom). In comparison, our blue light PRC has a slightly broader and slightly later phase advance region.
To compare these two PRCs exactly, the duration of the light pulses and the size of the bins have to be taken into account mathematically. This exercise reveals that their three highest points in the advance region (at 08.00, 10.00 and 12.00 h) include pulses that started as early as 05.30 h and ended as late as 14.30 h, whereas our three highest points in the advance region (at about 10.30, 13.30 and 16.30 h) include light pulses that started as early as 09.00 and ended as late as 20.00. On the other hand, the two delay portions are more similar. The slight difference in timing between the advance portions of these two PRCs could be because of the spectral composition of the light stimulus (blue vs. white), but it could also be due to the numerous methodological differences between the studies including sampling frequency (30 min saliva samples vs. 90 min urine sampling) and sample size. The increased amplitude in this white light PRC compared to our blue light PRC could be due to their increased duration and intensity light stimulus. The amplitude of their advance and delay regions looks similar, because their horizontal ‘zero’ line (at about –1 on the y-axis) is the average of all the points. Whether this average represents the average free-run can be debated. If the line is drawn higher, then the magnitude of the advances decreases while the magnitude of the delays increases; if lower, then the opposite changes occur. Our zero line actually represents no shift from the blue light, no difference from the free-run, because we obtained the free-running period of each individual subject. Therefore, we can better compare our advance and delay portions, and we see a slightly higher amplitude advance portion.
The Kripke et al. PRC to white light appears to have a ‘dead zone’ from 14.00 to 22.00 h, but a glance at the individual points in the panel above (their Fig. 5A) shows that this is a time when there are both advances and delays which average out rather than a time of no phase shifts. This is a time when advances predominate in our blue light PRC. In summary, our blue light PRC has an advance portion that is delayed by a few hours compared to the white light PRC of Kripke et al.
It is even more difficult to compare our blue light PRC to the white light PRCs of Czeisler et al. (1989) and Khalsa et al. (2003), because these PRCs were generated in re-entrainment protocols rather than the classic PRC generating protocol in which a stimulus is applied to free-running animals. In their PRCs, the 8 h sleep/dark episodes were either advanced or delayed by various amounts while bright white light was applied in the centre of the waking episode. In the PRC by Czeisler and colleagues (1989) there were 5 h episodes of very intense light (∼10,000 lux) for 3 days plus 4 days in which the 8 h sleep/dark episode was shifted. For the PRC by Khalsa and colleagues (2003) sleep/dark was shifted for 2 days while a single light ‘pulse’ (6.7 h, ∼10,000 lux) was applied. In these protocols, even if bright light was not applied the circadian rhythms would gradually phase advance or phase delay to re-entrain to the shifted sleep/dark episodes, to the new 24 h LD cycle. Thus, bright light was used to hasten re-entrainment to the shifted LD cycle, and the PRC really describes how far the rhythms shifted after a few days of re-entrainment. There were larger phase shifts in the ‘3 pulse’ PRC (Czeisler et al. 1989) than the ‘1 pulse’ PRC (Khalsa et al. 2003) as expected, because the final phase was measured much later in the re-entrainment process. For more discussion see Beersma & Daan (1993).
The shape of our blue light PRC (Fig. 5) is similar to the white light PRC of Khalsa et al. (2003) (Fig. 4A) in that there are broad advance and delay zones and no extended dead zone. But it is clear that our blue light PRC is delayed by several hours compared to the white light PRC. To visualize this, draw vertical lines for typical sleep times on Fig. 4A of Khalsa et al. at 19 and 3 (equivalent to the 8 h baseline sleep episode starting 5 h before the temperature minimum, which is at 0, and ending 3 h after the temperature minimum) and compare it to our Fig. 5.
For an even more precise comparison note that we plotted the time of the beginning of the 2 h light train on the x-axis in order to compare to the pill administration times of our melatonin PRCs, whereas Khalsa et al. plotted the midpoint of the 6.7 h light exposure. Therefore, imagine our PRC shifted to the right by 1 h (but leave the sleep lines in place) which would occur if we plotted our PRC according to the middle of the 2 h light train. Or imagine the Khalsa et al. PRC shifted to the left by 3.35 h, which would occur if they plotted their PRC according to the start of the 6.7 h light pulse. Either way, our PRC is phase delayed by several hours compared to theirs.
Also note that the Khalsa et al. PRC has a horizontal dotted line to represent no phase shift which is below 0 on the y-axis (at −0.54 h). This is calculated from the mean free-running period of 24.18 h derived from another study (Czeisler et al. 1999), and thus the phase shifts in this PRC are automatically corrected for an average free-run (0.18 × 3 = 0.54). Just like for the PRC of Kripke et al. the placement of this horizontal line affects the amplitude of the advance and delay portions, but does not affect their timing. Using their horizontal ‘zero’ lines we see that the white light Kripke et al. and Khalsa et al. PRCs have a slightly larger amplitude delay portion than advance portion, whereas our blue light PRC has a slightly larger amplitude advance portion compared to the delay portion.
The placement of this horizontal ‘zero’ line also affects the PRC crossover point between delays and advances. If the line is placed higher, then the crossover point becomes later; if it's lower the crossover point becomes earlier. Nevertheless, using the published zero lines we see that the white light PRCs of Kripke et al. and Khalsa et al. and our blue light PRC have crossover points falling within the second half of the usual sleep period. The crossover point of the human light PRC has been of interest to clinicians and researchers alike because they usually do not want to give light on the ‘wrong side’ of the PRC. The temperature minimum is widely used as an estimate for the crossover point, and this is still a useful estimate. Obviously, the later the crossover point within sleep, then the more likely that light slightly before the usual time of waking, which is usually intended to help circadian rhythms advance, could possibly cause unintended delays. According to our PRC a good time to give blue light to produce a phase advance is to start it soon after usual wake up time. However, our blue light PRC suggests that blue light later in the day can also be effective.
An earlier model of the goLITE (P2 or M2) was previously tested in a few phase shifting protocols. This model differs from the BLU model used in our current study with a slightly different spectral distribution and higher available intensities. We tested this earlier model goLITE in our phase advancing protocol for reducing jet lag when flying east, for early morning work shifts or to help night owls and patients with the DSPD to get on an earlier schedule. In this protocol, sleep is advanced by 1 h per day for 3 days and an intermittent light stimulus covering 3.5 h (four 30 min light pulses each separated by 30 min room lighting) is administered upon awakening (Burgess et al. 2003; Revell et al. 2006b). Two subjects completed this protocol in the Chicago lab with the goLITE P2 set at 100% intensity and placed directly in front of them on a desk about 41–51 cm from their eyes on a background of dim ceiling lighting which resulted in 90–120 lux, dependent upon angle of gaze. In both cases, phase advances ∼2 h were obtained (unpublished observations) which is slightly more than the mean phase shift when we used a bright white light stimulus ∼5000 lux from a large light box (screen size 54 × 54 cm) in the same protocol (1.7 h, Revell et al. 2006b; 1.5 h, Burgess et al. 2003). This suggests that the tiny goLITE is at least as powerful as a large white light box, at least when both are combined with an advancing sleep/dark schedule.
In two studies, the goLITE P2 was administered upon awakening to try and phase advance adolescents (Crowley & Carskadon, 2010) and young adult ‘struggling night owls’ (Sharkey et al. 2011). Both studies failed to show a phase shifting effect of the goLITE. In the adolescent group, morning goLITE exposure for 1 h on a weekend (Saturday and Sunday) was combined with a large, but typical, delay of the sleep schedule (bedtime was delayed 1.5 h and wake time was delayed 3 h). Regardless of whether the goLITE was used, the DLMO delayed over the weekend, and the magnitude of the delays was not significantly different (38 vs. 46 min). In the night owls, 1 h morning light treatment was combined with a sleep schedule that was advanced 1–2.5 h, depending on the individual. The light box was either aimed towards the face (treatment group) or away from the face (control group). After 6 days, both groups exhibited significant phase advances that did not significantly differ between groups (1.4 vs. 1.5 h). In both studies it appears that the shift of the sleep/dark episodes had a more powerful effect on the circadian clock than the 1 h of goLITE exposure. Furthermore, in both studies, the light treatment was done at home and the small size of the goLITE would have made it easy for the subject to move out of range or for the light box to be knocked such that the light was aimed in the wrong direction. Preliminary results of a goLITE study on ‘relatively late chronotypes’ showed phase advances of about 50 min after three mornings of 30 min or 60 min blue light exposure (Geerdink et al. 2011). Details about the sleep schedule were not provided.
We have previously constructed three pulse PRCs to 0.5 and 3.0 mg doses of melatonin; these doses induced similar magnitude phase advances and delays to each other, but the optimal timing of the dose differed (Burgess et al. 2008, 2010). This is one of the few situations where the phase shifting abilities of melatonin and light have been directly compared within the same protocol. For the melatonin doses used, some individual phase shifts observed with exogenous melatonin were as large as those produced by the blue light. These data indicate that for the 0.5 and 3.0 mg doses melatonin is as efficient as our blue light stimulus at inducing phase shifts. It should be noted that the optimal light stimulus in terms of irradiance and pattern may not have been used such that the phase shifting ability of blue light was underestimated. In addition, the efficacy relationship between exogenous melatonin and light may vary depending on the dose and various lighting parameters. In both cases (melatonin pills, light boxes) the timing of sleep/dark and whether it is shifted, and the 24 h pattern of light exposure, especially more intense outdoor light, will have a major impact on the resultant phase shift.
Melatonin and light have both advantages and disadvantages for being used in the real world. Taking a melatonin pill is simple and less time-consuming but has the potential disadvantage of being a soporific. Light therapy can be time-consuming and inconvenient but will actually have beneficial mood and alertness boosting side effects. An intermittent light stimulus can be fitted around everyday tasks, and other activities such as eating, reading and working on a computer can be done during light exposure
In conclusion, our first PRC to a blue light stimulus will be a powerful tool in the practical application of light therapy to real-world scenarios of circadian misalignment.
Acknowledgments
We are grateful to Marissa Dziepak for data analyses. We thank the following people for assistance with data collection: Elisabeth Beam, Helen Burgess, Jillian Canton, Rose Diskin, Marissa Dziepak, Sarah Garcia, Heather Gunn, Heather Holly, Carlo Legasto, Jacqueline Munoz, Yelizaveta Sorokin, Jessica Stroup, Christina Suh and Nicole Woodrick. We thank our medical director Margaret Park, MD. We thank Apollo Health (Philips) for providing the goLITEs used in the study. This work was supported by NIH grant R01HL086934 to C.I.E. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The National Heart Lung and Blood Institute and the National Institutes of Health had no involvement in designing the study, data collection, data analysis and interpretation, writing of the manuscript, or in the decision to submit the manuscript for publication. Conflicts of interest: V.R. acts as a scientific advisor to the light box manufacturer Lumie Ltd (Cambridge, UK). Apollo Health and Philips did not provide any funds for this research.
Glossary
- DLMO
dim light melatonin onset
- DSPD
delayed sleep phase disorder
- ipRGC
intrinsically photosensitive retinal ganglion cell
- LD
light–dark
- PRC
phase response curve
Author contributions
Conception of the experiment (C.I.E.), design of the experiment (C.I.E., V.L.R.), analysis and interpretation of data (C.I.E., V.L.R., T.A.M.), drafting the article (C.I.E., V.L.R., T.A.M.), revising it critically for important intellectual content (C.I.E., V.L.R.), final approval of the version to be published (C.I.E., V.L.R., T.A.M.). This study was performed at the Biological Rhythms Research Laboratory (C.I.E., Director), Behavioural Sciences Department, Rush University Medical Center, Chicago, IL, USA.
References
- Ackermann K, Sletten TL, Revell VL, Archer SN, Skene DJ. Blue-light phase shifts PER3 gene expression in human leukocytes. Chronobiol Int. 2009;26:769–779. doi: 10.1080/07420520902929045. [DOI] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beersma DG, Daan S. Strong or weak phase resetting by light pulses in humans? J Biol Rhythms. 1993;8:340–347. doi: 10.1177/074873049300800407. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–328. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008;586:639–647. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab. 2010;95:3325–3331. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, Orgul S, Wirz-Justice A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 2010;27:1469–1492. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Dawson D, Lack L, Morris M. Phase resetting of the human circadian pacemaker with use of a single pulse of bright light. Chronobiol Int. 1993;10:94–102. doi: 10.3109/07420529309059697. [DOI] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F, Cooper HM. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron. 2007;53:677–687. doi: 10.1016/j.neuron.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI. How to get a bigger dose of bright light. Sleep. 2011;34:559–560. doi: 10.1093/sleep/34.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enezi J, Revell V, Brown T, Wynne J, Schlangen L, Lucas R. A ‘melanopic’ spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights. J Biol Rhythms. 2011;26:314–323. doi: 10.1177/0748730411409719. [DOI] [PubMed] [Google Scholar]
- Geerdink M, Beersma DGN, Hommes V, Gordijn MC. Phase advancing the human circadian system with short pulses (30 min) of blue light exposure. Abstracts of the XII Congress of the European Biological Rhythms Society, Oxford, UK. 2011:141. [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma K, Honma S, Wada T. Phase-dependent shift of free-running human circadian rhythms in response to a single bright light pulse. Experientia. 1987;43:1205–1207. doi: 10.1007/BF01945525. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Elliott JA, Youngstedt SD, Rex KM. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Guler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FA, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- Revell VL, Arendt J, Fogg LF, Skene DJ. Alerting effects of light are sensitive to very short wavelengths. Neurosci Lett. 2006a;399:96–100. doi: 10.1016/j.neulet.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Revell VL, Arendt J, Terman M, Skene DJ. Short-wavelength sensitivity of the human circadian system to phase-advancing light. J Biol Rhythms. 2005;20:270–272. doi: 10.1177/0748730405275655. [DOI] [PubMed] [Google Scholar]
- Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab. 2006b;91:54–59. doi: 10.1210/jc.2005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–365. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med. 2011;12:685–692. doi: 10.1016/j.sleep.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten TL, Revell VL, Middleton B, Lederle KA, Skene DJ. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythms. 2009;24:73–84. doi: 10.1177/0748730408328973. [DOI] [PubMed] [Google Scholar]
- Smith MR, Eastman CI. Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol Int. 2009;26:709–725. doi: 10.1080/07420520902927742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009;10:287–294. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman M. Blue in the face. Sleep Med. 2009;10:277–278. doi: 10.1016/j.sleep.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne MM, Blackman JD, Leproult R, Ofek G, L’Hermite-Baleriaux M, Refetoff S, Turek FW, Van Reeth O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol Endocrinol Metab. 1994;266:E953–E963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]


