Abstract
Objective
The purpose of this study was to compare the biliary enhancement dynamics of gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic-acid (Gd-EOB-DTPA) and mangafodipir trisodium (Mn-DPDP) for contrast-enhanced MR cholangiography (MRC) in healthy subjects.
Methods
15 healthy volunteers underwent MRI at 1.5 T with volumetric interpolated breath-hold examination sequence. Each volunteer was scanned once for each contrast agent. The signal-to-noise ratio (SNR) of the liver parenchyma and common hepatic duct (CHD) and the contrast-to-noise ratio (CNR) of CHD to liver parenchyma were evaluated and compared before and at several time points (5, 15, 30, 45, 60, 90, and 120 min) after injection of each agent.
Results
SNR was significantly higher for Gd-EOB-DTPA than for Mn-DPDP in liver parenchyma after 5 min and in CHD after 15 min (p<0.05). CNR of CHD to liver parenchyma using Gd-EOB-DTPA showed an initial decrease at 5 min post-injection followed by a steep increase to a peak at 15 min post-injection. CNR using Mn-DPDP showed a steady increase to a peak at 15 min post-injection without an initial decrease. At 15 min, the value of CNR was significantly higher for Gd-EOB-DTPA than for Mn-DPDP (p<0.05).
Conclusion
For both contrast agents, CNR reached a peak at 15 min after contrast injection. At this time point, CNR of Gd-EOB-DTPA was significantly higher than that of Mn-DPDP. Therefore, Gd-EOB-DTPA may provide better contrast-enhanced MRC than Mn-DPDP at 15 min after contrast administration.
The bile ducts are generally studied using fast spin echo T2 weighted sequences with half-Fourier reconstruction. This conventional MR cholangiography (MRC) examination is highly accurate in detecting biliary tree disease [1,2]. However, the conventional MRC has diagnostic limitations, which include poor visualisation of the intrahepatic biliary tree compared with the extrahepatic biliary tree [3], limited spatial resolution and not being able to provide functional information of the biliary tree.
Contrast-enhanced MRC has created interest in the field of MRC because of its potential to provide functional assessment and to improve the visualisation of the intrahepatic biliary trees [4-6]. The specific indications include pre-operative anatomical assessment of the biliary tree in preventing inadvertent complications in common laparoscopic cholecystectomy, and also in complex biliary surgical procedures such as biliary–enteric anastomosis and liver transplantation; post-operative assessment of the biliary tree after surgery when complications such as bile leak or inadvertent biliary tree stricture or ligation are suspected; and functional assessment of bile secretion and excretion [7-9].
Contrast-enhanced MRC is performed with hepatobiliary MR contrast agents such as mangafodipir trisodium (Mn-DPDP), gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic-acid (Gd-EOB-DPTA) or gadobenate dimeglumine (Gd-BOPTA), which are administered intravenously, taken up by the hepatocytes, and then excreted via the biliary system.
Only a small fraction of Gd-BOPTA (3–5% of the injected dose) is excreted into the biliary system [10]. On the other hand, 50–60% of Mn-DPDP and 50% of Gd-EOB-DTPA is eliminated via the biliary system [11-13]. To the best of our knowledge, no comparative study has investigated which of Mn-DPDP or Gd-EOB-DTPA has a greater influence on biliary signal intensity (SI) on T1 weighted MRI.
The purpose of this study was to evaluate and compare the time course of biliary enhancement of Gd-EOB-DTPA and Mn-DPDP for contrast-enhanced MRC in normal healthy volunteers.
Methods and materials
Subjects
After we obtained written informed consent, 15 healthy volunteers with an age range of 22–33 years (mean age 27 years) were included in this study under a protocol approved for human investigation by our institutional review board. Of these, 10 were male and 5 were female. To exclude unknown liver and renal dysfunction, serum bilirubin and creatinine were evaluated prior to MRI scanning.
MRI
Contrast-enhanced MRC was performed with a 1.5 T imaging system (MAGNETOM Sonata®; Siemens, Erlangen, Germany) using a torso phased array coil. Three-dimensional volumetric interpolated breath-hold examinations (VIBEs) were obtained at baseline and then sequentially at 5, 15, 30, 45, 60, 90 and 120 min after administration of contrast agent. The parameters were follows: repetition time (TR), 4.8 ms; echo time (TE), 2.26 ms; flip angle, 10°; field of view (FOV), 34 cm; matrix size, 256×134 or 256×140; slice thickness, 4 mm; reconstruction interval, 2 mm.
Contrast agents
Mn-DPDP MRC was obtained following an intravenous injection of Mn-DPDP (Teslascan®; Nycomed Amersham, Princeton, NJ) at the standard dose of 5 μmol kg−1 administered via a slow injection for 1–2 min followed by a 10-ml saline flush. In the case of Gd-EOB-DTPA MRC, Gd-EOB-DTPA (Primovist®, Schering, Germany) was infused at the standard dose of 25 μmol kg−1 of body weight using an automated injection at a rate of 2 ml s−1 followed by a 10-ml saline flush. The two examinations for each subject were performed at least 7 days apart.
Image analysis
One reviewer performed operator-defined region-of-interest (ROI) measurements of the SI of CHD, liver parenchyma and background noise using a picture archiving and communication system (PACS) monitor and digital imaging and communications in medicine (DICOM) imaging viewing software (PiViewTM v. 4; Infinitt, Seoul, Republic of Korea). Three ROIs were drawn on the most proximal portion of the CHD on each image, to include as much CHD as possible. The mean value of the SIs in the three ROIs was defined as the SI of the CHD. The SI of the liver parenchyma was similarly calculated, with the ROIs placed at the same anteroposterior level used in measuring the CHD, and avoiding inclusion of imaging artefacts and major vascular structures in the same slice. Background noise was measured on the same image using ROIs positioned lateral to the abdominal wall. The same set of ROIs was applied to pre- and post-contrast images obtained with each contrast agent, for each subject.
Signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were calculated using the following standard equations:
SNRCHD(t)=SICHD(t)
SDbackground noise(t)
SNRliver(t)=SIliver(t)
SDbackground noise(t)
CNR(t)=SICHD(t)–SIliver(t)
SDbackground noise(t)
SNR(t) and CNR(t) are the SNR and CNR, respectively, at the time point t (min) after injection of contrast agent; SICHD(t) is the SI of the CHD; SILiver(t) is the SI of the liver parenchyma; and SDbackground noise(t) is the standard deviation (SD) of background noise at this time.
Statistical analysis
At each time point, we performed a statistical analysis to compare the SNR of liver and CHD, and the CNR of CHD to liver between the two contrast media using a Wilcoxon signed-rank test. A p-value ≤0.05 was considered to indicate significant difference.
Results
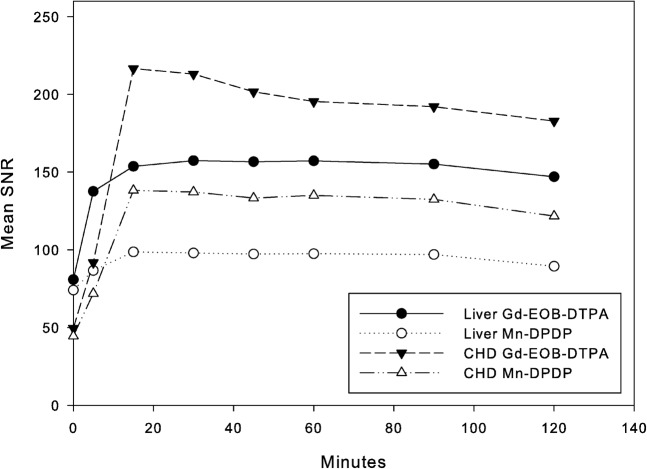
All subjects underwent both examinations without any adverse reactions or subjective symptoms. Representative MR images of both contrast agents are shown in Figure 1. Figure 2 and Table 1 show the time courses of the mean SNRs of liver parenchyma and CHD for both contrast media. SNR of liver parenchyma reached a peak at 15 min after injection of Mn-DPDP and 30 min after Gd-EOB-DTPA, before reaching a plateau. SNR of liver parenchyma began to decrease after 90 min for both contrast agents. Between 5 and 120 min, SNR in liver parenchyma was significantly higher for Gd-EOB-DTPA than for Mn-DPDP (p<0.05).
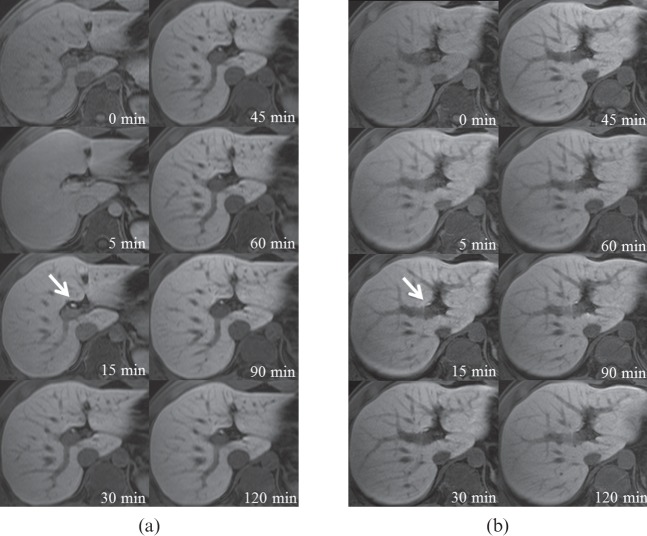
Figure 1.
Axial T1 weighted images with (a) Gd-EOB-DTPA and (b) Mn-DPDP of a 28-year-old male volunteer before and at 5, 15, 30, 45, 60, 90 and 120 min after contrast injection. Arrows indicate the proximal common hepatic duct. Gd-EOB-DTPA, gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid; Mn-DPDP, mangafodipir trisodium.
Figure 2.
Mean signal-to-noise ratios (SNRs) of the common hepatic duct (CHD) and liver parenchyma. The SNRs are significantly higher for Gd-EOB-DTPA than for Mn-DPDP in liver parenchyma after 5 min and in CHD after 15 min (p<0.05). Gd-EOB-DTPA, gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid; Mn-DPDP, mangafodipir trisodium.
Table 1. Signal-to-noise ratios for Gd-EOB-DTPA and Mn-DPDP at liver and common hepatic duct.
| Time | Liver |
Common hepatic duct |
||
| Gd-EOB-DTPA | Mn-DPDP | Gd-EOB-DTPA | Mn-DPDP | |
| Baseline | 80.8±14.6 | 74.1±15.4 | 49.6±15.7 | 44.5±9.4 |
| 5 min | 137.6±19.8a | 86.7±21.4a | 91.8±31.2 | 71.9±35.1 |
| 15 min | 153.7±26.0a | 98.7±18.9a | 216.6±37.0a | 138.2±27.8a |
| 30 min | 157.4±32.2a | 97.9±18.0a | 213.1±40.2a | 137.2±24.8a |
| 45 min | 156.7±29.1a | 97.3±20.6a | 201.7±36.6a | 133.3±22.3a |
| 60 min | 157.2±28.1a | 97.5±18.1a | 195.4±35.9a | 135.0±23.2a |
| 90 min | 155.2±30.0a | 97.0±19.8a | 192.1±41.3a | 132.4±21.5a |
| 120 min | 147.0±30.5a | 89.4±16.4a | 182.8±33.8a | 121.7±13.7a |
Gd-EOB-DTPA, gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid; Mn-DPDP, mangafodipir trisodium.
Data are mean±standard deviation.
ap<0.05 (Wilcoxon signed rank test was used).
For both contrast agents, SNR of CHD reached a peak at 15 min after injection, after which time SNR of CHD showed a gradual decrease for both contrast agents. Between 15 and 120 min, SNR in CHD was significantly higher for Gd-EOB-DTPA than for Mn-DPDP (p<0.05).
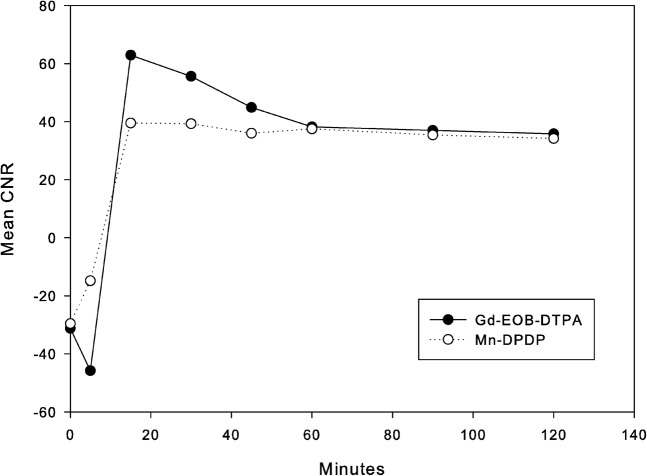
Figure 3 and Table 2 show the time courses of mean CNR of CHD to liver parenchyma for both contrast groups. CNR of Gd-EOB-DTPA showed an initial decrease in the 5 min after injection, a steep increase to a peak at 15 min, followed by a steep decrease. CNR using Mn-DPDP showed a steady increase to a peak at 15 min after injection, without an initial decrease, followed by a gradual decline. At 15 min, the value of CNR was significantly higher for Gd-EOB-DTPA than for Mn-DPDP (p<0.05).
Figure 3.
Mean contrast-to-noise ratios (CNRs) of common hepatic duct to liver parenchyma. The CNR of Gd-EOB-DTPA shows an initial decline and then steep increase to a peak at 15 min, whereas the CNR of Mn-DPDP shows a steady increase to a peak at 15 min. At a peak (15 min) the CNR of the Gd-EOB-DTPA is significantly higher than that of Mn-DPDP (p<0.05). Gd-EOB-DTPA, gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid; Mn-DPDP, mangafodipir trisodium.
Table 2. Contrast-to-noise ratios for Gd-EOB-DTPA and Mn-DPDP.
| Time | Contrast agent |
|
| Gd-EOB-DTPA | Mn-DPDP | |
| Baseline | −31.2±16.4 | −29.6±13.2 |
| 5 min | −45.8±22.8 | −14.8±30.7 |
| 15 min | 62.9±20.3a | 39.5±15.6a |
| 30 min | 55.6±23.2 | 39.3±21.0 |
| 45 min | 44.9±20.1 | 36.0±16.6 |
| 60 min | 38.2±19.1 | 37.5±19.3 |
| 90 min | 37.0±19.1 | 35.4±17.3 |
| 120 min | 35.8±22.0 | 34.2±15.0 |
Gd-EOB-DTPA, gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid; Mn-DPDP, mangafodipir trisodium.
Data are mean±standard deviation.
ap<0.05 (Wilcoxon signed rank test was used).
Discussion
During the past decade, MRC has proven to be an accurate imaging tool for evaluation of the extrahepatic biliary system [14]. Conventional MRC relies on the use of a heavily T2 weighted sequence to produce high SI in stationary tissues such as water and bile that have long T2 relaxation times. Tissues with short T2 relaxation times, such as liver and pancreas, and flowing blood, have low SI, allowing for optimal contrast between the hyperintense bile and hypointense background. The sensitivity and specificity of MRC for detection of choledocholithiasis are 90% and 100%, respectively [15]. Despite the impressive clinical performance reported in the literature, MRC has diagnostic limitations that include poor visualisation of the intrahepatic biliary tree compared with the extrahepatic biliary tree [3], variation in T2 weighting causing depiction of slow-flow vascular structures (such as the portal vein and hepatic vein) that may obscure adjacent biliary structures [16], and limited spatial resolution. However, detailed anatomical depiction of the non-distended intrahepatic biliary ductal system is occasionally required (e.g. in pre-operative evaluation of potential living liver donors) because biliary variants are seen in up to 45% of the population [17].
To overcome the above limitations, radiologists have developed new imaging strategies in recent years by using contrast agents that are excreted by the bile ducts for T1 weighted MRI [9]. In addition to functional assessment of the biliary tree, hepatobiliary contrast agents enable definition of intrahepatic ductal anatomy because it acts as a positive biliary contrast by differentiating hepatic vessels from bile ducts, which can be difficult on conventional MRC because of slow flow [5]. Traditional two-dimensional imaging using turbo spin-echo sequences to generate conventional T2 MRC source data results in low spatial resolution [1], whereas three-dimensional T1 weighted imaging using hepatobiliary contrast agents has improved resolution [5,6].
To the best of our knowledge, no studies have directly compared the time course of biliary enhancement of Gd-EOB-DTPA and Mn-DPDP for contrast-enhanced MRC in normal healthy volunteers.
Mn-DPDP is a manganese chelate developed as an MR contrast agent for the hepatobiliary system. The DPDP complex is chemically related to pyridoxal phosphate, a vitamin B6 analogue; therefore, uptake of Mn-DPDP into the hepatocytes via the vitamin B6 pathway was expected [18]. Gd-EOB-DTPA is a paramagnetic hepatobiliary contrast agent with hepatocellular uptake via the anionic transporter protein [19,20].
In the present study, the SNR curves of liver parenchyma and CHD showed a similar pattern between two contrast agents. But the SNRs were significantly higher for Gd-EOB-DTPA than for Mn-DPDP in liver parenchyma after 5 min and in CHD after 15 min. The shape of the CNR curve may give the impression that the CNR of Gd-EOB-DTPA exceeds that of Mn-DPDP at 15–45 min delay, but the statistical difference was noted only at 15 min.
Our results indicate that contrast-enhanced MRC using Gd-EOB-DTPA could be superior to that using Mn-DPDP at 1.5 T. This is an intriguing result, because the proportion of Gd-EOB-DTPA excreted into the biliary system is comparable to that of Mn-DPDP. Previous studies have shown that 50–60% of Mn-DPDP and 50% of Gd-EOB-DTPA were eliminated via the biliary system [11-13]; however, the T1 relaxivity of Mn-DPDP in aqueous solution is less pronounced than that of Gd-EOB-DTPA. The value of T1 relaxivity of Gd-EOB-DTPA is reported as 5.30 l mmol−1 s−1 in water, and that of Mn-DPDP as 2.8 l mmol−1 s−1 in water [20,21]. Another possible cause of the higher SNRs and CNRs observed with Gd-EOB-DTPA may be the injected dose difference between the two contrast agents at the standard dose. The administered dose of Gd-EOB-DTPA (25 μmol kg−1) was five times higher than that of Mn-DPDP (5 μmol kg−1).
A major limitation of the present study is the small number of volunteers. In addition, depiction of the biliary tree on contrast-enhanced MRC may show different results according to liver function, regardless of the administered contrast agents; however, only healthy volunteers with normally functioning livers were included in this study. We obtained serial images using both contrast agents, but no images were obtained more than 2 h after contrast administration. However, it has been reported that the optimal window for evaluating liver parenchyma and bile duct after injection is 15–20 min for Mn-DPDP contrast-enhanced MRC [4,5,22] and 20–30 min for Gd-EOB-DTPA contrast-enhanced MRC [12,23-25]. We did not perform a qualitative analysis, and we did not evaluate whether differences in the SNRs and CNRs between the two contrast agents would affect clinical practice.
In conclusion, the biliary enhancement dynamics to liver parenchyma of Gd-EOB-DTPA differed from that of Mn-DPDP in healthy volunteers. CNR of Gd-EOB-DTPA showed an initial decline and then a steep increase to a peak at 15 min. However, CNR of Mn-DPDP showed a steady increase to a peak at 15 min. At 15 min, the value of CNR was significantly higher for Gd-EOB-DTPA than for Mn-DPDP. Therefore, contrast-enhanced MRC using Gd-EOB-DTPA may provide more adequate images than that of Mn-DPDP at 15 min after contrast administration.
References
- 1.Irie H, Honda H, Tajima T, Kuroiwa T, Yoshimitsu K, Makisumi K, et al. Optimal MR cholangiopancreatographic sequence and its clinical application. Radiology 1998;206:379–87 [DOI] [PubMed] [Google Scholar]
- 2.Varghese JC, Liddell RP, Farrell MA, Murray FE, Osborne H, Lee MJ. The diagnostic accuracy of magnetic resonance cholangiopancreatography and ultrasound compared with direct cholangiography in the detection of choledocholithiasis. Clin Radiol 1999;54:604–14 [DOI] [PubMed] [Google Scholar]
- 3.Hintze RE, Adler A, Veltzke W, Abou-Rebyeh H, Hammerstingl R, Vogl T, et al. Clinical significance of magnetic resonance cholangiopancreatography (MRCP) compared to endoscopic retrograde cholangiopancreatography (ERCP). Endoscopy 1997;29:182–7 [DOI] [PubMed] [Google Scholar]
- 4.Mitchell DG, Alam F. Mangafodipir trisodium: effects on T2- and T1-weighted MR cholangiography. J Magn Reson Imaging 1999;9:366–8 [DOI] [PubMed] [Google Scholar]
- 5.Lee VS, Rofsky NM, Morgan GR, Teperman LW, Krinsky GA, Berman P, et al. Volumetric mangafodipir trisodium-enhanced cholangiography to define intrahepatic biliary anatomy. AJR Am J Roentgenol 2001;176:906–8 [DOI] [PubMed] [Google Scholar]
- 6.Carlos RC, Hussain HK, Song JH, Francis IR. Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid as an intrabiliary contrast agent: preliminary assessment. AJR Am J Roentgenol 2002;179:87–92 [DOI] [PubMed] [Google Scholar]
- 7.Laurent V, Corby S, Barbary C, Kermarrec E, Beot S, Regent D. New possibilities to study biliary tree and gallbladder: functional magnetic resonance cholangiography contrast-enhanced with mangafodipir trisodium (Mn DPDP). [In French.] J Radiol 2007;88:531–40 [DOI] [PubMed] [Google Scholar]
- 8.Fayad LM, Holland GA, Bergin D, Iqbal N, Parker L, Curcillo PG, 2nd, et al. Functional magnetic resonance cholangiography (fMRC) of the gallbladder and biliary tree with contrast-enhanced magnetic resonance cholangiography. J Magn Reson Imaging 2003;18:449–60 [DOI] [PubMed] [Google Scholar]
- 9.Lee VS, Krinsky GA, Nazzaro CA, Chang JS, Babb JS, Lin JC, et al. Defining intrahepatic biliary anatomy in living liver transplant donor candidates at mangafodipir trisodium-enhanced MR cholangiography versus conventional T2-weighted MR cholangiography. Radiology 2004;233:659–66 [DOI] [PubMed] [Google Scholar]
- 10.Kirchin MA, Pirovano GP, Spinazzi A. Gadobenate dimeglumine (Gd-BOPTA). An overview. Invest Radiol 1998;33:798–809 [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Gordon PB, Hustvedt SO, Grant D, Sterud AT, Martinsen I, et al. MR imaging properties and pharmacokinetics of MnDPDP in healthy volunteers. Acta Radiol 1997;38:665–76 [DOI] [PubMed] [Google Scholar]
- 12.Hamm B, Staks T, Muhler A, Bollow M, Taupitz M, Frenzel T, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology 1995;195:785–92 [DOI] [PubMed] [Google Scholar]
- 13.Schuhmann-Giampieri G, Mahler M, Roll G, Maibauer R, Schmitz S. Pharmacokinetics of the liver-specific contrast agent Gd-EOB-DTPA in relation to contrast-enhanced liver imaging in humans. J Clin Pharmacol 1997;37:587–96 [DOI] [PubMed] [Google Scholar]
- 14.Soto JA, Alvarez O, Munera F, Velez SM, Valencia J, Ramirez N. Diagnosing bile duct stones: comparison of unenhanced helical CT, oral contrast-enhanced CT cholangiography, and MR cholangiography. AJR Am J Roentgenol 2000;175:1127–34 [DOI] [PubMed] [Google Scholar]
- 15.Becker CD, Grossholz M, Becker M, Mentha G, de Peyer R, Terrier F. Choledocholithiasis and bile duct stenosis: diagnostic accuracy of MR cholangiopancreatography. Radiology 1997;205:523–30 [DOI] [PubMed] [Google Scholar]
- 16.Irie H, Honda H, Kuroiwa T, Yoshimitsu K, Aibe H, Shinozaki K, et al. Pitfalls in MR cholangiopancreatographic interpretation. Radiographics 2001;21:23–37 [DOI] [PubMed] [Google Scholar]
- 17.Gazelle GS, Lee MJ, Mueller PR. Cholangiographic segmental anatomy of the liver. Radiographics 1994;14:1005–13 [DOI] [PubMed] [Google Scholar]
- 18.Gallez B, Baudelet C, Adline J, Charbon V, Lambert DM. The uptake of Mn-DPDP by hepatocytes is not mediated by the facilitated transport of pyridoxine. Magn Reson Imaging 1996;14:1191–5 [DOI] [PubMed] [Google Scholar]
- 19.Weinmann HJ, Schuhmann-Giampieri G, Schmitt-Willich H, Vogler H, Frenzel T, Gries H. A new lipophilic gadolinium chelate as a tissue-specific contrast medium for MRI. Magn Reson Med 1991;22:233–7; discussion: 242 [DOI] [PubMed] [Google Scholar]
- 20.Schuhmann-Giampieri G, Schmitt-Willich H, Press WR, Negishi C, Weinmann HJ, Speck U. Preclinical evaluation of Gd-EOB-DTPA as a contrast agent in MR imaging of the hepatobiliary system. Radiology 1992;183:59–64 [DOI] [PubMed] [Google Scholar]
- 21.Elizondo G, Fretz CJ, Stark DD, Rocklage SM, Quay SC, Worah D, et al. Preclinical evaluation of MnDPDP: new paramagnetic hepatobiliary contrast agent for MR imaging. Radiology 1991;178:73–8 [DOI] [PubMed] [Google Scholar]
- 22.Kim KW, Park MS, Yu JS, Chung JP, Ryu YH, Lee SI, et al. Acute cholecystitis at T2-weighted and manganese-enhanced T1-weighted MR cholangiography: preliminary study. Radiology 2003;227:580–4 [DOI] [PubMed] [Google Scholar]
- 23.Carlos RC, Branam JD, Dong Q, Hussain HK, Francis IR. Biliary imaging with Gd-EOB-DTPA: is a 20-minute delay sufficient? Acad Radiol 2002;9:1322–5 [DOI] [PubMed] [Google Scholar]
- 24.Clement O, Muhler A, Vexler V, Berthezene Y, Brasch RC. Gadolinium-ethoxybenzyl-DTPA, a new liver-specific magnetic resonance contrast agent. Kinetic and enhancement patterns in normal and cholestatic rats. Invest Radiol 1992;27:612–19 [PubMed] [Google Scholar]
- 25.Takao H, Akai H, Tajima T, Kiryu S, Watanabe Y, Imamura H, et al. MR imaging of the biliary tract with Gd-EOB-DTPA: effect of liver function on signal intensity. Eur J Radiol 2011;77:325–9 [DOI] [PubMed] [Google Scholar]