Abstract
Objective
This study aims to compare dynamic conformal arc (DCA) plans based on different-percentage isodose surfaces (IDSs), normalised to 100% at the isocentre, for target coverage (TC; dose prescription) in stereotactic radiotherapy for large cystic brain metastases.
Methods
The DCA plans were generated for 15 targets (5 spherical models and 10 metastatic brain lesions) based on 90%, 80% and 70% IDSs for dose prescription to attain ≥99% TC values using the Novalis Tx platform. These plans were optimised mainly by leaf margin and/or collimator angle adjustment, while similar arc arrangements were used.
Results
TC values were equivalent among the three plans. Conformity index values were similar between the 80% and 70% plans, while they were worse in the 90% plans. Mean doses (Dmean) of the interior 3 mm rind structure were highest in the 70% plans, followed by the 80% plans and lowest in the 90% plans. Dmean of the exterior 3 mm rind structure and the ratio of 50%/100% isodose volumes (Paddick's gradient index values) were highest in the 90% plans, followed by 80% and lowest in the 70% plans.
Conclusions
These results suggest that the 70% IDS plans might be beneficial for both tumour control and reducing toxicity to surrounding normal tissue if appropriate dose conformity and precise treatment set-up are ensured. The 90% IDS plans are unfavourable in view of inferior dose gradient outside the target and should be limited to cases in which the target dose homogeneity is given the highest priority.
Stereotactic radiosurgery (SRS) and radiotherapy (SRT), either alone or combined with whole brain radiotherapy, are viable treatment options for brain metastases [1]. SRS/SRT for large brain metastases that were deemed inoperable poses therapeutic problems, including increased risk of injury of the surrounding normal tissue. It is therefore practical to administer lower doses to larger tumours and vice versa [2]. There are also concerns regarding lower doses (e.g. 50% of prescription dose) administered to the surrounding normal tissue. Some studies have reported a possible relation between irradiated normal brain volumes, such as 10–12 Gy volumes for SRS and 4 Gy volumes for SRT, and treatment-related toxicity [3,4]. Planning to attain steeper dose fall-off outside the target seems to be desirable.
There are various treatment modalities now available for intracranial SRS/SRT (referred to here as only SRT), and linear accelerator-based SRT is increased in sophistication due to technical developments such as high definition micro-multileaf collimator (mMLC), planning methods, and frameless image-guided treatment systems with high-precision treatment positioning [5,6]. The dynamic conformal arc (DCA) is one of the state-of-the-art techniques, in which dose prescription (target coverage, TC) is commonly defined at the specific percentage isodose surface (IDS) normalised to 100% at the isocentre [7,8]. However, the percentage IDS specification appears to vary substantially among institutions (Table 1) [4,7,9-21]. 80% or 90% IDSs are common, while IDS<80% is used in a few institutions. The target dose homogeneity becomes worse as the lower percentage IDS is selected for TC. However, the influence of differences in the percentage IDS specification on other dosimetric parameters (such as dose gradient outside the target or doses to the thin rind structure just interior to the target boundary) has not yet been elucidated.
Table 1. Variability in IDS (%) specification (100% at the isocentre) for dose prescription in mMLC-based SRS/SRT.
| Reference, year | SRS/SRT | Apparatus | Technique | Margin | IDS (%) specification | Dose |
| Giubilei et al [9], 2009 | SRT | 3DLinea mMLC | NA | 3 mm | Median 95% | 6 Gy×3 or 8 Gy×4 |
| Lindvall et al [10], 2005 | SRS/SRT | mMLC | static multibeam | 3 mm | 90% | 8 Gy×5 or 17 Gy (1–3 fractions) |
| Ernst-Stecken et al [4], 2006 | SRT | Novalis | DCA or static multibeam | 3 mm | 90% | 7 Gy×5 or 6 Gy×5 |
| Saitoh et al [11], 2009 | SRT | Accuknifeb mMLC | Static multibeam | 3 mm | 90% | 13 Gy×3 or 14 Gy×3 |
| Kim et al [12], 2011 | SRS/SRT | cm3® mMLC | DCA | 1 mm | Median 90% (84–98%) SRS/median 91% (82–96%) | Median 20 Gy (15–22 Gy); SRS, median 6 Gy (5–7 Gy)×6; SRT |
| Chitapanarux et al [13], 2003 | SRS | Novalis | DCA | 0 mm | Median 90% (80–90%) | Median 18 Gy (12–18 Gy) |
| Blonigen et al [3], 2010 | SRS | Novalis | DCA | NA | Usually 80% (80–100%)d | Mean 18 Gy (12–22 Gy) |
| Scorsetti et al [14], 2009 | SRT | 3DLinea mMLC | DCA | 4 mm | ≥ 80% | 4 Gy×6 or 4 Gy×7 |
| Marchetti et al [15], 2011 | SRT | 3DLinea 4 mm mMLC | NA | 2 mm | Median 80% | Median 8 Gy×3 |
| Chen et al [16], 2009 | SRS | Novalis | DCA | NA | 80% | Median 18 Gy (14–20 Gy) |
| Hazard et al [7], 2009 | SRS | m3c mMLC | DCA | NA | Median 80%, mean 82% (80–90%) | 18.9±2.6 Gy (mean±SD) |
| Molenaar et al [17], 2009 | SRS | Novalis | DCA | 2 mm | 80% | 21 Gy (<8 cm3), 18 Gy (8–13 cm3), 15 Gy (>13 cm3) |
| Hoefnagels et al [18], 2009 | SRS | Novalis | DCA | NA | 80% | 18–21 Gy |
| Kelly et al [19], 2011 | SRS/SRT | Novalis | NA | 0 mm | 70–80% | Median 13 Gy (8–16 Gy); SRS, 5 Gy×5; SRT |
| Kelly et al [20], 2012 | SRS/SRT | Novalis | NA | 0 mm | Median 78% (68–85%) | Median 18 Gy (15–18 Gy); SRS, 5 Gy×5 or 3 Gy×10; SRT |
| Valery et al [21], 2011 | SRS | m3c mMLC | DCA | NA | 70% | Median 13.4 Gy (8.2–15.0 Gy) |
DCA, dynamic conformal arc; IDS, isodose surface; mMLC, micro-multileaf collimator; NA, not available; ref, reference number; SD, standard deviation; SRS, stereotactic radiosurgery; SRT, stereotactic radiotherapy. Novalis, Novalis (BrainLAB AG, Feldkirchen, Germany).
aManufactured by 3D Line International, Milan, Italy.
bManufactured by DiREX Inc., Tokyo, Japan.
cManufactured by BrainLAB AG.
dNormalised to 100% at the maximum dose.
In this study, we generated and compared three distinct DCA plans based on different percentage IDSs (90%, 80% and 70%), normalised to 100% at the isocentre, for TC (dose prescription) with the similar values in SRT for large cystic brain metastases. In cystic lesions, treatment targets are regarded as viable tumour cells mainly located in the cyst wall, and the target dose inhomogeneity is considered to be not detrimental. In this study, we examined the relative merits of different percentage IDS-based plans, and considered the optimal percentage IDS selection for both tumour control and reducing toxicity to surrounding normal tissue.
Methods and materials
Treatment system, study population and planning method
The treatment system used was the Novalis Tx (BrainLAB AG, Feldkirchen, Germany; and Varian, Palo Alto, CA), a recently available dedicated platform for image-guided SRT and conventional radiotherapy, commissioned for the iPlan RT Dose version 4.1.2 (BrainLAB) treatment planning system (TPS) [5,22,23]. The iPlan Image version 4.1 (BrainLAB) was used specifically for image coregistration and target delineation.
Stereotactically localised CT scans were obtained in contiguous 1.25 mm slices. T1 weighted post-contrast MR images were acquired with 2 mm slices without fiducial markers and were coregistered with the CT scans by using a mutual information-based algorithm implemented in the TPS. The accuracy of image fusion in all cases was confirmed by the first author. Five spherical structures with diameters ranging from 20 to 40 mm in 5 mm increments were created on the planning CT images using the “draw sphere” tool. Ten metastatic brain lesions were chosen from the database of patients treated with SRS/SRT during the last 18 months. These 15 targets were used as planning objects without adjacent dose constraint. For metastatic brain lesions, clinical target volume (CTV) was defined as an enhanced lesion on MRI and was expanded to a planning target volume (PTV; referred to here as TV) with a 1 mm isotropic margin. The TVs of brain metastases ranged from 7.4 to 25.9 cm3, with a median value of 14.4 cm3. No margin was added to the spherical targets.
For each case, a DCA plan utilising five arcs, two of which were coplanar, was generated (e.g. 0°, 65°, 280°, 315° and 0° for couch positions; Figure 1). Three different plans were generated for each of the 15 targets by using 90%, 80% and 70% IDSs, normalised to 100% at the isocentre, to attain ≥99% target coverage values for each IDS as similar as possible. Plans were optimised mainly with leaf margin (0.1 mm increments) or collimator angle adjustments, while similar couch positions were used for the three non-coplanar arcs. The arc length (i.e. the range between the start and stop angles of the gantry) was set at 120° (30°–150° or 210°–330°) in all plans. To circumvent any dose interference resulting from simultaneous treatment of multiple targets, all cases were planned as a single lesion.
Figure 1.
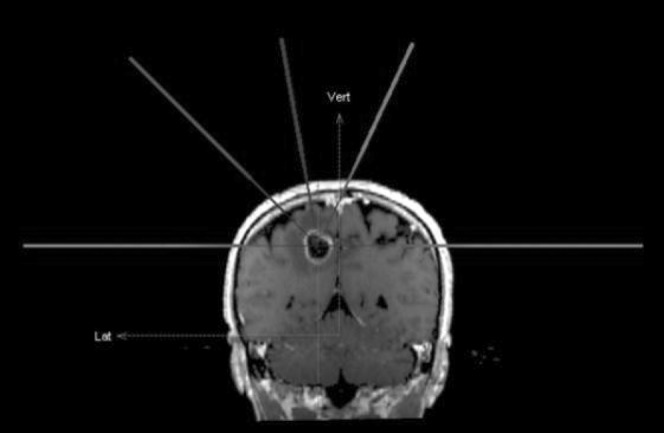
Frontal view of the adopted arc arrangement (couch positions).
Dose–volume histogram analyses
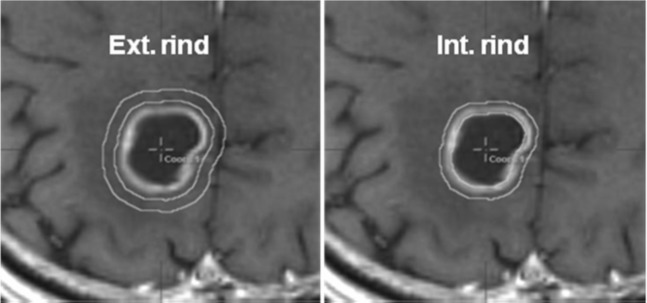
The dose calculation was based on a pencil-beam algorithm with radiological path length for tissue heterogeneity correction. The grid size of the dose–volume histogram (DVH) calculation was set to 1.0 mm. An expanded TV (eTV) was created with the addition of an isotropic margin >15 mm to the TV to directly compute the isodose volumes (IDVs) encompassed by the reference dose for each plan [8]. The exterior and interior 3-mm- thick rind structures around the target periphery were generated separately by using the “create wall” tool (Figure 2).
Figure 2.
(a) Exterior and (b) interior 3-mm-thick rind structures generated from the target boundary. Ext., exterior; Int., interior.
Conformity index (CI) was defined by the Radiation Therapy Oncology Group [24], and also described in Report 62 of the International Commission on Radiation Units and Measurements (ICRU) [25], as:
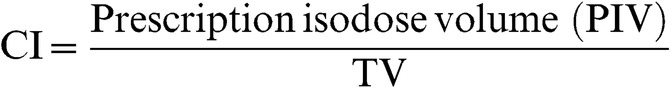 |
(1) |
where PIV corresponds to 100% IDV.
Homogeneity index (HI) was defined as Report 83 of the ICRU [26] as:
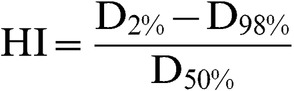 |
(2) |
where D2%, D98% and D50% are the doses (%) receiving at least 2%, 98% and 50% of the TV, respectively.
For dose–gradient analyses, the ratios of 50%/100% IDVs (known as Paddick's gradient index, GI) [27], 50% IDV/TV (modified GI) [28], 75%/100% IDVs, 75% IDV/TV, 25%/100% IDVs and 25% IDV/TV were examined as the prescription dose normalised to 100%. In comparison with Paddick's GI, a modified GI was valuable for considering the degree of dose conformity and for adjusting a possible false superior value for a specified IDS in cases with target over-coverage [28].
Statistical analyses
Statistical analyses were performed using PASW Statistics 18 software (SPSS Inc., Chicago, IL). Some variables were found to depart significantly from a normal distribution, based on the results from the Shapiro–Wilk test. Therefore, we applied non-parametric tests for the following analyses. The Wilcoxon signed-rank test was applied to compare paired variables. The Kruskal–Wallis (KW) test was used to compare variables among three groups. The Jonckheere–Terpstra (JT) test was also adopted to assess a trend in the dosimetric parameters among three groups with ordered percentage IDS-based plans. All p-values were calculated with two-sided tests, and p-values <0.05 were considered to be statistically significant unless otherwise specified.
Results
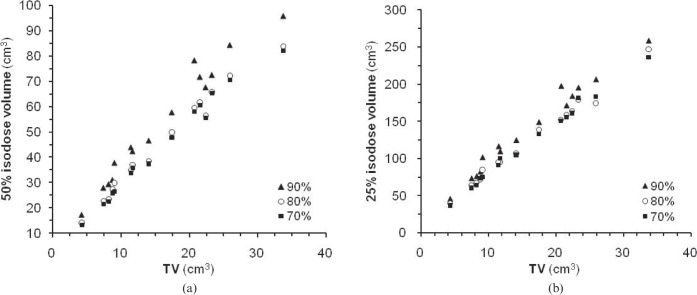
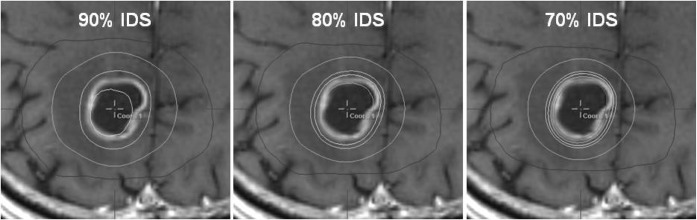
Dosimetric parameters for the 90%, 80% and 70% IDS-based plans are demonstrated in Table 2. TC values were equivalent among the three plans. CI values were similar between the 80% and 70% plans, whereas they were worse in the 90% plans. Mean dose (Dmean) values of the interior 3 mm rind structures were significantly higher in the order of the 70%, 80% and 90% plans. In contrast, Dmean values of the exterior 3-mm rind structures were significantly lower in the order of the 70%, 80% and 90% plans. The ratios of 75%/100% IDVs, 75% IDV/TV, 50%/100% IDVs, 50% IDV/TV, 25%/100% IDVs and 25% IDV/TV were also lower in the order of the 70%, 80% and 90% plans. The 50% IDV differences between the 90% and 80% plans were 3.3–18.9 cm3 (mean, 8.6 cm3), whereas those between the 80% and 70% were 0.4–3.3 cm3 (mean, 0.4 cm3; Figure 3a). The 25% IDV differences between the 90% and 80% plans were 5.5–45.5 cm3 (mean, 16.8 cm3), whereas those between the 80% and 70% were −8.4 to 10.9 cm3 (mean, 2.2 cm3; Figure 3b). An example of the dose distribution differences among the three plans for the same target is shown in Figure 4.
Table 2. Comparison of planning parameters at three levels of IDS.
| 90% |
80% |
70% |
|||||
| Parameter | Mean±SD (range) | p-valuea | Mean±SD (range) | p-valuea | Mean±SD (range) | KW test p-value | JT test p-value (↑/↓)b |
| Target coverage (%) | 99.1±0.1 | 99.2±0.1 | 99.2±0.1 | 0.601 | |||
| (99.0–99.3) | (99.0–99.6) | (99.0–99.4) | |||||
| 0.343 | 0.944 | ||||||
| CI | 1.22±0.06 | 1.16±0.05 | 1.16±0.05 | 0.027 | 0.005 | ||
| (1.14–1.30) | (1.08–1.23) | (1.07–1.24) | (↓) | ||||
| 0.001 | 0.691 | ||||||
| HI | 0.13±0.01 | 0.22±0.01 | 0.33±0.01 | <0.001 | <0.001 | ||
| (0.11–0.15) | (0.21–0.25) | (0.32–0.34) | (↑) | ||||
| 0.001 | 0.001 | ||||||
| TV Dmean (%) | 108.0±1.30 | 117.9±1.3 | 129.5±1.9 | <0.001 | <0.001 | ||
| (104.0–109.1) | (115.1–119.7) | (125.8–132.5) | (↑) | ||||
| <0.001 | <0.001 | ||||||
| Int. rind Dmean (%) | 106.7±0.70 | 113.5±1.2 | 121.1±2.1 | <0.001 | <0.001 | ||
| (105.5–108.0) | (111.6–115.4) | (117.6–124.3) | (↑) | ||||
| <0.001 | <0.001 | ||||||
| Ext rind Dmean (%) | 94.0±2.20 | 88.1±2.7 | 86.0±3.2 | <0.001 | <0.001 | ||
| (89.9–97.4) | (83.2–91.9) | (81.2–90.6) | (↓) | ||||
| <0.001 | <0.001 | ||||||
| 75% IDV/100% IDV | 1.72±0.18 | 1.52±0.08 | 1.45±0.06 | <0.001 | <0.001 | ||
| (1.27–2.08) | (1.41–1.70) | (1.32–1.57) | (↓) | ||||
| 0.006 | <0.001 | ||||||
| 75% IDV/TV | 2.10±0.26 | 1.76±0.12 | 1.68±0.12 | <0.001 | <0.001 | ||
| (1.46–2.46) | (1.54–1.93) | (1.46–1.84) | (↓) | ||||
| 0.001 | <0.001 | ||||||
| 50% IDV/100% IDV | 2.87±0.30 | 2.51±0.18 | 2.42±0.11 | <0.001 | <0.001 | ||
| (PGI) | (2.46–3.53) | (2.28–2.94) | (2.27–2.67) | (↓) | |||
| <0.001 | <0.001 | ||||||
| 50% IDV/TV | 3.50±0.38 | 2.91±0.24 | 2.80±0.19 | <0.001 | <0.001 | ||
| (mGI) | (2.84–4.17) | (2.48–3.29) | (2.43–3.09) | (↓) | |||
| <0.001 | <0.001 | ||||||
| 25% IDV/100% IDV | 7.47±0.85 | 6.87±0.69 | 6.69±0.44 | 0.012 | 0.003 | ||
| (6.15–9.23) | (5.59–8.35) | (5.88–7.4) | (↓) | ||||
| 0.001 | 0.078 | ||||||
| 25% IDV/TV | 9.12±1.02 | 7.96±0.74 | 7.75±0.54 | 0.001 | <0.001 | ||
| (7.68–11.18) | (6.74–9.34) | (7.01–8.58) | (↓) | ||||
| 0.001 | 0.054 | ||||||
CI, conformity index; Dmean, mean dose; Ext., exterior; HI, homogeneity index; IDS, isodose surface; IDV, isodose volume; Int., interior; JT test, Jonckheere–Terpstra test; KW test, Kruskal–Wallis test; mGI, modified gradient index; PGI, Paddick's gradient index; SD, standard deviation; TV, target volume.
Values are mean±SD (range).
Significant p-values are shown in bold.
aWilcoxon signed-rank test (p<0.016 considered significant based on Bonferroni's correction for multiple comparison).
bIncreased (↑) or decreased (↓) trends in the results from Jonckheere–Terpstra test.
Figure 3.
Target volumes (TVs) vs (a) 50% isodose volumes (IDVs) or (b) 25% IDVs (100% isodose=prescription dose).
Figure 4.
Isodose distribution differences for 90%, 80% and 70% isodose surface (IDS)-based plans for the same target (9.0 cm3). (a) From the outer line, 25%, 50%, 100% and 110% isodose line (IDLs), normalised to 100% at “the prescription dose”, shown in the 90% IDS plans. (b) 25%, 50%, 100%, 110% and 120% IDLs depicted in the 80% IDS plans. (c) 25%, 50%, 100%, 110%, 120% and 130% IDLs (e.g. 2.5, 5, 10, 11, 12 and 13 Gy at 100%=10 Gy) demonstrated in the 70% IDS plans.
When these dosimetric comparisons and statistical analyses were performed separately for 5 spherical targets and the 10 metastatic brain lesions, the results were similar (data not shown). Therefore, the results for all 15 targets are presented in their entirety in Table 2.
Discussion
The 90% plan showed worse CI values compared with the 80% and 70% plans. In the 90% plan inadequate TC was observed frequently at the caudal side of the target, and larger leaf margins were required to cover that, leading to target over-coverage. When a similar planning method is applied, 90% IDS-based plans might be disadvantageous for dose conformity. However, this fault may be remedied by manual leaf adjustment or modification of the target geometry for leaf adaptation [29].
The HI values for the 70% plans were properly higher (maximum value=1.5), but these fulfilled the Radiation Therapy Oncology Group (RTOG) criterion for SRT [24]. Furthermore, higher Dmean of the interior rind structure means steeper dose increase just inside the target boundary in the 70% plan. For cystic brain metastases, the dose to the interior rind structure will affect tumour control directly rather than the target Dmean or maximum dose (Dmax). Furthermore, the minimum dose (Dmin) of the CTV would be higher in the order of the 70%, 80% and 90% plans, considering a 1 mm PTV margin. As mentioned previously, large tumours force physicians to reduce prescription dose to tumour margin. However, 70% IDS-based planning may improve this restraint to some degree by increasing the Dmean, the integral dose of the interior rind structure and the Dmin of the CTV. In the meantime, for complex-shaped lesions with irregular surface, the 70% plan may involve the risk of spilling undesirable high doses into the normal tissue beneath the tumour. Similarly, lower percentage IDS-based plans should not be applied in cases requiring set-up margins >1 mm (PTV margin ≥2 mm). Application of the 70% plan might be limited to be used with the image-guided high-precision treatment with set-up margin ≤1 mm or adequately co-operative patients. In addition, post-resection cavity as an SRT indication may be similar to cystic brain metastases in view of the target periphery as a main target [20]. However, wall enhancement in the resection cavity may contain substantially normal brain tissue, especially in cases with gross total resection. Lower percentage IDS-based plans might also be unsuitable for these cases.
The degree of dose gradient outside the target was steeper in the order of the 70%, 80% and 90% plans. Considering the 50% and 25% IDV differences, the relative merit of the 70% plan compared with the 80% plan seemed to be small. In contrast, the 90% plan proved to be unfavourable in view of inferior dose gradient. Although inferior dose conformity (target over-coverage) in the 90% plan was considered to partially affect the higher Dmean of the exterior rind structure, 50% IDVs for the 90% plans were significantly larger than those for the 80% plans. These results suggest that the 90% plans should be limited to cases for which target dose homogeneity takes priority.
Taken together, these results indicate that differences in percentage IDS specification (100%=isocentre) for target coverage in treatment planning significantly affect dose distributions both inside and outside the target boundary even when the same prescription dose is administered to an IDS with the same TC values. Planners need to be aware of the significance of each percentage IDS selection for dose distribution, and select the optimal IDS on an individual case basis. In multiple target treatment, the actual isocentre dose may differ from the assigned dose to the isocentre due to possible dose interference. In these cases the relative percentage values for the IDS specified for TC should be confirmed by checking the actual isocentre dose. Lower than 80% IDS-based plans might be preferable for both tumour control and reducing toxicity to normal tissue if appropriate dose conformity and precise set-up accuracy are ensured. Given that the degree of dose homogeneity satisfied the RTOG recommendation, these plans might also be beneficial for mainly solid tumours. In clinical application, especially for SRT taking a longer treatment period, caution needs to be taken for possible target deviation due to alleviation of perilesional oedema by medication and for possible geometrical change of the target due to unexpected early tumour shrinking.
Conclusions
In this study, three distinct DCA planning methods based on 90%, 80% and 70% IDSs for target coverage, normalised to 100% at the isocentre, were compared. The 70% IDS-based plans showed steeper dose increase just inside the target boundary and also superior dose gradient outside the target. In contrast, the 90% IDS-based plans were unfavourable in view of the inferior dose gradient outside the target. These results suggest that the 70% IDS-based plans would be beneficial for both tumour control and reducing toxicity to surrounding normal tissues in SRT for large cystic brain metastases if superior dose conformity and precise set-up accuracy are ensured. These results warrant further investigations to determine whether lower percentage IDS-based plans can lead to better clinical outcomes.
References
- 1.Müller-Riemenschneider F, Bockelbrink A, Ernst I, Schwarzbach C, Vauth C, von derSchulenburg JM, et al. Stereotactic radiosurgery for the treatment of brain metastases. Radiother Oncol 2009;91:67–74 [DOI] [PubMed] [Google Scholar]
- 2.Wiggenraad R, Kanter AV, Kal HB, Taphoorn M, Vissers T, Struikmans H. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol 2011;98:292–7 [DOI] [PubMed] [Google Scholar]
- 3.Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2010;77:996–1001 [DOI] [PubMed] [Google Scholar]
- 4.Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol 2006;81:18–24 [DOI] [PubMed] [Google Scholar]
- 5.Chang Z, Wang Z, Wu QJ, Yan H, Bowsher J, Zhang J, et al. Dosimetric characteristics of novalis Tx system with high definition multileaf collimator. Med Phys 2008;35:4460–3 [DOI] [PubMed] [Google Scholar]
- 6.Jin JY, Yin FF, Tenn SE, Medin PM, Solberg TD. Use of the BrainLAB ExacTrac X-Ray 6D system in image-guided radiotherapy. Med Dosim 2008;33:124–34 [DOI] [PubMed] [Google Scholar]
- 7.Hazard LJ, Wang B, Skidmore TB, Chern SS, Salter BJ, Jensen RL, et al. Conformity of LINAC-based stereotactic radiosurgery using dynamic conformal arcs and micro-multileaf collimator. Int J Radiat Oncol Biol Phys 2009;73:562–70 [DOI] [PubMed] [Google Scholar]
- 8.Ohtakara K, Hayashi S, Hoshi H. Characterisation of dose distribution in linear accelerator-based intracranial stereotactic radiosurgery with the dynamic conformal arc technique: consideration of the optimal method for dose prescription and evaluation. Br J Radiol 2012;85:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giubilei C, Ingrosso G, D'Andrea M, Benassi M, Santoni R. Hypofractionated stereotactic radiotherapy in combination with whole brain radiotherapy for brain metastases. J Neurooncol 2009;91:207–12 [DOI] [PubMed] [Google Scholar]
- 10.Lindvall P, Bergström P, Löfroth PO, Henriksson R, Bergenheim AT. Hypofractionated conformal stereotactic radiotherapy alone or in combination with whole-brain radiotherapy in patients with cerebral metastases. Int J Radiat Oncol Biol Phys 2005;61:1460–6 [DOI] [PubMed] [Google Scholar]
- 11.Saitoh J, Saito Y, Kazumoto T, Kudo S, Ichikawa A, Hayase N, et al. Therapeutic effect of linac-based stereotactic radiotherapy with a micro-multileaf collimator for the treatment of patients with brain metastases from lung cancer. Jpn J Clin Oncol 2010;40:119–24 [DOI] [PubMed] [Google Scholar]
- 12.Kim YJ, Cho KH, Kim JY, Lim YK, Min HS, Lee SH, et al. Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys 2011;81:483–9 [DOI] [PubMed] [Google Scholar]
- 13.Chitapanarux I, Goss B, Vongtama R, Frighetto L, De Salles A, Selch M, et al. Prospective study of stereotactic radiosurgery without whole brain radiotherapy in patients with four or less brain metastases: incidence of intracranial progression and salvage radiotherapy. J Neurooncol 2003;61:143–9 [DOI] [PubMed] [Google Scholar]
- 14.Scorsetti M, Facoetti A, Navarria P, Bignardi M, De Santis M, Ninone SA, et al. Hypofractionated stereotactic radiotherapy and radiosurgery for the treatment of patients with radioresistant brain metastases. Anticancer Res 2009;29:4259–63 [PubMed] [Google Scholar]
- 15.Marchetti M, Milanesi I, Falcone C, De Santis M, Fumagalli L, Brait L, et al. Hypofractionated stereotactic radiotherapy for oligometastases in the brain: a single-institution experience. Neurol Sci 2011;32:393–9 [DOI] [PubMed] [Google Scholar]
- 16.Chen JC, Bugoci DM, Girvigian MR, Miller MJ, Arellano A, Rahimian J. Control of brain metastases using frameless image-guided radiosurgery. Neurosurg Focus 2009;27:E6. [DOI] [PubMed] [Google Scholar]
- 17.Molenaar R, Wiggenraad R, Verbeek-de Kanter A, Walchenbach R, Vecht C. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br J Neurosurg 2009;23:170–8 [DOI] [PubMed] [Google Scholar]
- 18.Hoefnagels FW, Lagerwaard FJ, Sanchez E, Haasbeek CJ, Knol DL, Slotman BJ, et al. Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol 2009;256:878–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly PJ, Lin YB, Yu AY, Ropper AE, Nguyen PL, Marcus KJ, et al. Linear accelerator-based stereotactic radiosurgery for brainstem metastases: the Dana-Farber/Brigham and Women's Cancer Center experience. J Neurooncol 2011;104:553–7 [DOI] [PubMed] [Google Scholar]
- 20.Kelly PJ, Lin YB, Yu AY, Alexander BM, Hacker F, Marcus KJ, et al. Stereotactic irradiation of the postoperative resection cavity for brain metastasis: a frameless linear accelerator-based case series and review of the technique. Int J Radiat Oncol Biol Phys 2012;82:95–101 [DOI] [PubMed] [Google Scholar]
- 21.Valery CA, Boskos C, Boisserie G, Lamproglou I, Cornu P, Mazeron JJ, et al. Minimized doses for linear accelerator radiosurgery of brainstem metastasis. Int J Radiat Oncol Biol Phys 2011;80:362–8 [DOI] [PubMed] [Google Scholar]
- 22.Wu QJ, Wang Z, Kirkpatrick JP, Chang Z, Meyer JJ, Lu M, et al. Impact of collimator leaf width and treatment technique on stereotactic radiosurgery and radiotherapy plans for intra- and extracranial lesions. Radiat Oncol 2009;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma J, Chang Z, Wang Z, Wu QJ, Kirkpatrick JP, Yin FF. ExacTrac X-ray 6 degree-of-freedom image-guidance for intracranial non-invasive stereotactic radiotherapy: comparison with kilo-voltage cone-beam CT. Radiother Oncol 2009;93:602–8 [DOI] [PubMed] [Google Scholar]
- 24.Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, et al. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 1993;27:1231–9 [DOI] [PubMed] [Google Scholar]
- 25.International Commission on Radiation Units and Measurements (ICRU) Prescribing, recording, and reporting photon beam therapy (supplement to ICRU report No. 50). Report no. 62 Bethesda, MD: ICRU; 1999 [Google Scholar]
- 26.International Commission on Radiation Units and Measurements (ICRU) Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). Report no. 83: Bethesda, MD: ICRU; 2010 [Google Scholar]
- 27.Paddick I, Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J Neurosurg 2006;105 Suppl. 194–201 [DOI] [PubMed] [Google Scholar]
- 28.Ohtakara K, Hayashi S, Hoshi H. Dose gradient analyses in Linac-based intracranial stereotactic radiosurgery using Paddick's gradient index: Consideration of the optimal method for plan evaluation. J Radiat Res 2011;52:592–9 [DOI] [PubMed] [Google Scholar]
- 29.Leavitt DD. Beam shaping for SRT/SRS. Med Dosim 1998;23:229–36 [DOI] [PubMed] [Google Scholar]