Abstract
Objective
The objective of this study was to determine the incidence of typical and atypical enhancement patterns of hepatocellular carcinomas (HCCs) on multiphasic multidetector row CT (MDCT) and to correlate the enhancement patterns and morphological image findings of HCC with the degree of tumour differentiation.
Methods
MDCT images of 217 patients with 243 surgically proven HCCs were evaluated through consensus reading by two radiologists. Our MDCT protocol was composed of precontrast, arterial, portal and delayed phases. The reviewers analysed the CT images for degree of attenuation; relative timing of washout; presence of dysmorphic intratumoral vessels, aneurysms and necrosis; tumour size; tumour margin; presence of pseudocapsule; intratumoral heterogeneity; and determined enhancement pattern. The imaging features were correlated with tumour differentiation using Fisher's exact test or the χ2 test.
Results
Among 243 HCCs, 137 (56.4%) showed the typical enhancement pattern of HCC, which is arterial enhancement and washout on portal or equilibrium phase images. In the arterial phase, 190 of 243 (78.2%) HCCs showed hypervascularity, with approximately three quarters of poorly differentiated (PD) (34 of 45, 75.6%) and moderately differentiated (MD) HCCs (92 of 123, 74.8%) showing washout during the portal or delayed phases, vs only 50% of well-differentiated (WD) HCCs (11 of 22; p<0.048). The presence of intratumoral vessels and aneurysms, tumour necrosis, attenuation of precontrast, the relative timing of washout, intratumoral attenuation heterogeneity, tumour margin and tumour size were correlated with the pathological differentiation of HCCs (p<0.05).
Conclusion
A typical enhancement of HCCs on MDCT was not unusual (43.6%) and WD and PD HCCs account for most of the atypical enhancement patterns. Early washout favoured MD and PD HCCs rather than WD HCCs, whereas in our study the presence of intratumoral aneurysm was a highly specific finding for PD HCC.
Hepatocellular carcinoma (HCC), one of the leading causes of cancer-related death, is the fifth most common tumour worldwide, and its incidence continues to rise [1-5]. In the era of diagnosis of HCC, the importance of radiological enhancement pattern of the tumour has been emphasised [1,6-9]. According to a recent recommendation by the American Association for the Study of Liver Diseases (AASLD), a tumour with a diameter >1 cm in a cirrhotic liver with typical enhancement pattern on dynamic study (four-phase multidetector CT or contrast-enhanced MRI), can be diagnosed as HCC without invasive biopsy [8-10]. The tumour is defined as having hyperattenuation present in the arterial phase followed by hypoattenuation or hypointensity (washout) of the tumour in the portal venous and delayed phases. Several researchers investigated external validation of AASLD criteria, but sensitivity (33–81.8%) varied and the study group was limited to small HCCs (<3 cm diameter) [11,12].
Moreover, several studies have revealed that the histopathological grade of HCC is an important prognostic factor, with poorly differentiated (PD) HCCs especially showing an unfavourable outcome [13-15]. However, as histological confirmation of the tumour grade prior to surgical treatment is not always possible owing to the risks of bleeding and track seeding [16,17], non-invasive estimation of the cellular differentiation of HCC prior to treatment can prove of vital importance not only in predicting the outcome, but also in determining the treatment strategy to be employed.
In the past decade, several studies have attempted to assess the histological grade of HCC using various modalities including spiral CT [18-20], contrast-enhanced ultrasound [21], invasive CT during hepatic arteriography (CTHA) and CT during arterioportography (CTAP) [22,23], and have demonstrated that the enhancement pattern could be correlated with histological differentiation. However, until now, relatively little attention has been given to investigation of the differences of enhancement patterns and imaging findings on non-invasive multiphasic multidetector row CT (MDCT) regarding different histological grades of HCC in a relatively large number of surgically confirmed lesions without limitation of tumour size. Thus, the aims of our study are to determine the incidence of typical and atypical enhancement pattern HCCs with MDCT, and to retrospectively correlate the enhancement patterns and morphological image findings of HCC seen on MDCT with the degree of tumour differentiation.
Methods and materials
Patients
Our institutional review board approved this retrospective study and waived the requirement for informed consent. During the period from January 2001 to December 2008, the pathology database of our hospital revealed 917 patients with 972 lesions identified as HCC. Among these patients, we selected our study population using the following inclusion criteria: (a) a pathology diagnosis of HCC through surgery such as tumour resection or liver transplantation; (b) available preoperative MDCT with eight or more channels using standardised protocols of dynamic liver CT; (c) a mean time interval between the pathology diagnosis and CT scans of no more than 6 weeks; and (d) no history of previous adjuvant treatment such as transcatheter arterial chemoembolisation, percutaneous ethanol injection or radiofrequency ablation for each HCC. According to these criteria, 700 patients were excluded for one of the following reasons: the use of an MDCT with four channels or fewer (n=426); an insufficient dynamic phase scan or the lack of a liver CT protocol (n=102); a history of previous adjuvant therapy such as transcatheter arterial chemoembolisation, percutaneous ethanol injection or radiofrequency ablation (n=166); and the presence of other pathology such as cholangiocarcinoma (n=3) or undifferentiated HCC (n=3). Finally, 217 patients with 243 lesions were enrolled in this retrospective study (171 males and 46 females; age range, 28–85 years; mean age, 58.4 years).
Among these 217 patients, 198 had one HCC, 14 had 2 HCCs, 4 had 3 HCCs and 1 had 5 HCCs. The pathology diagnosis of HCC was obtained in 204 patients through liver resection and in 13 patients through liver transplantation. Explanted livers were evaluated at 5 mm slice section intervals in the sagittal plane. Lesion diameter ranged from 1.0 to 18.0 cm (mean±standard deviation, 4.5±3.2 cm). The diagnosis was made according to the International Working Party criteria [24]. Histological differentiation of HCCs as well differentiated (WD), moderately differentiated (MD) or PD was evaluated by two clinically experienced liver pathologists based primarily on Edmondson and Steiner's grading [25]. The Child–Pugh score was recorded in all patients and was obtained within 0–180 days of each CT scan (mean delay=25.7 days). The detailed demographic characteristics of our study are summarised in Table 1.
Table 1. Demographic characteristics of the study population.
| Variables | Patients with surgically confirmed HCCs (n=217) |
| Age (mean±SD, years) | 58.4±9.7 (range 28–85) |
| Sex (M:F) | 171 (78%): 46 (22%) |
| Underlying cause of liver disease | |
| HBV infection | 178 (82%) |
| HCV infection | 11 (5%) |
| HBV and HCV coinfection | 1 (1%) |
| Alcoholism | 9 (4%) |
| Unknown | 17 (8%) |
| Normal | 1 (1%) |
| Child–Pugh score | |
| A | 137 (63%) |
| B | 75 (35%) |
| C | 5 (2%) |
| Type of pathological confirmation | |
| Liver resection | 204 (94%) |
| Transplantation | 13 (6%) |
| Cellular differentiation | |
| Well differentiated | 31 (13%) |
| Moderately differentiated | 147 (61%) |
| Poorly differentiated | 65 (27%) |
F, female; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; M, male.
Acquisition of CT images
214 patients (98.6%) underwent quadruple-phase MDCT including the unenhanced phase, arterial phase, portal venous phase and equilibrium phase. 3 patients (1.4%) underwent a liver protocol, triple-phase MDCT without unenhanced phase. As the data were obtained during the midst of the rapid development of MDCT technology, various MDCT scanners were used, i.e. 24 patients on an 8-MDCT scanner (LightSpeedTM Ultra; GE Medical Systems, Milwaukee, WI), 114 patients on a 16-MDCT scanner (LightSpeed 16; GE Medical Systems, n=3; Sensation 16; Siemens Medical Systems, Forchheim, Germany, n=105; or Brilliance 16; Philips Medical Systems, Eindhoven, Netherlands, n=6), and 79 patients on a 64-MDCT scanner (Sensation 64; Siemens Medical systems, n=3; Definition; Siemens Medical Systems, n=6; Brilliance 64; Philips Medical Systems, n=70). CT scans were obtained in the craniocaudal direction. The following CT parameters were used: detector configuration, 8×1.25, 16×1.5 and 64×0.625 mm; slice thickness, 2.5, 3.0 and 3.0 mm; reconstruction interval, 2.5, 3.0 and 3.0 mm; table speed, 13.5, 24.0 and 46.9 mm per rotation; 250, 200 and 175 mA effective current; rotation time, 0.5, 0.5 and 0.75 s; tube potential 120 kVp; and matrix size, 512×512.
CT images were obtained after an injection of 120 ml of iopromide (Ultravist® 370; Schering Korea, Seoul, Republic of Korea) at a rate of 3.0–4.0 ml s−1 using an automatic power injector (Envision CT; Medrad, Pittsburgh, PA). Arterial phase imaging was performed 15–19 s (15 s for 8-MDCT scanners, 17 s for 16-MDCT scanners and 19 s for 64-MDCT scanners) after achievement of 100 HU attenuation of the descending aorta measured using a bolus tracking method. A 30 to 33 s delay (30 s for 8-MDCT scanners, 33 s for 64-MDCT scanners) after the arterial phase was obtained for portal venous phase acquisition. The delay time was 180 s for equilibrium phase imaging following administration of a contrast medium.
CT image analysis
Three radiologists, i.e. two attending physicians (JML and SJK with 18 and 10 years of clinical experience, respectively) and a clinical fellow (JHB with 6 years of clinical experience), retrospectively reviewed the CT findings in consensus. They were blinded to the pathology results, and analysis was performed using the stack mode of a picture archiving and communications workstation. One of the authors not involved in the image analysis (JHL) reviewed the patients' medical records including the clinical features, laboratory findings and the results of the pathology findings. The pathology result and image analysis data were carefully compared using the information of tumour location and size from both reports, especially in patients who had more than one lesion, to avoid erroneous correlation. The scans were assessed using both soft-tissue (width, 400 HU; level, 70–100 HU) and narrow (width, 150 HU; level, 60–90 HU) window settings.
Enhancement pattern
To evaluate the characteristic enhancement pattern of various histological grades of HCC, the HCC attenuation in each phase was classified as hyperattenuation, isoattenuation or hypoattenuation compared with the surrounding liver parenchyma. On portal venous phase and equilibrium phase observation, each lesion was evaluated for the presence of washout, which was defined as when any part of the lesion showing hyperattenuation on arterial phase images becomes hypoattenuated relative to the adjacent liver parenchyma on portal- or equilibrium-phase imaging. A hypoattenuated area with no change in the degree of attenuation during the dynamic phase was defined as necrosis. If it was difficult to determine the degree of lesion attenuation through visual inspection of each image phase, quantitative analysis of the HCC was performed as a reference. Using the regions of interest (ROIs), tumour to liver attenuation was evaluated. Attenuation values were obtained in a 0.3 to 2.2 cm2 circular ROIs. To assess tumour attenuation, ROI cursors were carefully placed so as to contain as much of the most enhancing portions of the tumours as possible in order to avoid intralesional necrosis or adjacent liver parenchyma [19]. Measurement of liver parenchyma attenuation excluded visible portal or hepatic vessels, bile ducts, suspected calcifications and artefacts. At least three measurements were performed for each lesion and liver parenchyma, and the results were averaged. Lesions with heterogeneous enhancement were regarded as hyperattenuation when most of the lesion was enhanced during the arterial phase compared with the precontrast phase. The enhancement patterns of HCC were classified as typical enhancement, i.e. hyperattenuation on the arterial phase followed by washout in the portal or equilibrium phase, atypical enhancement including all of the other enhancement patterns such as arterial non-hyperattenuation (iso- or hypoattenuation) or no visible washout during the portal venous or equilibrium phases.
Morphological analysis of the imaging findings
In order to determine the most useful CT findings for differentiating the histological grade of HCCs, the following CT findings were analysed: (a) the presence of dysmorphic intratumoral vessels; (b) aneurysmal dilatation of dysmorphic intratumoral vessels; (c) the presence of intratumoral necrosis; (d) tumour size; (e) lesion attenuation on the precontrast phase; (f) tumour margin; (g) the presence of capsular enhancement; and (h) intratumoral heterogeneity of enhancement. Dysmorphic intratumoral vessels were defined as tortuous enhancing vasculature in a lesion, while being more enlarged or numerous than expected for the particular region of the liver. In addition, if there was focal dilatation of the intratumoral vessel diameter, such as an aneurysm, it was regarded as an intratumoral aneurysm. Pre-contrast tumour attenuation was assessed in the same manner as used in the enhancement pattern described above. The shape of the tumour margin was qualitatively evaluated and classified as having a smooth or irregular margin according to the CT findings for each lesion. In addition, the irregular margin group was categorised into two subgroups: (a) lobulated or (b) infiltrative. A thin (1–3 mm), hypoattenuated rim around a nodular HCC seen on the arterial phase and a hyperattenuated rim on delayed-phase images were considered to be capsules [26]. Intratumoral heterogeneity of enhancement was determined to be present when the attenuation of the enhancing portion of the lesion was heterogeneous.
Statistical analysis
To evaluate the differences in demographic data, the χ2 test was used. To determine the statistically significant CT features in order to differentiate the various cellular grades of HCC, statistical analysis was performed using the χ2 test and Fisher's exact test for categorical variables and the Student's t-test and analysis of variance for continuous variables. For the analysis, an overall test of the differences among the three groups followed by three pair-wise comparisons (WD to MD HCCs, MD to PD HCCs, WD to PD HCCs) was performed. The sensitivity and specificity of each significant CT criterion and a combination of the significant CT criteria to differentiate WD or PD HCCs from other histological groups among the typical enhancement groups of HCCs, i.e. those highly suspicious in the clinical setting of being HCC, were also calculated. In addition, the clinical and pathology findings were compared between each differentiation group of HCCs. The numbers used in this statistical analysis were the numbers of lesions. All statistical analyses were performed using commercial software (SPSS® v. 17.0; SPSS Inc., Chicago, IL and MedCalc for Windows, v. 8.0.0.1; Medcalc Software, Mariakerke, Belgium). A p-value <0.05 was considered to indicate statistical significance.
Results
Enhancement pattern
The overall enhancement patterns of 243 HCCs during both the arterial and portal venous phases or during the delayed phase are shown in Table 2. Among 243 HCCs, 137 (56.4%) showed the typical enhancement pattern of HCC described in the AASLD criteria [8]. The prevalence of HCCs showing the typical enhancement pattern differed significantly between groups based on pathology grading (p<0.016), with typical enhancement associated with MD and PD HCCs more than that associated with WD HCCs (MD or PD vs WD; p<0.020). Statistical difference was also observed between MD and WD HCCs (p=0.010), but not between MD and PD HCCs (p=0.210). When it comes to the underlying cause of cirrhosis-based analysis, the distribution of the histological grade of HCCs (p=0.701) and the prevalence of the typical enhancement pattern (p=0.612) showed no statistical differences.
Table 2. Distribution of typical and atypical enhancement of 243 hepatocellular carcinomas (HCCs) according to tumour differentiation.
| Category | WD HCC | MD HCC | PD HCC | p-valuea | |
| Typical enhancement group (n=137) | 11 (35%) | 92 (63%) | 34 (52%) | <0.016 | |
| Atypical enhancement group (n=106) | Iso- or hypoattenuation on arterial phase | 9 (29%) | 24 (16%) | 20 (31%) | |
| No washout until delayed phase | 11 (35%) | 31 (21%) | 11 (17%) | ||
| Total | 31 (100%) | 147 (100%) | 65 (100%) | ||
MD, moderately differentiated; PD, poorly differentiated; WD, well differentiated.
aAmong each differentiation group by typical vs atypical enhancement, calculated by the χ2 test.
Enhancement pattern during the arterial phase
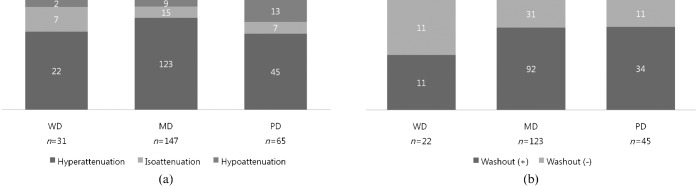
In the arterial phase, 190 (78.2%) of 243 HCCs showed hypervascularity (Figure 1a). Although the predominant enhancement pattern of all three groups of HCCs was arterial hyperattenuation, MD HCCs showed the highest proportion of typical hyperenhancement during the arterial phase (Figure 2). Atypical enhancement of HCCs on MDCT was not unusual (43.6%) and WD and PD HCCs account for most of the atypical enhancement patterns (Figures 4 and 5). Early washout favoured MD and PD HCCs rather than WD HCCs, whereas in our study the presence of intratumoral aneurysm was a highly specific finding for PD HCC. As the tumour grade progressed, the proportion of hyperenhancement increased from WD to MD HCCs, but decreased in the late stage of HCCs from MD to PD. Also, a significant difference in the proportion of hyperenhancement was noted between MD and PD HCCs (p<0.027). However, PD HCCs showed the highest proportion of hypoattenuation, which was significantly higher than MD HCCs (p<0.014) but not WD HCCs (p=0.223) (Figure 6).
Figure 1.
Chart showing the enhancement patterns of 243 hepatocellular carcinomas (HCCs) during the hepatic arterial phase (a) and washout patterns of 190 arterially hyperenhancing HCCs during portal venous or delayed phases (b). Although all three groups are predominantly hyperattenuated on arterial phase, hypoattenuation was more frequently seen in poorly differentiated (PD) HCCs than in the other two groups (PD vs moderately differentiated (MD) or well differentiated (WD); p<0.009) (a). The proportion of tumour washout is higher in MD or PD HCCs (p<0.027) than in WD HCCs (b). However, there is no significant difference between MD and PD HCCs (p=0.919).
Figure 2.
Chart shows the relative timing of washout of 190 hepatocellular carcinomas (HCCs). Results show a shift towards earlier washout concurrently with progression towards advanced HCC. d, delayed phase; MD, moderately differentiated; p, portal venous phase; PD, poorly differentiated; WD, well differentiated.
Figure 4.
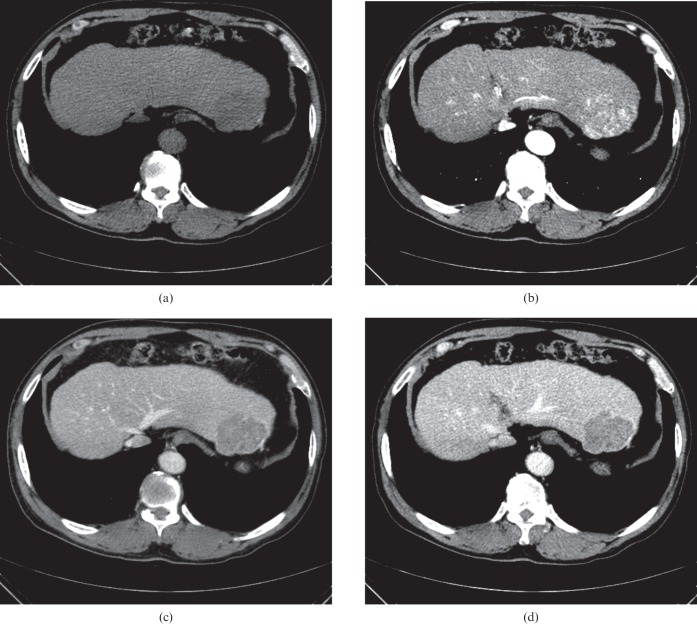
A 1.9-cm hepatocellular carcinoma (HCC) nodule in a 59-year-old woman with chronic hepatitis B. (a) Transverse precontrast, (b) late arterial, (c) portal venous and (d) equilibrium phase CT scans show subtle high attenuation (arrow) in segment V of the liver. The lesion could not be seen on the precontrast phase (a) and had no visible washout until the delayed phases (c, d). Pathology review of the resected liver revealed a well-differentiated HCC.
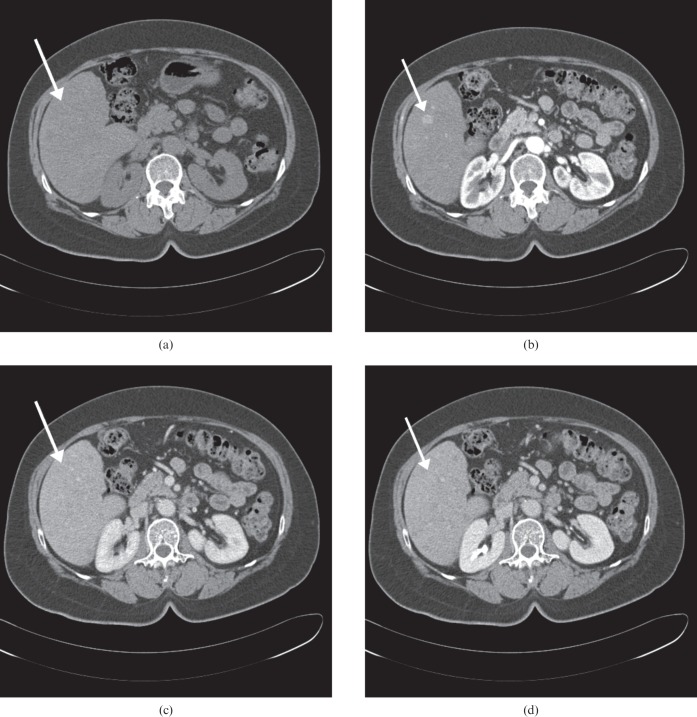
Figure 5.
Images of a 4 cm hepatocellular carcinoma (HCC) mass in a 62-year-old woman with hepatitis B-related cirrhosis. Axial CT scans obtained during the (a) precontrast, (b) late arterial, (c) portal venous, and (d) equilibrium phases show a bulging contour mass in segment III of the liver. The tumour shows overt low attenuation in the precontrast phase, isoattenuation in the arterial phase, and relatively early washout during the portal venous phase. Pathology review of the mass lesion revealed a poorly differentiated HCC.
Washout pattern during the portal venous and delayed phases
Among 190 hypervascular HCCs, 137 tumours (72.1%) showed washout during either portal venous or delayed phase (Figure 1b). There was a significant difference in the presence of washout in the cellular differentiation categories (p<0.048): only 50% of WD HCCs showed the typical washout pattern in the portal venous or delayed phases (11 of 22), while approximately three-quarters of PD (75.5%) and MD (74.8%) HCCs showed washout.
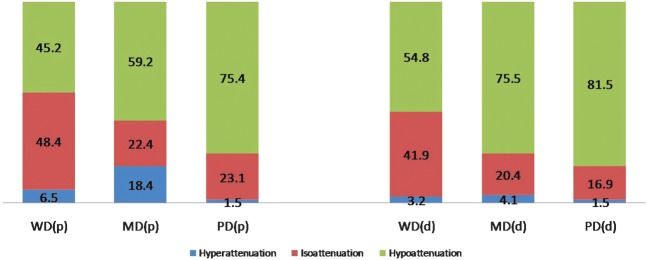
In addition, when it comes to the relative timing of washout, among 190 hypervascular tumours (Figure 3), as histological differentiation moved towards the more advanced categories from WD through MD to PD HCCs, there was a tendency for the relative timing of washout to shift towards “early” washout in the portal venous phase (45.2%, 59.2% and 75.4%, respectively). Significant differences were observed between PD and WD HCCs (p<0.007) and PD and MD HCCs (p<0.034), but there was no statistical difference between WD and MD HCCs (p=0.217). Figure 3 shows the relative timing of washout in 190 hypervascular HCCs.
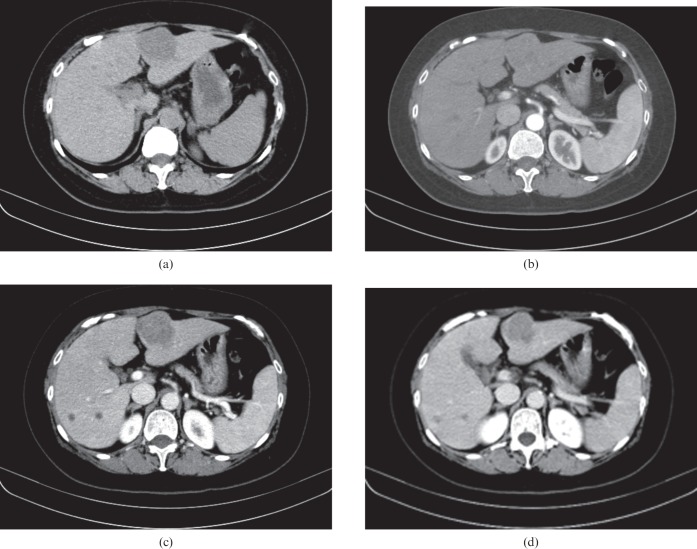
Figure 3.
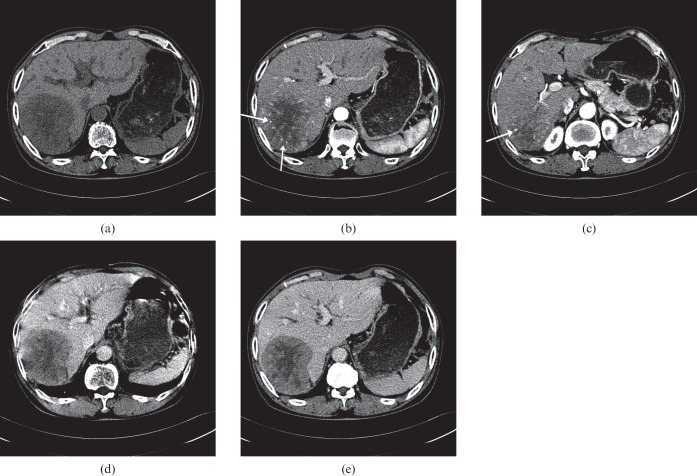
A 5.6 cm wide hepatocellular carcinoma (HCC) mass in a 69-year-old man with chronic hepatitis B. (a) Axial precontrast, (b) late arterial, (c) portal venous and (d) equilibrium phase CT scans show an overt arterial enhancement lesion in segment II of the liver. The lesion shows hypoattenuation in the precontrast phase and relatively early washout during the portal venous phase. Note the intratumoral heterogeneous enhancement (b) and multiple, high-density, dot-like attenuation [(arrows in (b)] in the tumour indicating intratumoral vessels. Pathology review of the resected liver revealed a moderately differentiated HCC.
Figure 6.
Images of a 9 cm hepatocellular carcinoma (HCC) mass in a 76-year-old man with hepatitis C-related cirrhosis. Transverse CT scans obtained during the (a) precontrast, (b, c) late arterial, (d) portal venous, and (e) equilibrium phases demonstrate a hypervascular HCC in the right posterior segment of the liver. The lesion shows prominent hypoattenuation in the precontrast phase with a central necrotic portion and relatively early washout during the portal venous phase. Note the intratumoral heterogeneous enhancement (b, c) and disproportionally enlarged vessels (arrows in b and c) in the tumor, thus indicating focal aneurysmal dilatation. Pathology review of the explanted liver revealed a poorly differentiated HCC.
Morphological characteristics
The morphological CT image findings and the histopathological characteristics of the 243 HCCs are summarised in Table 3. The presence of intratumoral vessels showed a significant difference between advanced HCCs and WD HCCs (p<0.001), i.e. PD and MD HCCs had more intratumoral vessels than WD HCCs. In addition, aneurysmal dilatation of intratumoral vessels was observed more often in PD HCCs than in MD HCCs. None of the WD HCCs showed intratumoral aneurysms (Figure 6).
Table 3. Summary of morphological CT findings in 243 hepatocellular carcinomas (HCCs).
| CT findings | WD HCC | MD HCC | PD HCC | p-valuea | |
| Intratumoral vessel | Absent | 22 (71%) | 53 (36%) | 23 (35%) | <0.001 |
| Present | 9 (29%) | 94 (64%) | 42 (65%) | ||
| Intratumoral aneurysm | Absent | 31 (100%) | 146 (99%) | 52 (80%) | <0.001 |
| Present | 0 (0%) | 1 (1%) | 13 (20%) | ||
| Tumour necrosis | Absent | 29 (93%) | 106 (72%) | 46 (71%) | 0.032 |
| Present | 2 (7%) | 41 (28%) | 19 (29%) | ||
| Intratumoral heterogeneity | Absent | 19 (61%) | 36 (24%) | 16 (24%) | <0.001 |
| Present | 12 (38%) | 111 (75%) | 49 (75%) | ||
| Tumour margin | Smooth | 12 (39%) | 66 (44%) | 17 (26%) | 0.036 |
| Irregular | |||||
| Lobulated | 7 (22%) | 44 (29%) | 23 (35%) | ||
| Infiltrative | 12 (38%) | 37 (25%) | 25 (38%) | ||
| Attenuation on precontrastb | Hypoattenuation | 11 (35%) | 100 (70%) | 47 (75%) | <0.001 |
| Isoattenuation | 18 (58%) | 40 (28%) | 15 (24%) | ||
| Hyperattenuation | 2 (6%) | 1 (1%) | 0 (0%) | ||
| Capsular enhancement | Absent | 25 (80%) | 95 (64%) | 48 (73%) | 0.135 |
| Present | 6 (19%) | 52 (35%) | 17 (26%) | ||
| Tumour size (cm) | 0–0.9 | 1 (3%) | 0 (0%) | 0 (0%) | 0.020 |
| 1–1.9 | 14 (45%) | 20 (14%) | 9 (14%) | ||
| ≥2.0 | 16 (52%) | 127 (86%) | 56 (86%) | ||
| Mean diameter (cm) | 3.02±2.88 | 4.69±3.22 | 4.92±3.52 | <0.02c | |
MD, moderately differentiated; PD, poorly differentiated; WD, well differentiated.
Percentages may not add up to 100% because of rounding.
aAmong the three differentiation groups of HCCs; calculated with the χ2 test.
bNine lesions (three patients) have no precontrast scan. All of the nine cases were non-WD groups (MD or PD HCCs).
cCalculated with the analysis of variance test.
With regards to intratumoral necrosis, PD and MD HCCs tended to contain more necrosis than WD HCCs (MD or PD vs WD; p<0.007). The mean diameters of HCCs, according to the degree of differentiation, were also significantly different among these three groups. PD (mean diameter 4.92±3.52 cm) and MD HCCs (mean diameter 4.69±3.22 cm) were significantly larger than WD HCCs (mean diameter 3.02±2.88 cm; MD vs WD, p<0.027; PD vs WD, p<0.022). There was, however, no significant difference between MD and PD HCCs (p<0.886). In terms of the categorical basis with a cut-off value of 2 cm, PD and MD HCCs were again significantly larger than WD HCCs (WD vs MD or PD; p<0.00001) [27].
Tumour attenuation during the precontrast phase also differed significantly between these three groups (p<0.0001). More than two-thirds of PD and MD HCCs showed hypoattenuation, while approximately one-third of the WD HCCs were hypoattenuated during the precontrast phase (PD vs WD, p<0.0004; MD vs WD, p<0.0004). In addition, the incidence of intratumoral heterogeneity was different among the three groups (p<0.0001): three-quarters of PD and MD HCCs showed intratumoral heterogeneity, while it was seen in only 38% (n=12) of WD HCCs (PD vs WD, p<0.001; MD vs WD, p<0.001). However, the presence of capsular enhancement near the tumour or tumour margin was not a significant factor for differentiating each histological grade of HCC (p=0.135).
Sensitivity and specificity values for the CT diagnosis of AASLD criteria
MDCT was able to make a correct diagnosis in 137 (56.4%) of 243 HCCs with typical enhancement pattern described in the AASLD criteria [8]. In addition, in an attempt to find possible CT findings which may be helpful in differentiating the histological grade of HCCs, each CT finding was assessed in the 137 lesions (11 WD, 92 MD and 34 PD HCCs) with the typical enhancement pattern [6]. There were no significant CT variables regarding enhancement pattern able to differentiate WD from MD or PD in the typical enhancement groups of HCCs. There were, however, three morphological CT findings that helped to differentiate PD from MD or WD HCCs; these included the presence of intratumoral aneurysm (p<0.001), the absence of capsular enhancement (p<0.010) and an irregular (lobulated or infiltrative) tumour margin (p<0.001). The sensitivity and specificity of each of these significant CT variables and their combination for differentiating PD from WD or MD HCCs are shown in Table 4a and Table 4b, respectively.
Table 4a. Sensitivity and specificity of CT findings in diagnosing poorly differentiated (PD) hepatocellular carcinomas (HCCs) from 137 tumours with the typical enhancement pattern.
| CT findings suggesting PD HCC | Sensitivity (%) | Specificity (%) |
| Presence of intratumoral aneurysm | 17.6 (6/34) | 99.0 (102/103) |
| Absence of capsular enhancement | 82.4 (28/34) | 41.8 (42/103) |
| Irregular tumour margin | 82.4 (28/34) | 47.6 (49/103) |
Data in parentheses are the numbers of lesions.
Table 4b. Sensitivity and specificity of combined CT findings in diagnosing poorly differentiated (PD) hepatocellular carcinomas (HCCs) in 137 HCCs with the typical enhancement pattern.
| Number of CT findings | PD HCC (n=34) | WD or MD HCC (n=103) | Sensitivity/specificity (%) |
| 1 | 32 (94.1%) | 71 (68.9%) | 94.1/31.1 |
| 2 | 27 (79.4%) | 45 (43.7%) | 79.4/56.3 |
| 3 | 3 (8.8%) | 0 (0%) | 8.8/100.0 |
MD, moderately differentiated; WD, well differentiated.
Data are numbers of patients with one or more of the following lesion findings at CT: presence of intratumoral aneurysm; absence of capsular enhancement; and irregular tumour margin.
Discussion
Our study provides a relatively large series of surgically proven HCCs as seen on multiphasic MDCT together with a correlation of the enhancement patterns of HCC with the degree of tumour differentiation. The validity of a recent recommendation by the AASLD that tumours >1 cm in diameter with typical enhancement patterns (hypervascular in the arterial phase with washout in the portal venous or delayed phases) on dynamic CT or MRI should be diagnosed as HCC without biopsy has rarely been studied [8]. In our study, only 56.4% of HCCs showed the typical enhancement pattern of HCC. Moreover, although 62.6% of MD HCCs showed classic enhancement features, it was seen in only 35.4% of WD and 52.3% of PD HCCs. In other words, when the non-invasive imaging diagnostic criteria for HCC proposed by the AASLD were applied to the HCCs in our study, approximately half of all HCCs and two-thirds of WD HCCs could not be diagnosed with MDCT [20].
The high incidence of atypical enhancement pattern in WD or PD HCCs observed in our study is a similar characteristic to that seen in previous studies using CT [19,28], ultrasonography [29,30] and MRI [16]. More specifically, WD HCCs in our series showed the largest percentage of atypical enhancement pattern. This may be explained by the haemodynamic changes which occur during the multistep hepatocarcinogenesis process [27,31-33]. In the initial phase of hepatocarcinogenesis, the normal vascular supply including the portal vein and hepatic artery decreases while an abnormal neoplastic artery caused by tumour angiogenesis develops [33]. Thus, several atypical enhancement patterns may appear, such as hypo- or isoattenuation in the arterial phase and iso- or hyperattenuation in the portal venous or delayed phases during this transition. In addition, there have been a few prior studies investigating the vascular changes of the late stage of hepatocarcinogenesis. A recent study by Asayama et al [22] analysed the enhancement pattern of 60 HCCs on CTHA and on CTAP, and demonstrated that arterial blood flow decreased in the late stage of HCC development from MD to PD HCCs, whereas it increased in the early stage from WD to MD HCCs. Their results using CTHA and CTAP show some similarity with our results, but were relatively limited by the invasive nature of the imaging technique used. In our study, the percentage of arterial hyperattenuation increased from WD to MD HCCs, whereas it decreased from MD to PD HCCs, with a statistical difference between MD and PD HCCs (p<0.027). Interestingly, PD HCCs had the largest percentage of hypoattenuation in the arterial phase among the three differentiation groups. The reason for this phenomenon is still not clear, but these findings might support the hypothesis that the arterial blood supply of an HCC increases in the early stage of advancement but, conversely, decreases in the late stage of advancement, as suggested by Asayama et al [22].
In addition, we found that as histological differentiation moved towards more advanced categories from WD through MD to PD HCCs, there was a tendency for the relative timing of washout to shift towards early washout in the portal venous phase (45.2%, 59.2% and 75.4%, respectively) with significant differences between PD and WD HCCs and PD and MD HCCs (Figure 3). Another study describing 112 HCCs using contrast-enhanced ultrasonography observed a similar finding in that a tendency towards early washout was correlated with poor differentiation, which is in agreement with our study [21]. In our study, as the pathological tumour grade worsened, the percentage of hypoattenuation in the portal phase (early washout) showed a stepwise increase among 190 hypervascular tumours (Figure 3) with statistical significance among all three pathological groups, i.e. more early washout as the tumour grade progressed. This tendency towards an increase in washout was also observed in the delayed phase. Interestingly, owing to a significant increase in the proportion of washout between the portal and delayed phases of MD HCCs, the prominent washout discrepancy was demonstrated between WD HCCs and MD or PD HCCs in the delayed phase, which might suggest the added value of delayed-phase imaging not only for detection, but also for the characterisation of HCCs [7,34].
Our study results demonstrated that six morphological CT findings as well as tumour size differed significantly in each histological grade group. Those morphological CT features include the presence of intratumoral vessels, the presence of an intratumoral aneurysm, tumour necrosis, attenuation on precontrast imaging, tumour margin and intratumoral heterogeneity. The presence of an intratumoral vessel was most often observed in more advanced HCCs, including PD (65%) and MD HCCs (64%). In addition, 13 of 14 HCCs with aneurysmal dilatation of the internal vessels were PD HCCs. A possible explanation for the higher incidence of intratumoral vessels and aneurysmal dilation of the internal vessels in advanced HCCs could be that those vessels may represent a distortion of the vascular architecture rather than an increased number of abnormal, unpaired arteries, and might correlate with the degree of tumour differentiation. A previous study also reported that a presence of abnormal internal vessels within the tumour indicated an HCC with a positive predictive value of 90% and a specificity of 98% [35].
In addition, only 2 of 31 cases of WD HCCs showed tumour necrosis on CT, whereas more than one-quarter of the advanced HCCs (MD and PD) had an intratumoral necrotic portion, with a statistically significant difference. This finding is consistent with the well-known fact that, microscopically, WD tumour cells resemble hepatocytes and form trabeculae, cords and nests, whereas in PD HCCs, there are discohesive, pleomorphic, anaplastic and giant cells which have scant stroma and central necrosis caused by poor vascularisation [25,36]. Furthermore, in our series, more than two-thirds of PD and MD HCCs were hypoattenuating in the unenhanced phase, whereas only 11 of 31 WD HCCs were hypoattenuating in the precontrast phase, but with considerable overlap. Although several investigators have reported the usefulness of unenhanced phase images, especially in the detection of HCCs [7,37], to our knowledge there are no detailed reports that discuss the role of the precontrast phase in the characterisation of HCCs. The reason that advanced HCCs show hypoattenuation in the precontrast phase is unclear, although presumably it is because advanced HCCs tend to be large, have heterogeneous organisation and contain haemorrhage, necrosis and fibrous or cystic degeneration compared with WD HCCs.
In our study, most of the advanced HCCs (86%) were >2 cm in diameter, whereas approximately half (48%) of the WD HCCs were <2 cm in diameter. Moreover, approximately 92% (183 of 199) of HCCs >2 cm in diameter were MD (n=127) or PD (n=56) HCCs. More than half (58%) of the small HCCs (<2 cm) showed an atypical enhancement pattern, which agrees with the results of a previous study [19]. Meanwhile, large HCCs (≥2 cm) tended to have a typical enhancement pattern but with considerable overlap, i.e. large HCCs had an atypical enhancement pattern (80 of 199, 40%) and small HCCs had a typical enhancement pattern (18 of 44, 41%).
There are some limitations to our study. First, it is limited by its retrospective nature and by our limited control regarding patient selection. However, our study provides a relatively large series of pathologically and surgically proven HCCs seen on contrast-enhanced multiphasic MDCT, which can prevent any potential sampling error of percutaneous biopsy. Second, we did not analyse false-positive or false-negative diagnoses of MDCT. However, our major goal was to correlate the enhancement patterns and imaging features with the cellular differentiation of HCCs. Third, we used various types of multidetector helical CT scanners and different CT variables, although appropriate arterial timing for the accurate analysis of tumour attenuation was feasible in each CT scan. Fourth, there were relatively few (2%, 5 of 243) Child–Pugh CHCCs, possibly because our study population was based on patients who had undergone surgery. Finally, in our study, even though the predominant HCC enhancement patterns and several morphological CT findings differed significantly among different histological grades of HCC, considerable overlap was also noted. However, our results demonstrate some important features which can be helpful for suggesting tumour differentiation, including the presence of intratumoral aneurysm, the presence of tumour necrosis and intratumoral vessels indicating MD or PD HCC.
In conclusion, in our study the predominant enhancement patterns of HCCs differed according to the cellular differentiation, and WD and PD HCCs accounted for most of the atypical enhancement patterns. Early washout favoured MD and PD HCCs rather than WD HCCs, whereas the presence of intratumoral aneurysm was a highly specific finding for PD HCC. Therefore, awareness of the characteristic enhancement patterns and imaging findings of HCC may help in obtaining the correct diagnosis and in determining the optimal treatment strategy.
Acknowledgments
We thank Bonnie Hami (USA) and Chris Woo for their editorial assistance.
Footnotes
This work was supported by a National Research Foundation of Korea Grant founded by the Korean Government (2010-0023435).
References
- 1.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421–30 [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745–50 [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907–17 [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94:153–6 [DOI] [PubMed] [Google Scholar]
- 5.Velazquez RF, Rodriguez M, Navascues CA, Linares A, Perez R, Sotorrios NG, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology 2003;37:520–7 [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36 [DOI] [PubMed] [Google Scholar]
- 7.Iannaccone R, Laghi A, Catalano C, Rossi P, Mangiapane F, Murakami T, et al. Hepatocellular carcinoma: role of unenhanced and delayed phase multi-detector row helical CT in patients with cirrhosis. Radiology 2005;234:460–7 [DOI] [PubMed] [Google Scholar]
- 8.Sherman M. The radiological diagnosis of hepatocellular carcinoma. Am J Gastroenterol 2010;105:610–12 [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrero JA, Hussain HK, Nghiem HV, Umar R, Fontana RJ, Lok AS. Improving the prediction of hepatocellular carcinoma in cirrhotic patients with an arterially enhancing liver mass. Liver Transpl 2005;11:281–9 [DOI] [PubMed] [Google Scholar]
- 11.Forner A, Vilana R, Ayuso C, Bianchi L, Sole M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97–104 [DOI] [PubMed] [Google Scholar]
- 12.Leoni S, Piscaglia F, Golfieri R, Camaggi V, Vidili G, Pini P, et al. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am J Gastroenterol 2010;105:599–609 [DOI] [PubMed] [Google Scholar]
- 13.Jonas S, Bechstein WO, Steinmuller T, Herrmann M, Radke C, Berg T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001;33:1080–6 [DOI] [PubMed] [Google Scholar]
- 14.Nakajima Y, Shimamura T, Kamiyama T, Kimura J, Sato N, Matsushita M, et al. Evaluation of surgical resection for small hepatocellular carcinomas. Am J Surg 1996;171:360–3 [DOI] [PubMed] [Google Scholar]
- 15.Oishi K, Itamoto T, Amano H, Fukuda S, Ohdan H, Tashiro H, et al. Clinicopathologic features of poorly differentiated hepatocellular carcinoma. J Surg Oncol 2007;95:311–16 [DOI] [PubMed] [Google Scholar]
- 16.Park HS, Lee JM, Kim SH, Chang S, Kim SJ, Han JK, et al. Differentiation of well-differentiated hepatocellular carcinomas from other hepatocellular nodules in cirrhotic liver: Value of SPIO-enhanced MR imaging at 3.0 Tesla. J Magn Reson Imaging 2009;29:328–35 [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto K, Moriyasu F, Kamiyama N, Yamada M, Iijima H. Correlation between parametric imaging using contrast ultrasound and the histological differentiation of hepatocellular carcinoma. Hepatol Res 2008;38:273–80 [DOI] [PubMed] [Google Scholar]
- 18.Amano S, Ebara M, Yajima T, Fukuda H, Yoshikawa M, Sugiura N, et al. Assessment of cancer cell differentiation in small hepatocellular carcinoma by computed tomography and magnetic resonance imaging. J Gastroenterol Hepatol 2003;18:273–9 [DOI] [PubMed] [Google Scholar]
- 19.Yoon SH, Lee JM, So YH, Hong SH, Kim SJ, Han JK, et al. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. AJR Am J Roentgenol 2009;193:W482–9 [DOI] [PubMed] [Google Scholar]
- 20.Woodall CE, Scoggins CR, Loehle J, Ravindra KV, McMasters KM, Martin RC. Hepatic imaging characteristics predict overall survival in hepatocellular carcinoma. Ann Surg Oncol 2007;14:2824–30 [DOI] [PubMed] [Google Scholar]
- 21.Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology 2007;244:898–906 [DOI] [PubMed] [Google Scholar]
- 22.Asayama Y, Yoshimitsu K, Nishihara Y, Irie H, Aishima S, Taketomi A, et al. Arterial blood supply of hepatocellular carcinoma and histologic grading: radiologic-pathologic correlation. AJR Am J Roentgenol 2008;190:W28–34 [DOI] [PubMed] [Google Scholar]
- 23.Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, et al. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR Am J Roentgenol 1999;172:969–76 [DOI] [PubMed] [Google Scholar]
- 24.Terminology of nodular hepatocellular lesions International Working Party. Hepatology 1995;22:983–93 [DOI] [PubMed] [Google Scholar]
- 25.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462–503 [DOI] [PubMed] [Google Scholar]
- 26.Lim JH, Choi D, Park CK, Lee WJ, Lim HK. Encapsulated hepatocellular carcinoma: CT-pathologic correlations. Eur Radiol 2006;16:2326–33 [DOI] [PubMed] [Google Scholar]
- 27.Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol 2009;44Suppl. 19:112–18 [DOI] [PubMed] [Google Scholar]
- 28.Li CS, Chen RC, Tu HY, Shih LS, Zhang TA, Lii JM, et al. Imaging well-differentiated hepatocellular carcinoma with dynamic triple-phase helical computed tomography. Br J Radiol 2006;79:659–65 [DOI] [PubMed] [Google Scholar]
- 29.Jang HJ, Yu H, Kim TK. Contrast-enhanced ultrasound in the detection and characterization of liver tumors. Cancer Imaging 2009;9:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu GJ, Xu HX, Lu MD, Xie XY, Xu ZF, Zheng YL, et al. Correlation between enhancement pattern of hepatocellular carcinoma on real-time contrast-enhanced ultrasound and tumour cellular differentiation on histopathology. Br J Radiol 2007;80:321–30 [DOI] [PubMed] [Google Scholar]
- 31.Honda H, Tajima T, Kajiyama K, Kuroiwa T, Yoshimitsu K, Irie H, et al. Vascular changes in hepatocellular carcinoma: correlation of radiologic and pathologic findings. AJR Am J Roentgenol 1999;173:1213–17 [DOI] [PubMed] [Google Scholar]
- 32.Matsui O. Imaging of multistep human hepatocarcinogenesis by CT during intra-arterial contrast injection. Intervirology 2004;47:271–6 [DOI] [PubMed] [Google Scholar]
- 33.Kondo F. Histological features of early hepatocellular carcinomas and their developmental process: for daily practical clinical application: Hepatocellular carcinoma. Hepatol Int 2009;3:283–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furlan A, Marin D, Vanzulli A, Patera GP, Ronzoni A, Midiri M, et al. Hepatocellular carcinoma in cirrhotic patients at multidetector CT: hepatic venous phase versus delayed phase for the detection of tumour washout. Br J Radiol 2011;84:403–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nino-Murcia M, Olcott EW, Jeffrey RB, Jr, Lamm RL, Beaulieu CF, Jain KA. Focal liver lesions: pattern-based classification scheme for enhancement at arterial phase CT. Radiology 2000;215:746–51 [DOI] [PubMed] [Google Scholar]
- 36.Rosai J, editor. Rosai and Ackerman's surgical pathology. 9th edn. St Louis, MO: Mosby; 2004 [Google Scholar]
- 37.Doyle DJ, O'Malley ME, Jang HJ, Jhaveri K. Value of the unenhanced phase for detection of hepatocellular carcinomas 3 cm or less when performing multiphase computed tomography in patients with cirrhosis. J Comput Assist Tomogr 2007;31:86–92 [DOI] [PubMed] [Google Scholar]