Abstract
Catecholaminergic neurons within the central nervous system are an integral part of stress-related neurocircuitry, and the nucleus of the solitary tract (NTS) plays a critical role in cardiovascular regulation. We tested the hypothesis that NTS catecholaminergic neurons attenuate psychological stress-induced increases in blood pressure and promote neuroendocrine activation in response to psychological stress. Anti-dopamine-β-hydroxylase antibody conjugated to the neurotoxin saporin (DSAP) or saline vehicle was microinjected into the NTS to lesion catecholaminergic neurons in male Sprague–Dawley rats, and 17 days later the rats were subjected to 60 min of restraint stress for five consecutive days. DSAP treatment significantly enhanced the integrated increase in mean arterial pressure during restraint on the first (800 ± 128 and 1115 ± 116 mmHg (min) for saline- and DSAP-treated rats) and fifth days (655 ± 116 and 1035 ± 113 mmHg (min) for saline- and DSAP-treated rats; P < 0.01 for overall effect of DSAP treatment) of restraint. In contrast, after 60 min of restraint plasma corticosterone concentration was significantly lower in DSAP-treated compared with saline-treated rats (25.9 ± 7 compared with 46.8 ± 7 μg dl−1 for DSAP- and saline-treated rats; P < 0.05). DSAP treatment also significantly reduced baseline plasma adrenaline concentration (403 ± 69 compared with 73 ± 29 pg ml−1 for saline- and DSAP-treated rats), but did not alter the magnitude of the adrenaline response to restraint. The data suggest that NTS catecholaminergic neurons normally inhibit the arterial pressure response, but help maintain the corticosterone response to restraint stress.
Key points
Exaggerated cardiovascular responses to stress increase risk for hypertension and cardiovascular disease, but the mechanisms controlling the magnitude of this response are not understood.
Catecholaminergic neurons located in the hindbrain area termed the nucleus of the solitary tract (NTS) modulate the control of blood pressure and are activated by psychological stress, but their role in modulating the cardiovascular response to stress is unknown.
In this study we lesioned these NTS catecholaminergic neurons and measured the cardiovascular and hormonal responses to psychological stress in rats.
We showed that lesioning these neurons increases baseline blood pressure and causes an exaggerated blood pressure response to acute or repeated psychological stress, suggesting that physiological or pathophysiological inhibition of these neurons could lead to exaggerated stress responses and hypertension.
These results help us understand the mechanisms that contribute to enhanced cardiovascular responses to psychological stress.
Introduction
Noradrenergic and adrenergic neurotransmission within the brain can modulate the cardiovascular, neuroendocrine, behavioural and metabolic responses to psychological stress (Koepke & DiBona, 1986; Pacak et al. 1995; Morilak et al. 2005; Rauls et al. 2005; Ritter et al. 2006; Rinaman, 2007). Previous work indicates that neuronal cell bodies which synthesize noradrenaline and adrenaline are found in a limited number of brain regions, all of which are involved in cardiovascular regulation. These regions include the A2 noradrenergic and C2 adrenergic neurons within the nucleus of the solitary tract (NTS), the A5 noradrenergic neurons in the ventrolateral pons and the A1 noradrenergic and C1 adrenergic neurons of the ventral medulla (Sawchenko & Swanson, 1982; Nieuwenhuys, 1985; Cunningham & Sawchenko, 1988). Lesioning the A5 and C1 neurons does not modulate the cardiovascular response to psychological stress such as restraint (Vianna & Carrive, 2010). However, the contribution of NTS catecholaminergic neurons to this response is unknown. NTS catecolaminergic neurons are activated in response to both physiological (systemic) and psychological (emotional) stressors (Pacak et al. 1995; Dayas et al. 2001a), and could influence the physiological response to stress by way of projections to forebrain regions including the paraventricular nucleus and the amygdala (Cunningham & Sawchenko, 1988; Riche et al. 1990).
The physiological response to stress includes increases in blood pressure, heart rate, sympathetic nerve activity and circulating catecholamines, and activation of the hypothalamic–pituitary–adrenal (HPA) axis leading to increases in glucocorticoids (cortisol and corticosterone) (Pacak et al. 1995). NTS neurons can mediate the stress-induced activation of the HPA axis when the stress is physiological or systemic in nature (Ulrich-Lai & Herman, 2009). It is also possible that they contribute to HPA axis activation in response to a psychological stress, but this remains unconfirmed (Dayas et al. 2001b; Ulrich-Lai & Herman, 2009). Silencing NTS catecholaminergic neurons leads to an increase in baseline blood pressure, indicating that these neurons exert a tonic inhibitory influence on blood pressure (Duale et al. 2007); whether they also inhibit the cardiovascular response to psychological stress is unknown. The present study tested the hypothesis that NTS catecholaminergic neurons inhibit psychological stress-induced increases in blood pressure and heart rate and promote neuroendocrine activation in response to psychological stress. NTS catecholaminergic neurons were lesioned using an anti-dopamine-β-hydroxylase antibody conjugated to the neurotoxin saporin (DSAP), an established, selective and effective method to lesion noradrenaline- and adrenaline-synthesizing neurons within selected brain regions including the NTS (Wrenn et al. 1996; Madden et al. 1999; Rinaman & Dzmura, 2007). Rats were subsequently subjected to restraint stress on five consecutive days, which is categorized as a psychological stress in rats (Dayas et al. 2001a).
Methods
Ethical approval
All animal housing, handling, surgical and experimental procedures were conducted within an Association for the Assessment and Accreditation of Laboratory Care (AALAC) internationally accredited animal care facility at the University of Florida, in accordance with the USA's Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of the University of Florida approved all animal housing, handling and surgical and experimental procedures.
Animals
Experiments were performed in 52 adult male Sprague–Dawley rats purchased from Charles River Laboratories at 275–300 g. Rats were housed in a Specific Pathogen Free animal care facility with a 12:12 h light–dark cycle. All experimental and surgical procedures were conducted within the AALAC-accredited animal care facility.
Surgical procedures
General
All surgical procedures were performed using a mixture of oxygen (1 litre min−1) and isoflurane (5% for induction and 2–3% for maintenance). Carprofen (5 mg kg−1, s.c.; Pfizer Inc., New York) and penicillin G were administered (s.c.) prior to surgery. Carprofen (5 mg kg−1 day−1, s.c.) was administered for 2 days after major surgery and for 1 day following femoral catheter implantation. The rats were returned to their home cages upon full recovery from anaesthesia.
Implantation of telemetry transducers
Telemetry transducers for the measurement of arterial pressure, heart rate and activity (model PA-C40, Data Sciences International, St Paul, MN, USA) were implanted into the descending aorta via a midline abdominal incision. The aorta was isolated and briefly occluded, and the tip of the catheter was inserted using a 21-gauge needle. Surgical glue (3M Vetbond Tissue Adhesive) and a nitrocellulose patch were applied to secure the catheter in place. The transducer was sutured to the abdominal muscle and the incision closed in layers.
NTS microinjection
Anti-dopamine-β-hydroxylase conjugated to saporin (DSAP) is an immunotoxin that can be used to selectively lesion catecholaminergic neurons (Wrenn et al. 1996; Madden et al. 1999; Rinaman & Dzmura, 2007). DSAP (Advanced Targeting Systems, San Diego, CA, USA) was diluted to a working concentration of 0.22 ng nl−1 in 0.1 m sterile phosphate buffered saline (pH 7.4). Rats were anaesthetized and placed in a stereotaxic frame with the head ventroflexed at an angle of approximately 40o to allow for surgical exposure of the dorsal surface of the hindbrain. Bilateral microinjections were made with a glass micropipette (1.2/0.68 mm OD/ID, World Precision Instruments Inc., Sarasota, FL, USA) pulled to obtain a tip diameter of 30–50 μm. The micropipette tip was positioned at calamus scriptorius (rostral–caudal), 250 μm lateral to midline, and 500 μm ventral to the dorsal surface of the medulla. These coordinates were selected to target the region of the NTS that expresses c-Fos in response to restraint stress (Dayas et al. 2001a). DSAP (22 ng in 100 nl) or sterile 0.9% saline (100 nl) was delivered bilaterally into NTS by micro syringe pump over 60 s (Stoelting Co., Wood Dale, IL, USA). The incision was sutured closed. Rats continued to gain weight following the surgery, and body weight at the conclusion of the studies averaged 467 ± 6 g and 481 ± 11 g for saline- and DSAP-treated rats, respectively.
Arterial catheter implantation
Indwelling arterial catheters were implanted to obtain blood samples for the measurement of plasma corticosterone and catecholamine concentrations. A small skin incision was made and a Teflon-tipped catheter was introduced into the femoral artery, and then advanced to the descending aorta. The catheter was tunnelled subcutaneously to exit between the scapulae, sutured in place, filled with sterile heparin (1000 U ml−1), and closed with a sterile plug. The incision was sutured in layers.
Experimental protocols
Protocol 1: immunohistochemistry to characterize the efficacy of DSAP
Microinjection of saline (n= 6) or DSAP (n= 7) was performed as described above, except that three of the DSAP-treated rats received a higher dose of DSAP (27.5 ng in 125 nl per side). Rats were humanely killed 23–26 days later and the brain tissue processed for immunohistochemistry as described below.
Protocol 2: cardiovascular data
Rats recovered from the telemetry surgery for a minimum of 2 weeks, and then control baseline arterial pressure and heart rate measurements were recorded for 7 days. NTS microinjections were performed on day 8 (saline n= 12, DSAP n= 12), and then the animals were subjected to 60 min of restraint stress on days 21–25. Restraint stress was performed in the morning between 08.00 and 12.00 h and consisted of placing each rat in a Plexiglas tube that provided ample circulation of air.
Protocol 3: neuroendocrine data
A second set of experiments was performed to determine if lesioning NTS catecholaminergic neurons also modulates the neuroendocrine response to restraint stress. Since DSAP had similar effects on the arterial pressure response to restraint on the first and fifth days of restraint, the neuroendocrine response was determined only for day 1 of stress (novel restraint). NTS microinjections were performed on day 1 (saline n= 8, DSAP n= 7), arterial catheters were implanted on day 13, and then the animals were subjected to 60 min of restraint stress on day 17. Blood samples (600 μl each) for the measurement of plasma corticosterone, adrenaline and noradrenaline were obtained 5 min prior to restraint and at 10 and 60 min of restraint. The volume was replaced with sterile isotonic saline after each sample was obtained. Samples were drawn while the rat remained in his home cage by connecting the arterial catheter to a long piece of tubing at least 2 h prior to the collection of any blood. Thus, the rats could move freely (except while restrained) and were unaware of the sampling procedure. Blood samples were aliquoted into separate tubes containing either 0.5 μl of heparin for measurement of corticosterone or 6 μl of glutathione-EGTA for measurement of catecholamines. The samples were kept on ice then centrifuged at 4°C, and the plasma was stored at −80°C until being assayed.
Immunohistochemistry
All DSAP-treated rats, all the saline-treated rats from protocol 1, and five of the saline-treated rats from protocols 2 and 3 were deeply anaesthetized with isoflurane and perfused with ice cold 0.1 m phosphate-buffered saline (PBS; pH 7.4) followed with 4% paraformaldehyde. The remaining saline-treated rats were humanely killed with an overdose of isoflurane and death was then ensured with a bilateral thoracotomy. Perfused brains were post-fixed in 4% paraformaldehyde and cryoprotected in 30% sucrose. The hindbrains were sectioned at 40 μm and deposited in series into four wells, then stored in cryoprotectant at −20°C. Brains from protocols 2 and 3 were sectioned from approximately −14.6 mm through −12.8 mm relative to Bregma while brains from protocol 1 were sectioned from approximately −14.6 mm through −8.8 mm relative to Bregma (Paxinos & Watson, 1998). Immunohistochemical labelling of dopamine-β-hydroxlase (DβH) was performed on brain sections from protocols 2 and 3 to determine the adequacy of the lesion within the NTS and to verify that catecholaminergic neurons in the corresponding ventrolateral medulla (VLM) remained intact (Rinaman & Dzmura, 2007). Brains from protocol 1 were also used to determine if microinjection of DSAP into the NTS affected the number of catecholaminergic neurons in the rostral ventrolateral medulla (RVLM), the A5 region or the locus coeruleus. Free-floating sections were incubated with a mouse anti-DβH primary antibody (1:1000 or 1:5000, Millipore, Billerica, MA, USA) for 24 to 48 h at 4°C. Sections were then incubated with biotinylated goat anti-mouse secondary antibody and the signal was visualized using avidin–biotin complex and 3,3′-diaminobenzidine substrate (Vectastain Elite ABC kit and DAB substrate kit for peroxidase, Vector Laboratories, Burlingame, CA, USA) to produce a brown reaction product.
The selectivity of DSAP for catecholaminergic neurons was verified by determining the effect of DSAP on 11β-hydroxysteroid dehydrogenase (11βHSD2)-positive neurons in the NTS, as these neurons are adjacent to the catecholaminergic neurons (Geerling et al. 2006). Hindbrain sections were mounted on slides, incubated with a sheep anti-11βHSD2 primary antibody (1:30,000, Millipore, Billerica, MA, USA) for 48 h at 4°C, followed by a fluorophore-conjugated donkey anti-sheep secondary antibody (DyLite 488, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). This antibody sometimes produces non-specific nuclear staining, so only neurons with cytoplasmic and dendritic staining were counted (Geerling et al. 2006). Immunohistochemistry for choline acetyltransferase was also performed on sections from two rats to confirm previous reports that the cholinergic neurons in the adjacent dorsal motor nucleus of the vagus were not lesioned by the catecholamine-specific neurotoxin (Wrenn et al. 1996). These sections were incubated for 40–48 h at 4°C in rabbit anti-choline acetyltransferase antibody (1:400, Millipore), and then processed as described above for DβH. To count DβH-positive neurons, photomicrographs were obtained using an Olympus BX41 system microscope and DP71 digital camera (Olympus America, Melville, NY, USA). Images of the NTS and ventrolateral medulla were taken at 100× total magnification at three rostral to caudal levels (approximately at calamus scriptorius and 0.5 mm rostral and 0.4 mm caudal to this anatomical landmark according to the atlas of Paxinos & Watson (1998). DβH-positive neuronal cell bodies were counted bilaterally at all three levels in each rat. DSAP-treated rats were considered to be significantly lesioned if no more than 70% of the DβH-positive neurons remained in the NTS compared with the average number of neurons present in the saline-treated animals. The caudal ventrolateral medulla (CVLM) was considered intact if at least 70% of the neurons were present in DSAP-treated rats compared with the average number of neurons present in the saline-treated animals. DβH-positive neurons were also counted bilaterally in the RVLM (Bregma −12.7 mm), the A5 region (Bregma −10.0 and −9.7 mm) and the locus coeruleus (Bregma −9.7 mm). All locations relative to Bregma are approximate, based on Paxinos & Watson (1998). Photomicrographs were taken of all sections with 11βHSD2-positive neurons in rats from protocol 1. The total number of 11βHSD2-positive neurons in the three sections with the largest number of 11βHSD2-positive neurons in each rat was determined.
Hormone assays
Plasma corticosterone was measured using a commercially available double antibody 125I radioimmunoassay kit (MP Biomedicals, Solon, OH, USA). Plasma adrenaline and noradrenaline were measured by HPLC by the Vanderbilt University Hormone Assay and Analytical Services Core (Nashville, TN, USA).
Data acquisition and analysis
Arterial pressure (mmHg) and heart rate (beats min−1) data were collected for 20 s every 10 min for 24 h per day, except for the 2 h periods beginning 30 min prior to restraint stress on the first and last days of stress during which time data were collected continuously at 500 Hz. Twenty-four hour baseline mean arterial pressure and heart rate values were averaged into hourly bins and divided into daytime (light) and nighttime (dark) periods for analysis. Arterial pressure and heart rate values during periods of restraint were excluded from the analysis of the 24 h baseline data shown in Fig. 3. Mean arterial pressure and heart rate responses to stress were calculated as follows. Data were averaged into 5 min bins starting at 30 min prior to restraint and continuing through to 30 min after the termination of stress. The average baseline value was calculated during the 30 min prior to stress, excluding the final 3 min. Periods during which the telemetry system recorded animal movement were excluded from the baseline data. The average baseline was then subtracted from the values measured during the 60 min of stress and the 30 min recovery period following stress. Average results are expressed as means ± SEM. The 30 min baseline data prior to stress were also used for determination of spontaneous baroreflex sensitivity and spectral analysis of blood pressure and heart rate variability using the freely available HemoLab software (http://www.haraldstauss.com/HemoLab/HemoLab.html). Blood pressure data for the baseline periods preceding restraint stresses 1 and 5 were visually inspected and artifact-free segments of at least 10 min duration were extracted. Sampling rate of the datasets was increased to 1000 Hz using spline interpolation as described by Bhatia et al. (2010). The gain of the baroreceptor reflex was determined using the sequence technique. Sequences were defined as a minimum of three consecutive (beat-by-beat) increases or decreases in systolic blood pressure that were associated with simultaneous changes in pulse interval. Sequences with increases and decreases in systolic blood pressure were pooled. No threshold for changes in systolic blood pressure or pulse interval was used. Only sequences with a correlation coefficient of >0.8 for the linear correlation between systolic blood pressure and pulse interval were included in the analysis, and the slope of the linear correlation was taken as the gain of the baroreceptor reflex (Bhatia et al. 2010).
Figure 3. Baseline mean arterial pressure (A) and heart rate (B) measured 24 h per day and divided into the light and dark periods.
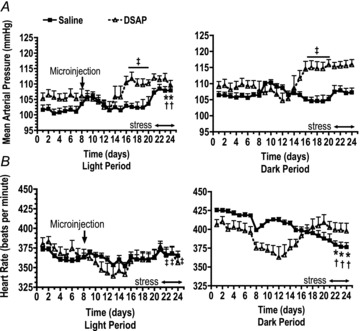
Bilateral NTS microinjections of saline (filled squares and continuous lines) or DSAP (open triangles and dashed lines) were performed on day 8, and rats were subjected to daily restraint stress on days 21–25. Data for day 25 are not shown because the experiments ended after the morning stress on that day. †P≤ 0.05 compared with the baseline control period for saline-treated rats. *P≤ 0.05 compared with the pre-stress period for saline-treated rats. ‡P≤ 0.05 compared with the baseline control period for DSAP-treated rats.
Statistical significance was evaluated using one-way or two-way ANOVA as appropriate (SPSS software, IBM). Linear regression analysis was performed to determine the relationship between the number of DβH-positive NTS neurons and the integrated increase in mean arterial pressure in response to 60 min of restraint stress in the DSAP-treated rats. Post hoc analyses were performed using Duncan's test. When multiple one-way ANOVA was required due to significant interactions between main effects, a Bonferroni adjustment was used. Differences were considered significant at P < 0.05. Two saline-treated rats from protocol 2 were removed from the study because the quality of the signal from the telemetry transmitter diminished following the microinjection such that average pulse pressure fell below 25 mmHg, and stress data from one rat were removed due to loss of a good signal during stress. Data from three saline-treated rats are not included in the 24 h results because of a power failure that resulted in the loss of several days’ worth of data. Two DSAP-treated rats from protocol 2 had insufficient NTS lesions (greater than 70% of cells remaining) and their data were excluded from the analyses, except for the linear regression analysis described above. Data from one animal were excluded from protocol 2 due to substantial (>70%) loss of VLM neurons. The plasma sample for the measurement of plasma catecholamines in one DSAP-treated animal was lost during processing.
Results
Results from protocol 1
Microinjection of the low dose of DSAP (22 ng per side) significantly reduced the number of DβH-positive neurons at all levels of the NTS by 87%, while the higher dose (27.5 ng per side) caused a reduction of 97% (Table 1). The low dose of DSAP did not reduce the average number of DβH-positive neurons in the VLM, while the high dose reduced the number by 28% (Table 1). Low dose DSAP had no effect on the number of DβH-positive neurons in the RVLM or locus coeruleus, but significantly reduced the number of such neurons in the A5 region by 30% (Table 2 and Fig. 1). High dose DSAP significantly reduced DβH-positive neurons in all three regions (Table 2). Neither dose of DSAP diminished the number of 11βHSD2-positive neurons in the NTS; neuronal counts were 45 ± 3, 42 ± 6 and 46 ± 7 for saline-, low dose DSAP- and high dose DSAP-treated rats, respectively (see Fig. 1 for sample photomicrographs).
Table 1.
Average number of DβH-positive neurons in the nucleus of the solitary tract and ventrolateral medulla in rats from protocol 1
| Rostral–caudal level | ||||
|---|---|---|---|---|
| Treatment group | −0.4 mm | CS | +0.5 mm | Total |
| NTS saline | 25 ± 2 | 36 ± 6 | 129 ± 4 | 190 ± 11 |
| NTS DSAP (LD) | 5 ± 1* | 8 ± 3* | 12 ± 5* | 25 ± 8* |
| NTS DSAP (HD) | 2 ± 2* | 3 ± 3* | 0 ± 0* | 5 ± 4* |
| VLM saline | 27 ± 1 | 28 ± 3 | 33 ± 3 | 89 ± 4 |
| VLM DSAP (LD) | 25 ± 2 | 26 ± 2 | 23 ± 6 | 74 ± 8 |
| VLM DSAP (HD) | 15 ± 3†* | 19 ± 3 | 19 ± 1 | 53 ± 7†* |
DβH, dopamine-β-hydroxylase; NTS, nucleus of the solitary tract; VLM, ventrolateral medulla; DSAP, anti-dopamine-β-hydroxylase conjugated to saporin; LD, low dose of 22 ng per side; HD, high dose of 27.5 ng per side. Rostral to caudal levels are relative to calamus scriptorius (CS).
P < 0.05 for DSAP compared with saline group;
P < 0.05 for LD compared with HD DSAP.
Table 2.
Average number of DβH-positive neurons in rostral ventrolateral medulla, A5 region and locus coeruleus in rats from protocol 1
| Treatment group | RVLM | A5 | LC |
|---|---|---|---|
| Saline | 40 ± 2 | 60 ± 3 | 36 ± 4 |
| DSAP (LD) | 37 ± 1 | 42 ± 9* | 34 ± 5 |
| DSAP (HD) | 23 ± 1†* | 24 ± 5* | 14 ± 5†* |
DβH, dopamine-β-hydroxylase; RVLM, rostral ventrolateral medulla; A5, the A5 noradrenergic region; LC, locus coeruleus; LD, low dose of 22 ng per side; HD, high dose of 27.5 ng per side; DSAP, anti-dopamine-β-hydroxylase conjugated to saporin.
P < 0.05 for DSAP compared with saline group;
P < 0.05 for LD compared with HD DSAP.
Figure 1.
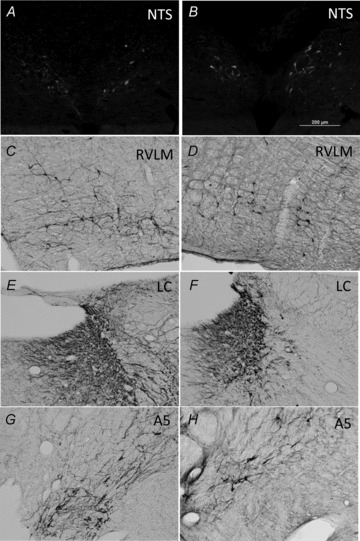
Example photomicrographs from saline-treated (A, C, E and G) and DSAP-treated (B, D, F and H) rats for labelling of 11βHSD2-positive neurons in the NTS (A and B), or the DβH-positive neurons in the RVLM (C and D), locus coeruleus (E and F) or A5 region (G and H)
Results from protocols 2 and 3
As in Protocol 1, microinjection of DSAP (22 ng per side) significantly reduced the total number of DβH-positive neurons (Fig. 2, Table 3). Conversely, the number of DβH-positive neurons in the ventrolateral medulla was not altered by DSAP treatment (Fig. 2, Table 3). Cholinergic neurons appeared to be unaffected by the DSAP treatment as has been previously reported (data not shown) (Wrenn et al. 1996).
Figure 2. DβH-positive NTS (A and B) and ventrolateral medulla (C and D) neurons in a saline-treated rat (A and C) and a DSAP-treated rat (B and D).
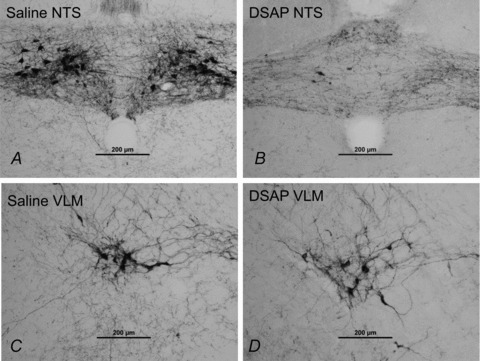
The scale bars represent 200 μm.
Table 3.
Average number of DβH-positive neurons in the nucleus of the solitary tract and ventrolateral medulla in rats from protocols 2 and 3
| Rostral–caudal level | ||||
|---|---|---|---|---|
| Treatment group | −0.4 mm | CS | +0.5 mm | Total |
| NTS saline | 36 ± 4 | 63 ± 7 | 122 ± 5 | 221 ± 7 |
| NTS DSAP | 10 ± 3* | 19 ± 3* | 38 ± 5* | 61 ± 9* |
| VLM saline | 23 ± 2 | 26 ± 3 | 33 ± 3 | 83 ± 6 |
| VLM DSAP | 26 ± 2 | 29 ± 1 | 34 ± 2 | 89 ± 4 |
DβH, dopamine-β-hydroxylase; NTS, nucleus of the solitary tract; DSAP, anti-dopamine-β-hydroxylase conjugated to saporin; VLM, ventrolateral medulla. Rostral to caudal levels are relative to calamus scriptorius (CS).
P < 0.05 for DSAP compared with saline group.
Average baseline mean arterial pressure during the control period prior to the microinjection of saline or DSAP (days 1–7, Fig. 3A) was not different between treatment groups (101 ± 1 mmHg and 106 ± 2 mmHg, P= 0.11, for the saline- and DSAP-treated rats, respectively, during the light period, with corresponding values of 106 ± 1 and 108 ± 2 mmHg, P= 0.23, during the dark period). Analysis of mean arterial pressure over the time course of the experiment revealed a significant interaction between time and treatment, so one-way ANOVA with a Bonferroni adjustment for multiple means comparisons was performed separately on each treatment group. Saline treatment alone (prior to any stress) had no significant effect on baseline blood pressure, while DSAP significantly increased mean arterial pressure during both the light period (P= 0.038) and the dark period (P= 0.04). Repeated restraint stress significantly increased average baseline mean arterial pressure in saline-treated rats during the light period on days 23–24, and tended to increase baseline mean arterial pressure by day 24 during the dark period, but the change was not statistically significant (P= 0.08). In contrast, repeated stress did not further increase baseline blood pressure in DSAP-treated rats.
Baseline heart rate fell significantly during the 7 day control period during both the light and dark periods, so days 5–7 were used to represent baseline control values. Average baseline heart rate during the control period prior to the microinjection of saline or DSAP (days 5–7, Fig. 3B) was not significantly different between treatment groups (359 ± 3 mmHg and 368 ± 9 mmHg, P= 0.46, for the saline- and DSAP-treated rats, respectively, during the light period, with corresponding values of 419 ± 2 and 398 ± 9 mmHg, P= 0.09, during the dark period). Average baseline heart rate during the light period was similar in saline- and DSAP-treated rats throughout the experimental protocol, and there were no significant changes in heart rate relative to the control (days 5–7) or pre-stress (days 18–20) periods. (Fig. 3B). Baseline heart rate during the dark period decreased over time during the experiment in the saline-treated rats, and was significantly lower compared with both the control period and the pre-stress period on days 22–24. There was no significant change in heart rate over time in the DSAP-treated rats.
During the 30 min prior to stress, baseline mean arterial pressure was significantly higher (P= 0.02) in DSAP-treated rats (108 ± 2 and 107 ± 2 mmHg on days 21 and 25, respectively, n= 9) compared with saline-treated rats (103 ± 2 and 102 ± 2 mmHg on days 21 and 25 respectively, n= 9). Sixty minutes of restraint stress significantly raised mean arterial pressure in all rats, but the magnitude of the increase was significantly greater in DSAP- compared with saline-treated rats from minutes 15–55 of the stress period, with no significant difference between day 1 and day 5 (P < 0.01, Fig. 4A). The total integrated increase in mean arterial pressure during stress was 800 ± 128 and 655 ± 116 mmHg (min) on days 21 and 25, respectively, for saline-treated rats and 1115 ± 116 and 1035 ± 113 mmHg (min) for DSAP-treated rats. DSAP treatment had an overall effect to enhance the arterial pressure response to stress (P < 0.01) with no significant effect of time (Day 1 compared with Day 5, P= 0.35) and no significant interaction between DSAP treatment and time (P= 0.78). Recovery from stress was assessed during the 30 min period immediately following the period of restraint. Blood pressure during the recovery period was significantly greater in DSAP- compared with saline-treated rats (P= 0.048, Fig. 4A). The total integrated increase in mean arterial pressure during the recovery period was 293 ± 48 and 361 ± 51 mmHg (min) on days 21 and 25 respectively for saline-treated rats and 419 ± 65 and 476 ± 68 mmHg (min) for DSAP-treated rats. The blood pressure increase during the recovery period was significantly greater on day 25 compared with day 21 for the first 10 min of the recovery period in both saline- and DSAP-treated rats.
Figure 4. Changes in mean arterial pressure (MAP; A) and heart rate (HR) in beats per minute (bpm; B) in response to 60 min of restraint stress and 30 min of recovery from stress on the first day (Day 21, left) and last day (Day 25, right) of repeated stress in saline-treated (continuous lines and squares) and DSAP-treated (dashed lines and open circles) rats.
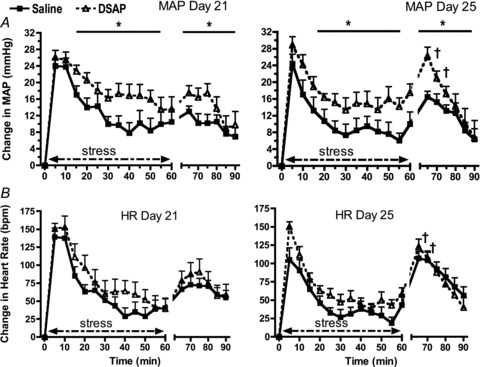
DSAP-mediated lesioning of NTS catecholaminergic neurons significantly enhanced the arterial pressure responses to stress and stress recovery (*P < 0.05). There were no effects of DSAP on the heart rate response to stress (B). The blood pressure and heart rate responses during the first 10 min of the recovery period were greater on day 25 compared with day 21 (†P < 0.05).
Baseline heart rate during the 30 min prior to stress was similar in DSAP-treated rats (356 ± 11 and 337 ± 9 beats min−1 on days 21 and 25, respectively) compared with saline-treated rats (358 ± 7 and 336 ± 5 beats min−1 on days 21 and 25, respectively), but fell significantly between the first and fifth days of repeated restraint (P= 0.02). There were no effects of DSAP on the heart rate increase during either the stress (P= 0.09; Fig. 4B) or recovery period (P= 0.83). The heart rate response during the first 10 min of the recovery period was greater on day 25 compared with day 21.
Spontaneous baroreflex sensitivity and spectral analysis of heart rate and blood pressure variability were assessed during the 30 min baseline period prior to the first and fifth stresses. There were no effects of either DSAP or stress on resting spontaneous baroreflex sensitivity (Fig. 5A). DSAP also failed to influence the power of the very low and low frequency components of blood pressure variability, but repeated stress reduced power in both frequency components regardless of treatment (Fig. 5B and C). DSAP had no significant effect on the low frequency component of heart rate variability, but it increased the power of the high frequency range prior to the first stress (P= 0.04). Repeated stress decreased the power of low and high frequency components in both the saline and DSAP groups (Fig. 5D and E). The ratio of low to high frequency power for heart rate variability was not affected significantly either by DSAP or stress (Fig. 5F).
Figure 5. Spontaneous baroreceptor reflex (BRR) sensitivity determined by the sequence method (A), spectral analysis of blood pressure variability (BPV: B: very low frequency component, VLF; C: low frequency component, LF) and spectral analysis of heart rate variability (D: low frequency component, LF; E: high frequency component, HF; F: ratio of LF and HF).
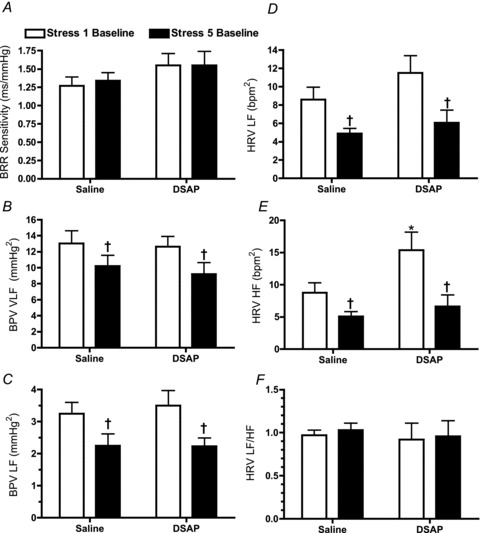
†P < 0.05 for the fifth stress compared with the first stress; *P < 0.05 for the Saline compared with DSAP.
Linear regression analysis was performed to determine if there was a relationship between the number of remaining NTS catecholaminergic neurons and the integrated response to restraint stress in the DSAP-treated rats at different rostral to caudal locations of the neurons within the NTS (Fig. 6). Data from the first day of stress were used. There was a significant negative correlation between the integrated stress response and the number of remaining NTS catecholaminergic neurons for the NTS as a whole (total), and at the two more rostral levels examined (approximately at calamus scriptorius and 0.5 mm rostral to calamus scriptorius), while there was no similar relationship for the more caudal NTS catecholaminergic neurons (approximately 0.4 mm caudal to calamus scriptorius). No significant correlations were found between the numbers of NTS catecholaminergic neurons and the mean arterial pressure response to stress in the saline-treated rats (data not shown).
Figure 6. Linear regression analysis of the relationship between the number of catecholaminergic neurons in the NTS and the average integrated increase in mean arterial pressure during the first day of restraint stress in DSAP-treated rats.
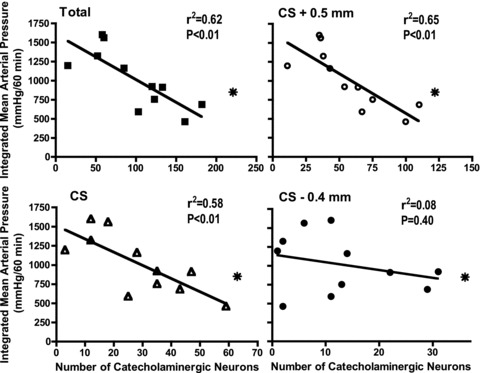
These factors were significantly correlated in the NTS as a whole (total, filled squares) as well as at the level of calamus scriptorius (CS, open triangles) and at 0.5 mm rostral to calamus scriptorius (CS + 0.5 mm, open circles), but not at 0.4 mm caudal to calamus scriptorius (CS – 0.4 mm, filled circles). The average values in the saline-treated rats are noted with an asterisk in each graph. Note the different scales on the x-axis.
Plasma adrenaline, noradrenaline and corticosterone were all significantly increased above baseline at both 10 and 60 min of restraint stress (P < 0.05, Fig. 7). DSAP treatment dramatically reduced plasma adrenaline at all time points (P < 0.01, Fig. 7A), but did not alter the magnitude of the adrenaline response to restraint. There were no significant effects of DSAP treatment on plasma noradrenaline (Fig. 7B). Plasma corticosterone concentration was significantly reduced in DSAP- compared with saline-treated rats after 60 min of stress (P= 0.05, Fig. 7C).
Figure 7.
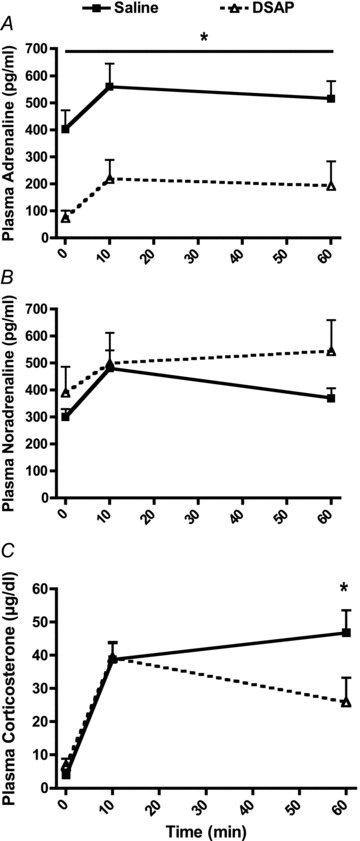
Plasma concentrations for adrenaline (A), noradrenaline (B) and corticosterone (C) at baseline (time = 0) and at 10 and 60 min of novel restraint stress (*P < 0.05 for saline compared with DSAP)
Discussion
This is the first study to investigate the role of NTS catecholaminergic neurons in mediating the cardiovascular response to an emotional stressor. The results support the conclusions that NTS catecholaminergic neurons normally limit the arterial pressure response to acute restraint stress during both the stress and recovery periods, while having the opposite effect on stress-induced corticosterone secretion, in agreement with the initial hypothesis. There is strong existing evidence that hindbrain catecholaminergic neurons mediate the HPA axis response to systemic (physiological) stressors, at least in part due to direct projections from the NTS catecholaminergic neurons to the paraventricular nucleus (Rinaman & Dzmura, 2007; Ulrich-Lai & Herman, 2009). Other data indicate that the NTS can promote the corticosterone response to restraint stress (Dayas et al. 2001b; Bechtold et al. 2009), although not via direct catecholaminergic projections to the paraventricular nucleus (Dayas et al. 2001b). Thus, our results suggest that NTS catecholamergic neurons with projections to a region or regions other than the paraventricular nucleus of the hypothalamus can promote the corticosterone response to an emotional stressor.
Microinjection of DSAP is an established method for lesioning catecholaminergic neurons (Wrenn et al. 1996; Madden et al. 1999; Rinaman & Dzmura, 2007); we assessed the efficacy and specificity of the lesion in the present experiments. Microinjection of DSAP (22 ng per side) directly into the NTS substantially depleted the number of NTS catecholaminergic neurons without altering the number of adjacent 11βHSD2-positive neurons, indicating that the lesion was effective and specific for catecholaminergic neurons. This agrees with reports that intracerebroventricular administration of DSAP lesioned catecholaminergic neurons while leaving cholinergic neurons intact (Wrenn et al. 1996), and microinjection of DSAP into the RVLM lesioned adrenergic neurons without damaging neighbouring non-catecholaminergic neurons (Madden et al. 1999). Populations of catecholaminergic neurons in the CVLM, RVLM or locus coeruleus were not altered by NTS microinjection of DSAP. However, there was a 30% reduction in A5 neurons, complementing a report that 37% of DβH-positive cells in the A5 region are retrogradely labelled following microinjection of tracer into the NTS (Thor & Helke, 1988). The other catecholaminergic cell groups with substantial projections to the NTS are in the ventrolateral medulla (A1 and C1), but these neurons were not lesioned in our experiments, in agreement with previous work using the same dose of DSAP microinjected into the NTS (Rinaman & Dzmura, 2007). It is unlikely that the partial lesion of A5 neurons caused the increase in baseline arterial pressure or the enhanced arterial pressure response to stress. First, more complete lesions of the A5 neurons using DSAP microinjected into the spinal cord has no effect on the arterial pressure response to restraint (Vianna & Carrive, 2010). Second, selective activation of A5 neurons increases sympathetic nerve activity, so a loss of neurons would be likely to decrease rather than increase pressure (Kanbar et al. 2011). It is possible the lack of an increase in daytime baseline arterial pressure in the DSAP-treated rats was caused in part by the loss of A5 neurons. Also, it is possible that the overall reduction in plasma adrenaline in the DSAP-treated rats was mediated at least in part by the loss of A5 neurons, although these neurons account for less than 5% of the CNS projections to preganglionic sympathetic neurons that innervate the adrenal gland (Strack et al. 1989). Microinjection of DSAP into the NTS also attenuated the corticosterone response to restraint at the 60 min time point, indicating that catecholaminergic neurons in the NTS and/or A5 regions are important for maintaining the corticosterone response to a prolonged stress. The A5 region does not project to the medial parvocellular paraventricular nucleus (Byrum & Guyenet, 1997). However, the A5 region could influence hypothalamic–pituitary–adrenal function indirectly via projections to the NTS and central nucleus of the amygdala (Byrum & Guyenet, 1997; Jankord & Herman, 2008). It is probable that the loss of NTS catecholaminergic neurons contributed to the attenuated corticosterone response to stress, but a role of the A5 neurons cannot be excluded. Two additional points must be considered. First, the loss of the NTS catecholaminergic neurons could alter the perceptions of stress. These neurons project to the limbic forebrain and are involved in the formation of memories having an emotional component (Rinaman, 2011). Second, DSAP lesions could alter the function and connectivity of neighbouring neurons producing non-specific changes in functional output. Nonetheless, the validity of our results is supported by the fact that we obtained very similar effects on baseline arterial pressure compared to those reported by Duale et al. (2007), who used a viral vector-based approach to silence the NTS catecholaminergic neurons.
The integrated blood pressure response to stress was inversely correlated to DβH-positive neuron numbers at the rostral-caudal levels of calamus scriptorius and area postrema. Catecholaminergic neurons in these subregions of the NTS send projections to several midbrain and forebrain areas that can influence both HPA axis activation and blood pressure regulation, including the paraventricular nucleus and other hypothalamic nuclei, the amygdala, the bed nucleus of the stria terminalis and the parabrachial nucleus (Sawchenko & Swanson, 1982; Cunningham & Sawchenko, 1988; Riche et al. 1990). Evidence from both human and animal studies suggests the amygdala can moderate the magnitude of the stress response (Kubo et al. 2004; McDougall et al. 2005; Gianaros et al. 2008; Ulrich-Lai & Herman, 2009). However, relatively little is know regarding the actions of adrenergic receptors on blood pressure regulation in these specific brain regions. Global activation of α-adrenergic receptors in the PVN and amygdala can cause increases in blood pressure, which cannot explain the results observed in the present study (Koepke et al. 1987; Zhang & Felder, 2008). However, it is possible that selective blockade of α-adrenergic receptors in neurons receiving input from the NTS would have different effects. Further studies are required to determine the roles of the amygdala and other nuclei in mediating effects of NTS catecholaminergic neurons on the cardiovascular and HPA axis responses to stress.
Silencing or lesioning NTS catecholaminergic neurons in rats has been reported to either increase (Itoh & Bunag, 1993; Duale et al. 2007) or have no effect on (Talman et al. 1980; Itoh et al. 1992; Itoh & Bunag, 1992) baseline mean arterial pressure. The present study reports that DSAP significantly elevated baseline mean arterial pressure by about 5 mmHg, which is slightly less than the increases of 7–13 mmHg reported in the studies describing increases in pressure following chronic silencing or lesioning of NTS catecholaminergic neurons (Itoh & Bunag, 1992; Duale et al. 2007). All three studies indicating that NTS catecholaminergic neurons tonically inhibit baseline mean arterial pressure measured arterial pressure in the same animals before and after inhibiting or lesioning the neurons, and two of these studies, including the present one, measured blood pressure with radiotelemetry. These methodological approaches allow for detection of the relatively small differences in mean arterial pressure.
Previous studies have not investigated the role of NTS catecholaminergic neurons in the control of baseline blood pressure during repeated stress. The data in this paper show that DSAP-induced lesions of NTS neurons blocked a small chronic stress-induced increase in baseline mean arterial pressure. One possible explanation is that the increase in baseline blood pressure with repeated stress in the saline-treated rats was due to inhibition of the catecholaminergic neurons that are normally restraining baseline blood pressure, and in DSAP-treated rats this mechanism was inoperable. Thus, both the increase in baseline pressure prior to stress in the DSAP-lesioned rats and the increase in baseline pressure following stress in the saline-treated rats could be due to the same mechanism: an attenuation or removal of the restraining influence of the NTS catecholaminergic neurons on blood pressure. Another possibility is that stress recruits NTS catecholaminergic neurons that are normally silent but have an excitatory effect on baseline blood pressure, and these neurons have also been lesioned by the DSAP treatment. There is ample evidence that the NTS catecholaminergic neurons are a heterogeneous population of neurons with divergent projections sites (Dayas et al. 2001a; Hermes et al. 2006), and the principle that noradrenergic neurons can be recruited in a state-dependent manner is also established (Morilak et al. 2005). Additional research is required to determine if subpopulations of NTS catecholaminergic neurons can be recruited by repeated stress or other related factors such as elevated levels of stress hormones.
The mechanism for the elevated baseline pressure and enhanced stress response in the DSAP-treated rats cannot be determined by the present study. There were no significant differences in baseline values for noradrenaline, or in stress-induced increases in either noradrenaline or adrenaline in DSAP- compared with saline-treated rats. However, baseline blood pressure was elevated in the DSAP-treated rats, so the lack of suppression of baseline noradrenaline in these rats suggests that noradrenaline was elevated relative to blood pressure. There were no changes in sympathetic tone to the vasculature or the heart reflected in blood pressure and heart rate variability as determined by spectral analysis. These results are in agreement with Duale et al. (2007) who reported that silencing NTS catecholaminergic neurons in normotensive WKY rats elevated baseline blood pressure in the absence of evidence of elevated baseline sympathetic nerve activity as determined by spectral analysis of systolic blood pressure. There were also no changes in the spontaneous barororeflex control of heart rate. However, the enhanced blood pressure responses to stress and the impaired recovery of blood pressure following stress in the DSAP-treated rats could be due in part to attenuated baroreceptor reflex function that cannot be measured with the relatively insensitive methods employed in this study. Future experiments will need to determine the effect of DSAP lesions of NTS catecholaminergic neurons using the modified Oxford method (Moffitt et al. 2005). Surprisingly, lesioning of the NTS catecholaminergic neurons resulted in a substantial inhibition of baseline plasma adrenaline levels, although the magnitude of the adrenaline response to restraint was unaffected. Neurons projecting to the preganglionic neurons that innervate the adrenal medulla do not arise from the NTS, so the reduction in adrenaline was not due to direct destruction of these neurons (Strack et al. 1989). The role of the NTS in specific regulation of adrenal nerve activity warrants further investigation.
Perspectives
Exaggerated cardiovascular responses to acute stress predict future hypertension and cardiovascular disease in vulnerable individuals (Chida & Steptoe, 2010). Knowledge of mechanisms underlying enhanced stress responsiveness would enable the identification of at risk individuals and allow for the development of targeted interventions. Genetic factors influence the magnitude of the cardiovascular and neuroendocrine responses to stress, and several of the identified contributing genes mediate synthesis and signalling of catecholamines (Jabbi et al. 2007a,b; Mueller et al. 2011). The present results demonstrate that catecholaminergic neurons within the NTS both modulate the magnitude of the blood pressure response to acute stress and influence the control of baseline blood pressure. Factors that attenuate the inhibitory effects of NTS catecholaminergic neurons on baseline blood pressure and stress could thus contribute to exaggerated stress responses and hypertension. Future investigations can reveal what regulates the activity of these neurons and also which projection sites and neurotrasmitter and neurotrophic receptors mediate the actions of these neurons on stress reactivity. Together, this new information can guide the development of targeted and effective therapies to identify, prevent and treat stress-induced cardiovascular disease.
Acknowledgments
This work was funded by the National Institutes of Health, National Heart, Lung and Blood Institute (USA) grant no. R01 HL076807 (D.A.S.) and American Heart Association Greater Southeast Affiliate grant no. 10GRNT4460047 (D.A.S.).
Glossary
- CS
calamus scriptorius
- CVLM
caudal ventrolateral medulla
- DβH
dopamine-β-hydroxylase
- DSAP
anti-dopamine-β-hydroxylase
- HR
heart rate
- HPA
hypothalamic–pituitary–adrenal axis
- 11βHSD2
11-β-hydroxysteroid dehydrogenase
- LC
locus coeruleus
- MAP
mean arterial pressure
- NTS
nucleus of the solitary tract
- RVLM
rostral ventrolateral medulla
- VLM
vetrolateral medulla
Author contributions
The experiments were conducted in the laboratory of D.A.S. at the University of Florida. D.A.S. initiated the conception and design of the experiments, participated in experimental procedures, performed data analysis and interpretation and assisted in writing the manuscript. D.L.D. assisted with the conception and design of the experiments, participated in experimental procedures, and assisted with writing the paper. M.M. participated in developing immunohistochemistry protocols, performing experimental procedures and editing the manuscript. B.E. performed the spectral and baroreflex analyses and assisted with writing the manuscript. All authors have approved the submitted version of the manuscript.
References
- Bechtold AG, Patel G, Hochhaus G, Scheuer DA. Chronic blockade of hindbrain glucocorticoid receptors reduces blood pressure responses to novel stress and attenuates adaptation to repeated stress. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1445–R1454. doi: 10.1152/ajpregu.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia V, Rarick KR, Stauss HM. Effect of the data sampling rate on accuracy of indicies for heart rate and blood pressure variability and barorelfex function in resting rats and mice. Physiol Meas. 2010;31:1885–1201. doi: 10.1088/0967-3334/31/9/009. [DOI] [PubMed] [Google Scholar]
- Byrum CE, Guyenet PG. Afferent and efferent connections of the A5 noradrenergic cell group in the rat. J Comp Neurol. 1997;261:529–542. doi: 10.1002/cne.902610406. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Compar Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001a;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Medullary neurons regulate hypothalamic corticotropin-releasing factor cell responses to an emotional stressor. Neuroscience. 2001b;105:707–719. doi: 10.1016/s0306-4522(01)00213-5. [DOI] [PubMed] [Google Scholar]
- Duale H, Waki H, Howorth P, Kasparov S, Teschemacher AG, Paton JFR. Restraining influence of A2 neurons in chronic control of arterial pressure in spontaneously hypertensive rats. Cardiovasc Res. 2007;76:184–193. doi: 10.1016/j.cardiores.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Kawata M, Loewy AD. Aldosterone-sensitive neurons in the rat central nervous system. J Comp Neurol. 2006;494:515–527. doi: 10.1002/cne.20808. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes SM, Mitchell JL, Aicher SA. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonomic targets in the rat brain. Exp Neurol. 2006;198:539–551. doi: 10.1016/j.expneurol.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Itoh H, Alper RH, Bunag RD. Baroreflex changes produced by serotonergic or catecholaminergic lesions in the rat nucleus tractus solitarius. J Pharmacol Exp Ther. 1992;261:225–233. [PubMed] [Google Scholar]
- Itoh H, Bunag RD. Catecholaminergic nucleus tractus solitarius lesions in anesthtied rats alters baroreflexes differently with age. Mech Ageing Dev. 1992;64:69–84. doi: 10.1016/0047-6374(92)90097-w. [DOI] [PubMed] [Google Scholar]
- Itoh H, Bunag RD. Age-related reduction of reflex bradycardia in conscious rats by catecholaminergic nucleus tractus solitarius lesions. Mech Ageing Dev. 1993;67:47–63. doi: 10.1016/0047-6374(93)90111-4. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Kema IP, van der Pompe G, te Meerman GJ, Ormel J, den Boer JA. Catechol-o-methyltransferase polymorphism and susceptibility to major depressive disorders modulates psychological stress response. Psychiatr Genet. 2007a;17:183–193. doi: 10.1097/YPG.0b013e32808374df. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema IP, Hartman C, van der Pompe G, Minderaa RB, Ormel J, den Boer JA. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Mol Psychiatry. 2007b;12:483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann NY Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbar R, Depuy SD, West GH, Stornetta RL, Giuyenet PG. Regulation of visceral sympathetic tone by A5 noradrenergic neurons in rodents. J Physiol. 2011;589:903–917. doi: 10.1113/jphysiol.2010.198374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepke JP, DiBona GF. Central adrenergic receptor control of renal function in conscious hpertensive rats. Hypertension. 1986;8:133–141. doi: 10.1161/01.hyp.8.2.133. [DOI] [PubMed] [Google Scholar]
- Koepke JP, Jones S, DiBona GF. α2-Adrenoceptors in amygdala control renal sympathetic nerve activity and renal function in spontaneously hypertensive rats. Brain Res. 1987;404:80–88. doi: 10.1016/0006-8993(87)91357-6. [DOI] [PubMed] [Google Scholar]
- Kubo T, Okatani H, Nishigori Y, Hagiwara Y, Fukumori R, Goshima Y. Involvement of the medial amygdaloid nucleus in restraint stress-induced pressor responses in rats. Neurosci Lett. 2004;354:84–86. doi: 10.1016/j.neulet.2003.09.061. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Ito S, Rinaman L, Wiley RG, Sved AF. Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats using anti-DβH-saporin. Am J Physiol Regul Comp Physiol. 1999;277:R1063–R1075. doi: 10.1152/ajpregu.1999.277.4.R1063. [DOI] [PubMed] [Google Scholar]
- McDougall SJ, Widdop RE, Lawrence AL. Central autonomic integration of psychological stressors: focus on cardiovascular modulation. Autonom Neurosci Basic Clin. 2005;123:1–11. doi: 10.1016/j.autneu.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Moffitt JA, Grippo AJ, Johnson AK. Baroreceptor reflex control of heart rate in rats studied by induced and autogenic changes in arterial pressure. Am J Physiol Heart Circ Physiol. 2005;288:H2422–H2430. doi: 10.1152/ajpheart.00057.2004. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Mueller A, Straler J, Armbruster D, Lesch K-P, Brocke B, Kirschbaum C. Genetic contributions to acute autonomic stress responsiveness in children. Int J Psychophysiol. 2011;88:302–308. doi: 10.1016/j.ijpsycho.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. Chemoarchitecture of the Brain. New York: Springer-Verlag; 1985. [Google Scholar]
- Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol. 1995;16:89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Rauls RA, Tan Y, Knuepfer MM. Central β-adrenoreceptors mediate phasic and sustained components of hemodynamic responses to acute behavioral stress. Brain Res. 2005;1048:98–107. doi: 10.1016/j.brainres.2005.04.057. [DOI] [PubMed] [Google Scholar]
- Riche D, de Pommery J, Menetrey D. Neuropeptides and catecholamines in efferent projections of the nuclei of the solitary tract in the rat. J Comp Neurol. 1990;293:399–424. doi: 10.1002/cne.902930306. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol. 2007;28:50–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–R235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1495–R1503. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, Li A-J. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav. 2006;89:490–500. doi: 10.1016/j.physbeh.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organisation of noradrenergic pathways to the paraventricular and supraoptic nuclei in the rat. Brain Res Rev. 1982;4:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989;491:274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- Talman WT, Snyder D, Reis DJ. Chronic lability of arterial pressure produced by destruction of A2 catecholaminergic neurons in the rat brainstem. Circ Res. 1980;46:6. doi: 10.1161/01.res.46.6.842. [DOI] [PubMed] [Google Scholar]
- Thor KB, Helke CJ. Catecholamine-synthesizing neuronal projections to the nucleus tractus solitarius of the rat. J Comp Neurol. 1988;268:264–280. doi: 10.1002/cne.902680210. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna DML, Carrive P. Cardiovacular and behavioral responses to conditioned fear and restraint are not affected by retrograde lesions of A5 and C1 bulbospinal neurons. Neuroscience. 2010;166:1210–1218. doi: 10.1016/j.neuroscience.2010.01.039. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Picklo MJ, Lapppi DA, Robertson D, Wiley RG. Central noradrenergic lesioning using ant-DBH-saporin: anatomical findings. Brain Res. 1996;740:175–184. doi: 10.1016/s0006-8993(96)00855-4. [DOI] [PubMed] [Google Scholar]
- Zhang Z-H, Felder RB. Hypothalamic CRF and norepinephrine mediate sympathetic and cardiovascular responses to acute injection of TNF-α in the rat. J Neuroendocrinol. 2008;20:978–987. doi: 10.1111/j.1365-2826.2008.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


