Abstract
The neural inspiratory activity originates from a ventrolateral medullary region called the pre-Bötzinger complex (preBötC), yet the mechanism underlying respiratory rhythmogenesis is not completely understood. Recently, the role of not only neurons but astrocytes in the central respiratory control has attracted considerable attention. Here we report our discovery that an intracellular calcium rise in a subset of putative astrocytes precedes inspiratory neuronal firing in rhythmically active slices. Functional calcium imaging from hundreds of preBötC cells revealed that a subset of putative astrocytes exhibited rhythmic calcium elevations preceding inspiratory neuronal activity with a time lag of approximately 2 s. These preinspiratory putative astrocytes maintained their rhythmic activities even during the blockade of neuronal activity with tetrodotoxin, whereas the rhythm frequency was lowered and the intercellular phases of these rhythms were decoupled. In addition, optogenetic stimulation of preBötC putative astrocytes induced firing of inspiratory neurons. These findings raise the possibility that astrocytes in the preBötC are actively involved in respiratory rhythm generation in rhythmically active slices.
Key points
Autonomic respiratory rhythm is essential to maintain lives and is generated in the lower brainstem. The ventrolateral medullary region, called the pre-Bötzinger complex (preBötC), is the kernel for respiratory rhythm generation. Despite previous extensive studies focusing on neurons, the mechanism of how respiratory rhythm is generated has not been fully understood.
Here we show that non-neuronal glial cells (a subset of putative astrocytes) in the preBötC are periodically activated preceding inspiratory neuronal activity, periodic activity of putative astrocytes persists during blockade of neuronal activity, and stimulation of astrocytes in the preBötC induces inspiratory neuronal firings.
These findings together with the previous report that blockade of astrocytic metabolism abolishes inspiratory neural output suggest that astrocytes are functionally involved in respiratory rhythm generation.
These results will help us better understand how respiratory rhythm is generated and how respiratory output is disturbed in various pathological conditions.
Introduction
Medullary slice preparations made of neonatal rodents that contain the pre-Bötzinger complex (preBötC), termed rhythmically active slices (Del Negro et al. 2011), exhibit spontaneous inspiratory-related rhythmic activity in vitro (Smith et al. 1991) and have been widely used to investigate the mechanism of respiratory rhythm generation. Although various hypotheses have been proposed to explain the mechanism of rhythmogenesis in this region, many of which are based on single neuron kinetics and neuronal network dynamics (Koshiya & Smith, 1999; Feldman & Del Negro, 2006; Pace et al. 2007; Koizumi & Smith, 2008; Rubin et al. 2009; Morgado-Valle et al. 2010; Del Negro et al. 2011), no consensus exists among researchers in this area. Although inhibitory synaptic transmission is necessary in network-driven rhythmogenesis in mature animal preparations (Smith et al. 2007), inspiratory-related activity persists even after inhibitory synaptic transmission is blocked in rhythmically active slices (Shao & Feldman, 1997). It is also reported that rhythmogenesis requires subthreshold currents, such as persistent sodium current (INaP) or non-specific cationic current (ICAN) (Del Negro et al. 2005). However, even if both INaP and ICAN were abolished, the rhythm could be maintained by boosting neural excitability, suggesting that pacemaker neurons are not essential for rhythmogenesis (Del Negro et al. 2005). These experimental results suggest the involvement of a currently unknown regulatory factor.
Recent progresses of glial cell physiology has revealed that astrocytes play active roles in various brain functions by driving neuronal synchronization (Parri et al. 2001; Angulo et al. 2004; Fellin et al. 2004; Tian et al. 2005). Hülsmann et al. (2000) reported that blockade of astrocytic metabolism abolishes inspiratory-related neural output in rhythmically active slices, suggesting that astrocytic metabolic support is necessary for the maintenance of respiratory rhythm. Also, it has been indicated that astrocytes play chemosensory, modulatory and regulatory roles in the respiratory network in the medulla oblongata (Neusch et al. 2006; Szoke et al. 2006; Ballanyi et al. 2010; Erlichman et al. 2010; Gourine et al. 2010; Huxtable et al. 2010; Wenker et al. 2010; Gourine & Kasparov, 2011; Mulkey & Wenker, 2011). Härtel et al. (2009) showed that astrocytes in the ventral respiratory group are activated by neuromodulators that also stimulate respiratory neurons, suggesting that thus modulated astrocytes influence respiratory neurons in the ventral respiratory group. In humans, abnormalities of brainstem astrocytes correlate with disturbances in central respiratory control, e.g. abnormal proliferation of brainstem astrocytes with central neurogenic hyperventilation (Rodriguez et al. 1982) and immaturity of brainstem astrocytes with sudden infant death syndrome (Zhang et al. 1992).
These reports led us to further investigate the role of astrocytes in respiratory control, and we conducted large-scale simultaneous analyses of inspiratory-related activities of putative neurons and astrocytes in rhythmically active slices. Here we show that a subset of putative astrocytes in the preBötC is activated preceding inspiratory neuronal firing.
Methods
Animal experiment ethics
All experiments were performed with the approval of the Animal Experiment Ethics Committee at the University of Tokyo (approval numbers: 19-35 and 19-43) and were in accordance with the University of Tokyo guidelines for the care and use of laboratory animals.
Preparations
Rhythmically active slices were prepared from rats for calcium imaging (n= 16) or from mice for optogenetic experiments (n= 30) between postnatal days 1 and 3. Briefly, animals were deeply anaesthetized with diethyl ether, and the brainstem was dissected and mounted with its rostral side down on an agar block, and transferred to a vibratome (VT1200S, Leica, Heidelberg, Germany). The brainstem was serially sectioned until the most caudal portion of the inferior olive, which approximately corresponded to the third or fourth hypoglossal nerve rootlet. Then a transverse slice containing the preBötC was prepared with a thickness of 550–650 μm. The rostral surface of the slice should coincide with the rostral end of the preBötC or be within approximately 50 μm rostral to the preBötC (see Fig. 1A in Ruangkittisakul et al. (2008)). The slice was placed in a submerged-type recording chamber with its rostral side up, and continuously superfused with oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid (aCSF) containing (in mm): 118 NaCl, 3 KCl, 1.2 CaCl2, 1 MgCl2, 1 NaH2PO4, 25 NaHCO3 and 30 d-glucose (∼330 mosmol l−1, pH 7.4). The temperature was maintained at 28°C. The concentration of potassium ions was then elevated to 8 mm to activate and maintain rhythmic activity (Smith et al. 1991).
Figure 1. Large-scale multi-cell calcium imaging from the preBötC.
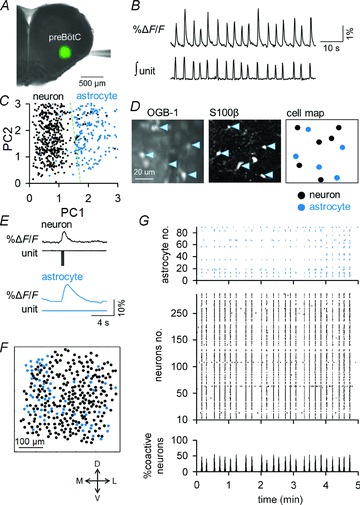
A, bolus injection of OGB1 (green) into the preBötC of a rhythmically active slice. B, spontaneous fluctuations in the mean fluorescence of the preBötC (top) correlated with the integrated inspiratory unit activity (bottom). C, Functional classification of neurons and astrocytes by a principal component (PC) analysis of the calcium waveforms. Putative neurons and putative astrocytes are indicated in black and blue, respectively. D, Immunohistochemical classification of neurons and astrocytes (right) based on S100β immunoreactivity (middle) after OGB1 imaging (left). E, simultaneous calcium imaging (ΔF/F) and cell-attached recording (unit) from a neuron (top) and an astrocyte (bottom). F, locations of 236 putative neurons and 90 putative astrocytes identified in a single video. G, raster plots of the calcium activity of the putative astrocytes and putative neurons that are represented in F. The bottom histogram indicates the percentage of coactivated putative neurons at a given time (bin = 0.2 s). Putative astrocytes and putative neurons are plotted as blue and black dots, respectively.
Calcium imaging
A glass pipette (1–3 MΩ) for dye loading was filled with aCSF containing of 200 μm Oregon Green 488 BAPTA-1 AM (OGB1, Invitrogen, Carlsbad, CA, USA), 2% Pluronic F-127 (Invitrogen) and 20% DMSO. The tip of the pipette was inserted into the centre of the preBötC region of rhythmically active slices. The point of pipette insertion was carefully determined making reference to Fig. 2C of Ruangkittisakul et al. (2009). Then, the dye solution was pressure-ejected over 3–5 min by manually controlling a 10 ml syringe press (30–70 kPa), following the calcium dye injection technique originally developed for the use in vivo (Stosiek et al. 2003) and later applied to the preBötC in vitro by the group of Ballanyi (Ruangkittisakul et al. 2006, 2008). The fluorophore was excited at 488 nm using a laser diode (2–8 mW, HPU50101, FITEL, Tokyo, Japan) and was recorded through a 507 nm long-pass emission filter. Images (512 × 512 pixels = 512 × 512 μm, 14-bit intensity) were captured at 5–50 frames s−1 using a Nipkow-disk confocal scanner unit (CSU-X1; Yokogawa Electric, Tokyo, Japan), a back-illuminated cooled CCD camera (iXon DU897; Andor, Belfast, Northern Ireland, UK), an upright microscope (ECLIPSE FN1; Nikon, Tokyo, Japan) and a water-immersion objective lens (16×, 0.8 NA or 40×, 0.8 NA, Nikon).
Figure 2. Preinspiratory activity of putative astrocytes.
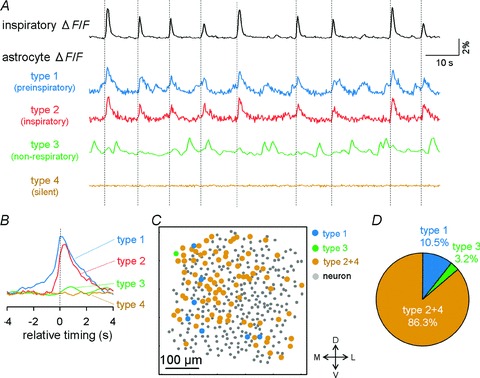
A, representative calcium ΔF/F traces of preinspiratory (type 1), inspiratory (type 2), non-respiratory (type 3) and silent putative astrocytes (type 4). Top: bulk ΔF/F fluctuations of the preBötC. B, peri-inspiratory ΔF/F responses of preinspiratory, inspiratory, non-respiratory and silent putative astrocytes. The traces were averaged from 20 successive events by aligning the onset of individual inspiratory events at 0 s. C, representative cell map of putative neurons and four types of putative astrocytes. We avoided strict separation of inspiratory and silence cells, and they are shown together as type 2+4. D, distribution of four types of putative astrocytes (n= 541 putative astrocytes from 14 movies).
Analysis of calcium dynamics
First, a region covering the whole preBötC was set in order to show average calcium rises in the entire preBötC. Second, a small region of interest (ROI) was carefully placed on (within) each recorded cell body, and the fluorescence intensity (F) was measured. In some cases, non-cellular structures were labelled with calcium dyes, and thus we carefully avoided placing ROIs on these structures based on the following two features. First, because the somata of cells are separated at a cell-to-cell distance at least 20 μm, the ROIs were not placed within 20 μm from the nearest ROI. Second, each ROI was set to be 50–75 μm2 in size to avoid placing ROIs on the small non-cellular structures. Some astrocytes contact vessels through their endfeet, and these endfeet were easily identified by eye and excluded from the analysis. In each ROI, the fluorescence intensity was spatially averaged. The change in fluorescence (ΔF/F) in a ROI was calculated as (Ft– F0)/F0, where Ft was the fluorescence intensity at a focused time point and F0 was the average baseline fluorescence intensity across the 10 s periods before and after the focused time point. No image compensation was made to cancel background inspiratory activity.
Functional classification of neurons and astrocytes
In calcium imaging experiments, recorded cells were often classified into neurons and astrocytes on the basis of distinct calcium rise patterns, i.e. faster and slower calcium rises in neurons and astrocytes, respectively (e.g. Pasti et al. 1997; Fellin et al. 2004, 2006; Bardoni et al. 2010). In the present study, however, we intended to classify the recorded cells into putative neurons and putative astrocytes more reliably, and conducted principal component analysis of the waveforms derived from the cellular calcium dynamics. The ΔF/F waveform was quantified with the following multivariate measures: the rise time constant, maximum rise amplitude and decay time constant of fluorescent calcium signals, based on the universally observed characteristics that the astrocytic calcium changes exhibit slower kinetics and higher amplitude rises compared with those of neurons (Pasti et al. 1997; Svoboda et al. 1997; Garaschuk et al. 2000; Parri et al. 2001; Stosiek et al. 2003; Fellin et al. 2004, 2006; Hirase et al. 2004; Nimmerjahn et al. 2004; Sasaki et al. 2007, 2011a; Bardoni et al. 2010; Takahashi et al. 2010). The dataset was decomposed into a lower dimensional space of the first two components of the principal component analysis, a linear data-reduction technique (Sasaki et al. 2008). The first two principal components were plotted in a figure. The calcium dynamics were automatically classified using a linear support-vector machine. The support-vector machine is one of the most widely used supervised learning methods for classification, initially conceived by Cortes & Vapnik (1995). Further, cell sizes and an identity factor were incorporated. The identity factor scores +1 or −1, according to subjective judgement of neurons or astrocytes based on their calcium waveforms and soma sizes (assuming soma diameter <10 μm as astrocyte and soma diameter >10 μm as neuron) (Ruangkittisakul et al. 2009; Huxtable et al. 2010) were used for post hoc confirmation of the cell types after automatic classification. Cells were thus classified into putative neurons and putative astrocytes, and this classification was validated in a selected sample by S100β immunoreactivity and by their responses to tetrodotoxin (see Results).
Classification of astrocyte types
Based on the relative timings of astrocytic activity to inspiratory neuronal activity, recorded putative astrocytes were classified into four types: preinspiratory cells (type 1), inspiratory cells (type 2), non-respiratory cells (type 3) and silent cells (type 4). Preinspiratory cells were defined as cells that were activated 0.5−2 s earlier than inspiratory neuronal activity. Inspiratory cells were defined as cells that were activated corresponding with inspiratory timings. Non-inspiratory cells were defined as cells that were activated irrespective of inspiratory timings. Silent cells were defined as cells that were not activated at least during our recording period of 5 min.
Electrophysiology
All electrophysiological recordings were carried out with a MultiClamp 700B amplifier and a Digidata 1440A digitizer controlled by pCLAMP 10 software (Molecular Devices, Union City, CA, USA). Spontaneous multi-unit activity was recorded from the preBötC using borosilicate glass pipettes (∼1 MΩ) and integrated along a time window of 300 ms (Funke et al. 2007). This integrated signal of spontaneous multi-unit activity has been widely used as neural output of inspiratory activity (Funke et al. 2007; Ruangkittisakul et al. 2009; Schnell et al. 2011). The onset of integrated signal faithfully coincided with that of average fluorescence signal recorded from the entire preBötC region, and thus the average fluorescence signal was used as an alternative of electrophysiologically recorded inspiratory neural output. For cell-attached recordings, borosilicate glass pipettes (4–7 MΩ) were filled with aCSF. For current-clamp recordings, the same pipettes were filled with an internal solution consisting of (in mm): 135 potassium gluconate, 4 KCl, 10 HEPES, 10 phosphocreatine-Na2, 0.3 Na2-GTP and 4 Mg-ATP (pH 7.2). Signals were low-pass filtered at 1–2 kHz, digitized at 10 kHz and analysed with pCLAMP 10 software.
Optogenetics
Megalencephalic leukoencephalopathy with subcortical cysts (MLC) is a neurological disorder caused by mutations in the MLC1 gene in human. MLC1 and its mouse homologue, Mlc1, are specifically expressed in astrocytes (Schmitt et al. 2003). To selectively excite astrocytes, we developed transgenic mice that expressed the light-activated ion channel, channelrhodopsin-2 (ChR2), fused to enhanced yellow fluorescent protein (YFP), under the control of the astrocyte-specific Mlc1 gene promoter. The ChR2(C128S mutant)–YFP fusion molecule, a gift from Karl Deisseroth (Berndt et al. 2009), was selectively expressed in astrocytes using the tetracycline transactivator (tTA)–tetO promoter strategy (Gossen et al. 1995), whereby two lines of mice were bred, one expressing an astrocyte-specific promoter driving tTA expression and the other expressing the tetO promoter driving the ChR2–YFP transgene. The transgene was induced by binding of tTA to tetO. Mlc1-tTA mice (Tanaka et al. 2010) expressing tTA under the control of the astrocyte specific gene (Mlc1) promoter were bred with the newly developed tetO-ChR2(C128S)–YFP mice (Tanaka et al. in press). ChR2 is a cation-selective membrane channel, and by photo-activation it rapidly opens to form a pore permeable to monovalent (Na+, K+ and H+) and divalent (Ca2+) cations (Figueiredo et al. 2011). To show the rapid channel opening in photo-stimulated astrocytes, photo-induced inward currents were recorded in patch-clamped astrocytes. As light activation of ChR2 could not be combined with fluorescent OGB1 imaging due to optical interference, inspiratory-related neural activity was electrophysiologically monitored from the preBötC. Since our experiments with photo-activation of astrocytes aimed to examine whether photo-activation induces neuronal firing and also preliminary experiments indicated that suppression of spontaneous inspiratory-related activity was more advantageous to obtain clearer responses, after we observed spontaneous firings of inspiratory neurons we lowered the superfusate potassium concentration from 8 mm to 4 mm that induced ‘in vitro apnoea’ (Smith et al. 1991). While a preBötC neuron from a rhythmically active slice was current-clamped, the centre part of the preBötC region, which was determined by referring to Ruangkittisakul et al. (2011), was illuminated with a circular spot (diameter ∼350 μm) of blue (488 nm; 6.6 mW) or green (540 nm; 19.5 mW) light to selectively stimulate and suppress the preBötC astrocytes for 3–4 s, respectively.
Immunostaining
Functional classification of cells recorded by calcium imaging into neurons and astrocytes was validated in a representative rhythmically active slice of rat by post hoc immunoreactivity analysis of S100β, which is an established astrocyte-specific marker protein in rodents (Huxtable et al. 2010; Aoyama et al. 2011; Sasaki et al. 2011a), though not necessarily astrocyte-specific in humans (Steiner et al. 2007). Also, ChR2–YFP-positive cells in rhythmically active slices of mouse were immunohistochemically examined to determine whether they were exclusively astrocytes.
A rhythmically active slice was fixed as a whole in 4% paraformaldehyde in 0.1 m phosphate buffer solution overnight at 4°C and permeabilized and blocked with 0.1% Triton X-100 and 5% goat serum at room temperature (Sasaki et al. 2011a). It was then incubated overnight with primary mouse monoclonal anti-S100β antibody (S2532, Sigma-Aldrich, 1:1000) and 1 μg ml−1 affinity-purified rabbit antibody to green fluorescent protein (gift from Takeshi Kaneko; Tamamaki et al. 2000) and labelled with secondary anti-rabbit IgG Alexa-488 (A11008, Invitrogen, 1:500), anti-mouse IgG Alexa-594 (A11032, Invitrogen, 1:500) and Neuro Trace 435/455 blue fluorescent Nissl (N21479, Invitrogen, 1:50), which specifically stains neuronal somata (Schmued et al. 1982), for 5 h at room temperature. The region where calcium imaging was conducted was identified based on the structural characteristics of the slice and the distribution of S100β-positive cells in the region that was presumed to be at the same depth as calcium imaging was observed under a confocal microscope (MRC-1024, Bio-Rad, CA, USA).
Results
Inspiratory-related activity patterns of astrocytes and neurons
The medullary region into which OGB1 was bolus-injected emitted green fluorescence, and coincided well with the preBötC in rhythmically active slices of rats (Fig. 1A). All tested slices spontaneously exhibited intermittent elevations of total fluorescence intensity in the dye-loaded region, the onset of which faithfully coincided with that of extracellularly recorded inspiratory neuron bursting, as previously shown (Koshiya & Smith, 1999; Ruangkittisakul et al. 2009) (Fig. 1B). These inspiratory-related rhythmic signals were monitored at 5–50 frames s−1 at a cellular resolution using a Nipkow-disk confocal microscope. Single videos were 243 ± 14 s in length, and included 161 ± 23 cells (120 ± 17 putative neurons; 41 ± 5 putative astrocytes) (n= 25 videos from 14 slices). The recorded cells were classified into putative astrocytes and putative neurons by principal component analysis of the waveforms derived from the cellular calcium dynamics and of the cell sizes (Fig. 1C). This functional cell classification was validated by post hoc immunohistochemical staining for the astrocytic marker S100β (Fig. 1D). Cell-attached patch-clamp recordings from these identified cells confirmed that the calcium elevations in putative neurons, but not in putative astrocytes, were associated with action potentials (9.5 ± 5.8 spikes per neuron in one inspiratory phase), thereby validating our classification methodology (Fig. 1E; putative neuron, n= 8 cells; putative astrocyte, n= 8 cells). Also, any cells classified as putative neurons did not show spontaneous calcium rises in the presence of tetrodotoxin, which further validated our cell classification (data not shown).
The spatial distribution and spatiotemporal activity patterns of 236 putative neurons and 90 putative astrocytes from one experiment are illustrated in Fig. 1F and G, respectively. The inspiratory-related modulation of calcium activity was evident in the putative neuronal populations, with a rhythm frequency of 0.16 ± 0.05 Hz and with 55 ± 19% of putative neurons participating in a single respiratory cycle (n= 14 videos).
Activity of putative astrocytes precedes inspiratory neuronal firing
A fraction of putative astrocytes also exhibited inspiratory-related modulation of their calcium activity in slices of rats (Fig. 1G). Thus, we analysed the temporal profile of the individual putative astrocytic responses. Trace plots of the inspiratory-related activity revealed that a subset of putative astrocytes were activated early relative to the inspiratory neuronal activity with a time lag of approximately 2 s (Fig. 2A and B and Supplementary video). The location of these preinspiratory putative astrocytes was unlikely to indicate spatial organization (Fig. 2C). In the present study, 10.5% of putative astrocytes were found to be preinspiratory, 3.2% of cells exhibited spontaneous calcium activity irrespective of inspiratory-related activity and 86.3% were either inspiratory or silent during the observation period. We avoided strict separation of inspiratory and silence cells when counting their numbers, because recorded cell activity could reflect a small contamination with background inspiratory fluorescence of neuropiles (Fig. 2D).
Intrinsic rhythmic activity of preinspiratory putative astrocytes
To analyse activity patterns of putative astrocytes without the influence of neuronal firings, we blocked neuronal activity by bath application of 1 μm tetrodotoxin, a voltage-sensitive Na+ channel inhibitor in slices of rats. Preinspiratory and inspiratory putative astrocytes maintained their periodic calcium rises even during the blockade of neuronal activity with tetrodotoxin, whereas the intercellular phases of these rhythmic activities were decoupled (Fig. 3A). Tetrodotoxin also reduced the frequency of calcium rises in preinspiratory, inspiratory and non-respiratory putative astrocytes (Fig. 3B). The activity patterns of the preinspiratory and inspiratory putative astrocytes tended to be more periodic than that of non-respiratory putative astrocytes both before and after application of tetrodotoxin, although their differences were not statistically significant when their rhythm regularities were assessed by the coefficient of variation (CV) in inter-activity intervals (Fig. 3C). The regularity of rhythmic activity of preinspiratory putative astrocytes was similar to that of inspiratory neuronal activity (Fig. 3C). It was interesting to find that tetrodotoxin induced rhythmic calcium activity with unknown mechanisms in a small number of putative astrocytes that were silent before application of tetrodotoxin (bottom trace in Fig. 3A).
Figure 3. Intrinsic rhythmic activities of preinspiratory putative astrocytes.
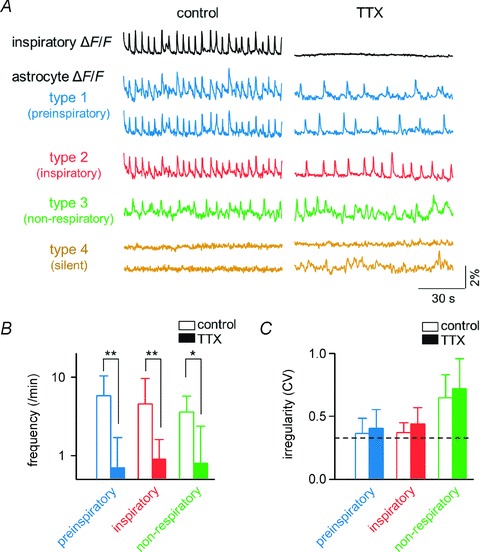
A, representative overall inspiratory ΔF/F traces (top) and calcium ΔF/F traces of preinspiratory, inspiratory, non-respiratory and silent putative astrocytes before and after application of tetrodotoxin. Note that the bottom silent trace exhibits activity after application of tetrodotoxin. B, the mean frequency of calcium events in preinspiratory (blue, n= 20 cells), inspiratory (red, n= 175 cells) and non-respiratory putative astrocytes (green, n= 14 cells) in the presence (filled bar) or absence (open bar) of tetrodotoxin. C, coefficient of variation (CV) of the time-intervals between two successive calcium events in preinspiratory, inspiratory and non-respiratory putative astrocytes in the presence or absence of tetrodotoxin. The dashed line indicates the CV of the inspiratory-related neuronal activity in the presence of tetrodotoxin, i.e. the irregularity of the inspiratory rhythm. *P < 0.05, **P < 0.01, paired t test.
Selective photo-stimulation of preBötC astrocytes
To gain an insight into the functional role of preBötC astrocytes, we examined whether astrocytes in the preBötC were capable of activating inspiratory neurons, by selectively photo-stimulating astrocytes in the centre part of the preBötC in slices of ChR2(C128S)–YFP transgenic mice (Fig. 4A). As ChR2 is rapidly inactivated after cessation of light application, we employed the C128S point mutation of ChR2 (Berndt et al. 2009), which alters the photocycle, enabling bistable, step-like control of cell membrane potentials by two different wavelengths. This optical property is effective to mimic the slow kinetics (2–4 s) of astrocytic calcium dynamics. To confirm the specificity of transgene expression in astrocytes, we performed simultaneous Nissl staining that specifically stains neuronal somata and immunohistochemistry for YFP and for an astrocyte-specific marker S100β (Fig. 4B). In the preBötC, 45% of S100β-positive cells expressed YFP signals (n= 278 cells from 16 slices). On the other hand, there was no overlap between Nissl-positive cells and YFP signals (n= 895 cells from 16 slices). To further validate the specificity of transgene expression, whole-cell patch-clamp recordings were performed randomly from astrocytes and neurons in the presence of 1 μm tetrodotoxin, 100 μm Cd2+ and 50 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to avoid generation of action potentials, Ca2+-dependent vesicular releases and non-NMDA receptor-mediated transmission, respectively, if any. In astrocytes, 5 out of 9 cells exhibited inward currents in response to brief illumination with blue light, and the currents persisted until flashed with green light (Fig. 4C, top). The average amplitude of the photocurrents was 68 ± 37 pA (n= 5 astrocytes). In contrast, no current responses to photo-stimulation were observed in all neurons tested (n= 25 cells) (Fig. 4C, bottom). These histological and electrophysiological findings clearly demonstrate that ChR2(C128S)–YFP was expressed exclusively in a subset of astrocytes. Using this transgenic mouse line, we then investigated the effect of selective activation of preBötC astrocytes on excitability of neighbouring inspiratory neurons. Photo-activation of astrocytes elicited single or burst firing of action potentials in 8 of 16 preBötC inspiratory neurons in the ChR2(C128S)–YFP transgenic mice (n= 16 mice), but not in the wild-type mice (n= 9 cells from 9 mice) (Fig. 4D and E). In the eight responsive neurons, 75 ± 11% of trials with light stimulation elicited action potentials (Fig. 4F) and the latency from the stimulus onset to the first spike was 5.5 ± 0.9 s (Fig. 4G).
Figure 4. Astrocyte-driven action potentials in inspiratory neurons.
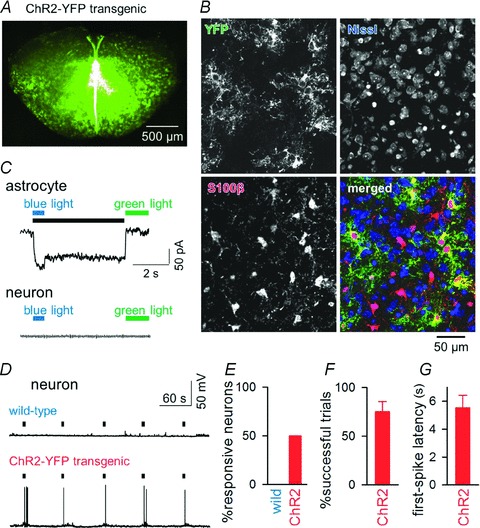
A, YFP fluorescence of a rhythmically active slice prepared from a Mlc1-tTA::tetO-ChR2(C128S)–YFP transgenic mouse. B, triple labelling of preBötC cells with an antibody against green fluorescent protein (i.e. YFP appearing in green) to indicate ChR2-expressing cells, with fluorescent Nissl to show neuronal somata (blue), and with an antibody against S100β to identify astrocytes (red). ChR2–YFP positivity was found exclusively in S100β-positive cells. Thus, ChR2 was expressed only in astrocytes. C, switch-on (blue light) and switch-off (green light) of a persistent inward current in a voltage-clamped ChR2-expressing astrocyte (top) and neuron (bottom). Voltages were clamped at −70 mV. The black bar indicates the activated period. D, photo-activation of astrocytes triggers action potentials in an inspiratory neuron from the preBötC in a ChR2(C128S)YFP transgenic (but not in a wild-type) mouse. Black bars indicate the activation periods of preBötC astrocytes. E, ratios of neurons that fired in response to photo-activation of astrocytes in wild-type (n= 9 neurons) and in ChR2(C128S)YFP transgenic mice (n= 16 neurons from 5 animals). F, ratios of the trials in which light stimulation elicited action potentials (n= 8 responsive neurons). G, latency from the light-stimulus onset to the first spike (n= 8 neurons).
Discussion
In the present study we have shown that a subset of putative astrocytes exhibited calcium elevations preceding inspiratory neuronal activity with a time lag of approximately 2 s. These preinspiratory putative astrocytes maintained their rhythmic activity even during the blockade of neuronal activity with tetrodotoxin, whereas their rhythm frequency was lowered and the intercellular phases of these rhythms were decoupled. Optogenetic stimulation of preBötC astrocytes induced firing of inspiratory neurons.
Recently, a number of studies by calcium imaging in the preBötC have been reported. However, none showed respiratory-related rhythmic calcium activity in astrocytes (e.g. Funke et al. 2007; Härtel et al. 2009; Ruangkittisakul et al. 2009), and the present study is the first to show respiratory-related stable rhythmic calcium activities in putative astrocytes. Therefore, the preinspiratory activity in putative astrocytes is also first reported here. Although it is not clear why respiratory-related rhythmic calcium activities in astrocytes have not previously been reported; it may be partly attributed to the experimental technique in previous studies, i.e. calcium indicator dyes were injected into the midline of the slice to selectively label neurons through axons of passage (Koizumi et al. 2008; Koshiya & Smith, 1999), imaging areas were rather small (Koizumi et al. 2008) or injected dyes preferentially stained neurons. On the contrary, we directly stained the preBötC region and conducted large-scale imaging, and the dye we used, OGB1, appears to preferentially stain astrocytes (Funke et al. 2007; Sasaki et al. 2011a). Recently, Schnell et al. (2011) analysed functional coupling between astrocytes and neurons, detected a rhythmic inwardly directed current that was entrained to neuron population discharges in 10% of preBötC astrocytes by whole-cell patch recording, and attributed the rhythmic current fluctuation to periodic elevations of extracellular potassium that were produced by efflux from rhythmically oscillating neurons and to consequent changes in potassium equilibrium potentials of astrocytes (Brockhaus et al. 1993; Okada et al. 2005). However, Schnell et al. (2011) did not detect periodic calcium rises in astrocytes coupled with neuronal rhythmic activities. Although they observed spontaneous calcium fluctuation in astrocytes, it did not propagate to or from other cells. To explain the discrepancy between their and our observations, further studies are needed.
Optogenetic stimulation is a powerful method to study the function of astrocytes (Figueiredo et al. 2011). It has been reported that optogenetic stimulation of chemosensory astrocytes near the ventral surface of the medulla oblongata induces respiratory augmentation (Gourine et al. 2010). In the present study we applied an optogenetic stimulation technique, and carefully focused the photo-stimulating region within the the preBötC. Thus, chemosensory astrocytes near the ventral surface should not have been excited. Although we could induce inspiratory neuronal firings by optogenetic stimulation of astrocytes in the preBötC, not only preinspiratory but all other types of astrocytes expressing ChR2 should have been excited with this technique. Also, the latency approximately 5 s after photo-stimulation to inspiratory neuronal firings, as well as the latency approximately 2 s after activation of preinspiratory putative astrocytes to inspiratory neuronal firings, appear too long, if assuming a pacemaking role in preinspiratory astrocytes to trigger inspiratory neurons especially in in vivo conditions where breathing rates are notably faster than 1 Hz in rodents. Further studies are necessary to examine the response of inspiratory neurons to selective activation of preinspiratory astrocytes and to clarify the role of astrocytes in respiratory control in in vitro and in vivo conditions.
Even if preinspiratory astrocytes play an active role in initiating inspiratory neuron firings, the notion does not necessarily contradict any previously proposed neuronal pacemaker or neuronal group pacemaker hypotheses (Koshiya & Smith, 1999; Feldman & Del Negro, 2006; Pace et al. 2007; Mironov, 2008; Del Negro et al. 2011). Although calcium influxes in the somata of inspiratory neurons have been shown to be a consequence of membrane depolarization and through a voltage-dependent calcium channels (Morgado-Valle et al. 2008), those in the dendrites precede somatic inspiratory activity with a time lag of a few hundred milliseconds (Mironov, 2008; Del Negro et al. 2011). According to the group pacemaker hypothesis, preBötC excitatory interneurons mutually interact to elevate dendritic calcium, activate ICAN and periodically trigger inspiration (Mironov, 2008; Rubin et al. 2009). Our findings raise an alternative possibility that the preinspiratory dendritic calcium elevation and depolarization are evoked, at least partly, by the preceding astrocytic activity (Sasaki et al. 2011b). Our observation that blockade of neuronal activity with tetrodotoxin slowed and decoupled the periodic calcium rises of preinspiratory putative astrocytes suggests that the neuronal network facilitates the occurrence of calcium transient of preinspiratory astrocytes and synchronizes them. Inspiratory neurons appear to have an influence on the rhythmic glial activities and thus preinspiratory astrocytes and inspiratory neurons seem to have mutual interactions in the preBötC.
These findings, together with previous reports that inhibition of astrocytic metabolism abolishes inspiratory-related neural output in rhythmically active slices (Hülsmann et al. 2000) and decreases respiratory frequency in in vivo rat pups (Young et al. 2005), raise the possibility that astrocytes are actively involved in respiratory rhythm generation in the preBötC. As astrocyte-driven neural synchronization has been reported in many other brain regions (Parri et al. 2001; Angulo et al. 2004; Fellin et al. 2004; Tian et al. 2005), we propose a hypothesis that preinspiratory astrocytes are actively involved in respiratory rhythm generation at least in rhythmically active slices. This issue must be thoroughly investigated in the future, in a precisely ‘calibrated’ rhythmically active slice that can generate an eupnoea-like inspiratory burst pattern in a more physiological superfusate potassium concentration (Ballanyi & Ruangkittisakul, 2009) and by experiments including long-term calcium imaging and patch-clamp recording from astrocytes that are identified, e.g. genetically (e.g. Härtel et al. 2009; Ruangkittisakul et al. 2009). Also, pharmacological modulation of the bursting rhythm by glial toxins and agonist/antagonist of possible gliotransmitter receptors must be thoroughly examined to clarify whether preinspiratory calcium rise in astrocytes leads inspiratory neuronal firings.
Acknowledgments
This work was supported in part by Grants-in-Aid for Science Research (nos. 20590218, 22115003, 22650080, 22680025, 23650218, 24300108 and 24500365) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Suzuken Memorial Foundation; the Kanae Foundation for the Promotion of Medical Science; the Daiichi-Sankyo Foundation of Life Science; Health and Labour Sciences Research Grants; and the Funding Program for Next Generation World-Leading Researchers (no. LS023). Authors declare no conflict of interest.
Glossary
- aCSF
artificial cerebrospinal fluid
- ChR2
channelrhodopsin-2
- CV
coefficient of variation
- F
fluorescence intensity
- ICAN
non-specific cationic current
- INaP
persistent sodium current
- MLC
megalencephalic leukoencephalopathy with subcortical cysts
- OGB1
Oregon Green 488 BAPTA-1 AM
- PC
principal component
- preBötC
pre-Bötzinger complex
- ROI
region of interest
- tTA
tetracycline transactivator
- YFP
yellow fluorescent protein
Author contributions
The experiments were performed at the laboratory of Y.I.
Y.O.: Conception of the experiments and drafting the article. T.S. and Y.O.: Collection, analysis and interpretation of data and drafting the article. N.T., M.S. and S.U.: Collection of data. K.F.T.: Development of transgenic mice. N.M.: Supervision of experiments and critical revision of the article. Y.I.: Design of the experiments, analysis and interpretation of data, and drafting the article. All authors approved this manuscript.
Supplementary material
Supplementary video
References
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama R, Okada Y, Yokota S, Yasui Y, Fukuda K, Shinozaki Y, Yoshida H, Nakamura M, Chiba K, Yasui Y, Kato F, Toyama Y. Spatiotemporal and anatomical analyses of P2X receptor-mediated neuronal and glial processing of sensory signals in the rat dorsal horn. Pain. 2011;152:2085–2097. doi: 10.1016/j.pain.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Ruangkittisakul A. Structure-function analysis of rhythmogenic inspiratory pre-Bötzinger complex networks in ‘calibrated’ newborn rat brainstem slices. Respir Physiol Neurobiol. 2009;168:158–178. doi: 10.1016/j.resp.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Panaitescu B, Ruangkittisakul A. Control of breathing by ‘nerve glue’. Sci Signal. 2010;3:pe41. doi: 10.1126/scisignal.3147pe41. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Ghirri A, Zonta M, Betelli C, Vitale G, Ruggieri V, Sandrini M, Carmignoto G. Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn. J Physiol. 2010;588:831–846. doi: 10.1113/jphysiol.2009.180570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci. 2009;12:229–234. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K, Smith JC, Richter DW. Microenvironment of respiratory neurons in the in vitro brainstem–spinal cord of neonatal rats. J Physiol. 1993;462:421–445. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes C, Vapnik V. Support-vector networks. Mach Learn. 1995;20:273–297. [Google Scholar]
- Del Negro CA, Hayes JA, Rekling JC. Dendritic calcium activity precedes inspiratory bursts in preBötzinger complex neurons. J Neurosci. 2011;31:1017–1022. doi: 10.1523/JNEUROSCI.4731-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman JS, Leiter JC, Gourine AV. ATP, glia and central respiratory control. Respir Physiol Neurobiol. 2010;173:305–311. doi: 10.1016/j.resp.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Gomez-Gonzalo M, Gobbo S, Carmignoto G, Haydon PG. Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. J Neurosci. 2006;26:9312–9322. doi: 10.1523/JNEUROSCI.2836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Figueiredo M, Lane S, Tang F, Liu BH, Hewinson J, Marina N, Kasymov V, Souslova EA, Chudakov DM, Gourine AV, Teschemacher AG, Kasparov S. Optogenetic experimentation on astrocytes. Exp Physiol. 2011;96:40–50. doi: 10.1113/expphysiol.2010.052597. [DOI] [PubMed] [Google Scholar]
- Funke F, Dutschmann M, Müller M. Imaging of respiratory-related population activity with single-cell resolution. Am J Physiol Cell Physiol. 2007;292:C508–C516. doi: 10.1152/ajpcell.00253.2006. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasparov S. Astrocytes as brain interoceptors. Exp Physiol. 2011;96:411–416. doi: 10.1113/expphysiol.2010.053165. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel K, Schnell C, Hülsmann S. Astrocytic calcium signals induced by neuromodulators via functional metabotropic receptors in the ventral respiratory group of neonatal mice. Glia. 2009;57:815–827. doi: 10.1002/glia.20808. [DOI] [PubMed] [Google Scholar]
- Hirase H, Qian L, Barthó P, Buzsáki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2:E96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülsmann S, Oku Y, Zhang W, Richter DW. Metabolic coupling between glia and neurons is necessary for maintaining respiratory activity in transverse medullary slices of neonatal mouse. Eur J Neurosci. 2000;12:856–862. doi: 10.1046/j.1460-9568.2000.00973.x. [DOI] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Alvares TS, Ruangkittisakul A, Fang X, Hahn LB, Posse de Chaves E, Baker GB, Ballanyi K, Funk GD. Glia contribute to the purinergic modulation of inspiratory rhythm-generating networks. J Neurosci. 2010;30:3947–3958. doi: 10.1523/JNEUROSCI.6027-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Smith JC. Persistent Na+ and K+-dominated leak currents contribute to respiratory rhythm generation in the pre-Bötzinger complex in vitro. J Neurosci. 2008;28:1773–1785. doi: 10.1523/JNEUROSCI.3916-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J Neurosci. 2008;28:2353–2365. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- Mironov SL. Metabotropic glutamate receptors activate dendritic calcium waves and TRPM channels which drive rhythmic respiratory patterns in mice. J Physiol. 2008;586:2277–2291. doi: 10.1113/jphysiol.2007.149021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado-Valle C, Baca SM, Feldman JL. Glycinergic pacemaker neurons in preBötzinger complex of neonatal mouse. J Neurosci. 2010;30:3634–3639. doi: 10.1523/JNEUROSCI.3040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado-Valle C, Beltran-Parrazal L, DiFranco M, Vergara JL, Feldman JL. Somatic Ca2+ transients do not contribute to inspiratory drive in preBötzinger complex neurons. J Physiol. 2008;586:4531–4540. doi: 10.1113/jphysiol.2008.154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Wenker IC. Astrocyte chemoreceptors: mechanisms of H+ sensing by astrocytes in the retrotrapezoid nucleus and their possible contribution to respiratory drive. Exp Physiol. 2011;96:400–406. doi: 10.1113/expphysiol.2010.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neusch C, Papadopoulos N, Müller M, Maletzki I, Winter SM, Hirrlinger J, Handschuh M, Bähr M, Richter DW, Kirchhoff F, Hülsmann S. Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: impact on extracellular K+ regulation. J Neurophysiol. 2006;95:1843–1852. doi: 10.1152/jn.00996.2005. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods. 2004;1:31–37. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- Okada Y, Kuwana S, Kawai A, Mückenhoff K, Scheid P. Significance of extracellular potassium in central respiratory control studied in the isolated brainstem-spinal cord preparation of the neonatal rat. Respir Physiol Neurobiol. 2005;146:21–32. doi: 10.1016/j.resp.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Baele PL, Marsh HM, Okazaki H. Central neurogenic hyperventilation in an awake patient with brainstem astrocytoma. Ann Neurol. 1982;11:625–628. doi: 10.1002/ana.410110612. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Okada Y, Oku Y, Koshiya N, Ballanyi K. Fluorescence imaging of active respiratory networks. Respir Physiol Neurobiol. 2009;168:26–38. doi: 10.1016/j.resp.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Panaitescu B, Ballanyi K. K+ and Ca2+ dependence of inspiratory-related rhythm in novel ‘calibrated’ mouse brainstem slices. Respir Physiol Neurobiol. 2011;175:37–48. doi: 10.1016/j.resp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Ma Y, Bobocea N, Poon BY, Funk GD, Ballanyi K. Generation of eupnea and sighs by a spatiochemically organized inspiratory network. J Neurosci. 2008;28:2447–2458. doi: 10.1523/JNEUROSCI.1926-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JE, Hayes JA, Mendenhall JL, Del Negro CA. Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proc Natl Acad Sci U S A. 2009;106:2939–2944. doi: 10.1073/pnas.0808776106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Kuga N, Namiki S, Matsuki N, Ikegaya Y. Locally synchronized astrocytes. Cereb Cortex. 2011a;21:1889–1900. doi: 10.1093/cercor/bhq256. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Matsuki N, Ikegaya Y. Metastability of active CA3 networks. J Neurosci. 2007;27:517–528. doi: 10.1523/JNEUROSCI.4514-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Matsuki N, Ikegaya Y. Action-potential modulation during axonal conduction. Science. 2011b;331:599–601. doi: 10.1126/science.1197598. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Takahashi N, Matsuki N, Ikegaya Y. Fast and accurate detection of action potentials from somatic calcium fluctuations. J Neurophysiol. 2008;100:1668–1676. doi: 10.1152/jn.00084.2008. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Gofferje V, Weber M, Meyer J, Mössner R, Lesch KP. The brain-specific protein MLC1 implicated in megalencephalic leukoencephalopathy with subcortical cysts is expressed in glial cells in the murine brain. Glia. 2003;44:283–295. doi: 10.1002/glia.10304. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Swanson LW, Sawchenko PE. Some fluorescent counterstains for neuroanatomical studies. J Histochem Cytochem. 1982;30:123–128. doi: 10.1177/30.2.6174560. [DOI] [PubMed] [Google Scholar]
- Schnell C, Fresemann J, Hülsmann S. Determinants of functional coupling between astrocytes and respiratory neurons in the pre-Bötzinger complex. PLoS One. 2011;6:e26309. doi: 10.1371/journal.pone.0026309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bernstein HG, Bielau H, Berndt A, Brisch R, Mawrin C, Keilhoff G, Bogerts B. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci. 2007;8:2. doi: 10.1186/1471-2202-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K, Denk W, Kleinfeld D, Tank DW. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature. 1997;385:161–165. doi: 10.1038/385161a0. [DOI] [PubMed] [Google Scholar]
- Szoke K, Härtel K, Grass D, Hirrlinger PG, Hirrlinger J, Hülsmann S. Glycine transporter 1 expression in the ventral respiratory group is restricted to protoplasmic astrocytes. Brain Res. 2006;1119:182–189. doi: 10.1016/j.brainres.2006.08.089. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Sasaki T, Matsumoto W, Matsuki N, Ikegaya Y. Circuit topology for synchronizing neurons in spontaneously active networks. Proc Natl Acad Sci U S A. 2010;107:10244–10249. doi: 10.1073/pnas.0914594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Nakamura K, Furuta T, Asamoto K, Kaneko T. Neurons in Golgi-stain-like images revealed by GFP-adenovirus infection in vivo. Neurosci Res. 2000;38:231–236. doi: 10.1016/s0168-0102(00)00176-0. [DOI] [PubMed] [Google Scholar]
- Tanaka KF, Ahmari SE, Leonardo ED, Richardson-Jones JW, Budreck EC, Scheiffele P, Sugio S, Inamura N, Ikenaka K, Hen R. Flexible Accelerated STOP Tetracycline Operator-knockin (FAST): a versatile and efficient new gene modulating system. Biol Psychiatry. 2010;67:770–773. doi: 10.1016/j.biopsych.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka KF, Matsui K, Sasaki T, Sano H, Sugio S, Fan K, Hen R, Nakai J, Yanagawa Y, Hasuwa H, Okabe M, Deisseroth K, Ikenaka K, Yamanaka A. Expanding the repertoire of optogenetically targeted cells with an enhanced gene expression system. Cell Reports. doi: 10.1016/j.celrep.2012.06.011. in press. [DOI] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Kréneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK, Dreshaj IA, Wilson CG, Martin RJ, Zaidi SI, Haxhiu MA. An astrocyte toxin influences the pattern of breathing and the ventilatory response to hypercapnia in neonatal rats. Respir Physiol Neurobiol. 2005;147:19–30. doi: 10.1016/j.resp.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Zhang WD, Tsukamoto T, Yamada Y, Itakura Y, Oono T, Nagao M, Takatori T. Immunohistochemical classification of astrocytes in infants by glial fibrillary acidic protein staining and application to forensic practice. Nihon Hoigaku Zasshi. 1992;46:189–197. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


