Abstract
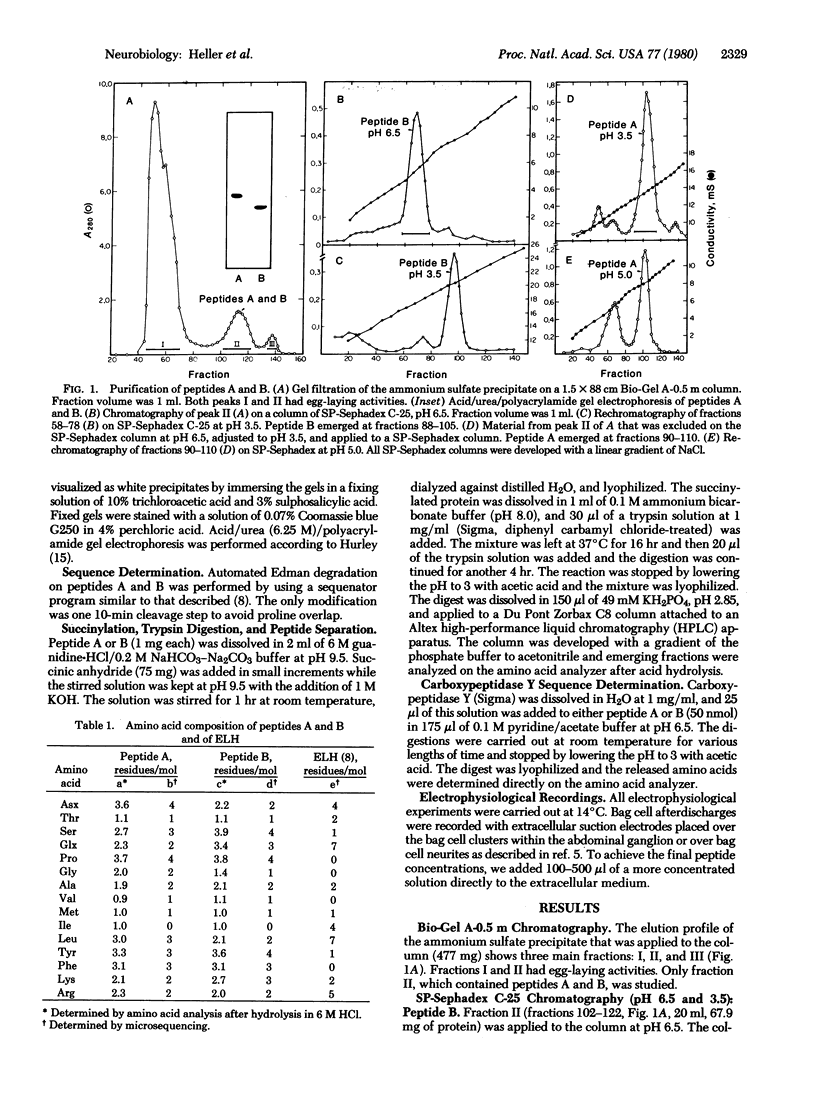
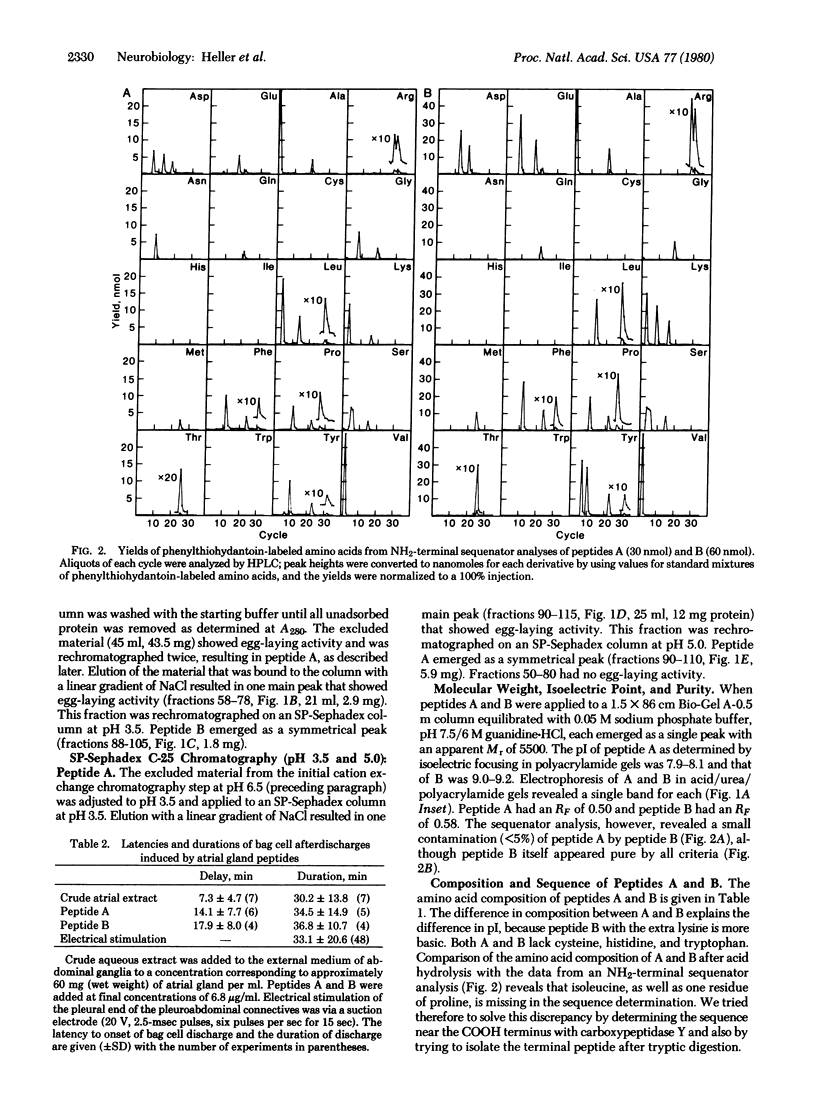
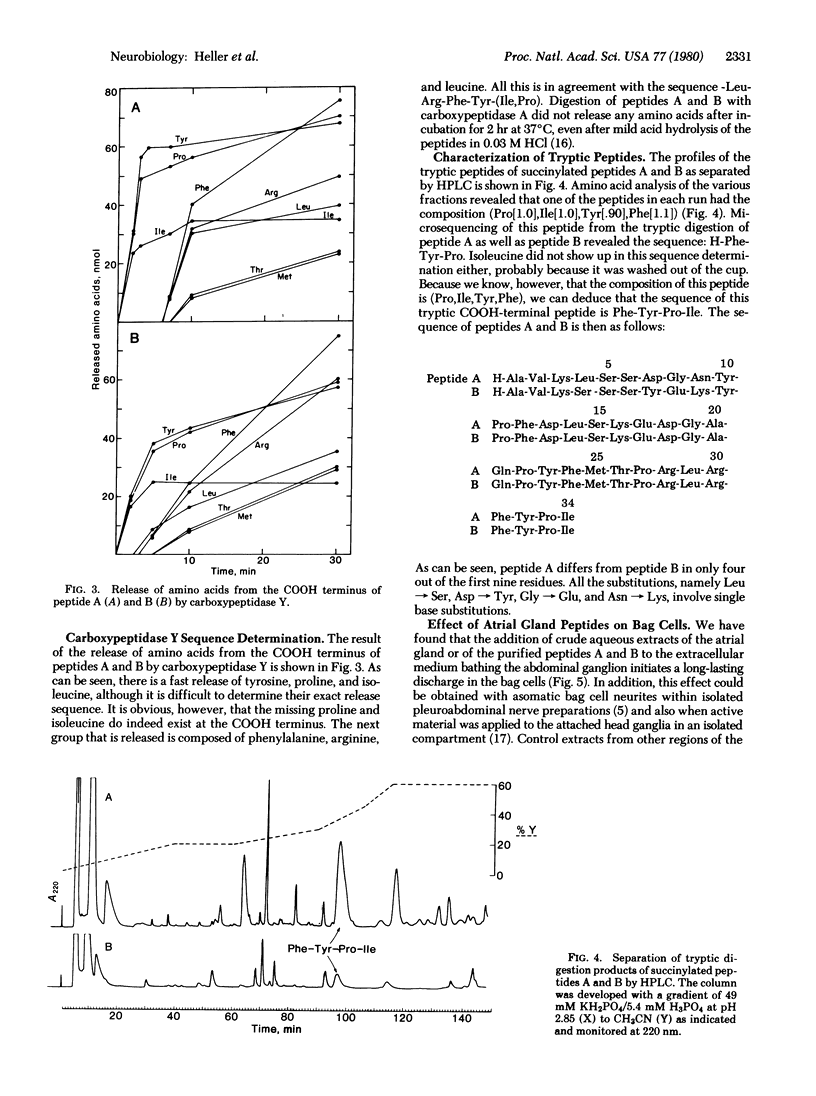
Two neuroactive peptides, A and B, have been isolated from the atrial gland in the reproductive tract of Aplysia. Each of the two peptides is able to induce egg-laying behavior in recipient animals. In vitro recordings from the abdominal ganglion show that both peptides also trigger longlasting discharges in the bag cell neurons at concentrations around 0.1 μM. The peptides were purified by a combination of ammonium sulfate precipitation, agarose gel filtration, and cation exchange chromatography. Each peptide has 34 amino acid residues. Microsequencing together with carboxypeptidase Y degradation and analysis of tryptic peptides revealed the following sequence for peptide A: H-Ala-Val-Lys-Leu-Ser-Ser-Asp-Gly-Asn-Tyr-Pro-Phe-Asp-Leu-Ser-Lys-Glu-Asp-Gly -Ala-Gln-Pro-Tyr-Phe-Met-Thr-Pro-Arg-Leu-Arg-Phe-Tyr-Pro-Ile. Peptide B differs from A in only four positions. The first nine residues of B are: Ala-Val-Lys-Ser-Ser-Ser-Tyr-Glu-Lys-, whereas residues 10-34 of B are identical to those of A. The calculated Mr of A is 3924 and that of B is 4032. The pI of peptide A as determined by isoelectric focusing in polyacrylamide gels is 7.9-8.1 and that of peptide B is 9.0-9.2. It is estimated that each atrial gland contains at least 150 μg of peptide A and 50 μg of B. Neither peptide resembles the egg-laying hormone isolated from bag cell neurons. It is postulated that the atrial gland peptides are released during copulation, and then by interacting with neuronal receptors in the head ganglia and pleuroabdominal connectives they cause the bag cells to afterdischarge, thereby releasing egg-laying hormone.
Keywords: atrial gland, egg-laying hormone, amino acid sequence, molluskan peptides
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audesirk T. E. Chemoreception in Aplysia californica. I. Behavioral localization of distance chemoreceptors used in food-finding. Behav Biol. 1975 Sep;15(1):45–55. doi: 10.1016/s0091-6773(75)92066-0. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Purification of a toxic protein from scorpion venom which activates the action potential Na+ ionophore. J Biol Chem. 1976 Sep 25;251(18):5528–5536. [PubMed] [Google Scholar]
- Chang M. M., Leeman S. E., Niall H. D. Amino-acid sequence of substance P. Nat New Biol. 1971 Jul 21;232(29):86–87. doi: 10.1038/newbio232086a0. [DOI] [PubMed] [Google Scholar]
- Chiu A. Y., Hunkapiller M. W., Heller E., Stuart D. K., Hood L. E., Strumwasser F. Purification and primary structure of the neuropeptide egg-laying hormone of Aplysia californica. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6656–6660. doi: 10.1073/pnas.76.12.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R. Carboxypeptidase Y in sequence determination of peptides. Methods Enzymol. 1977;47:84–93. doi: 10.1016/0076-6879(77)47010-1. [DOI] [PubMed] [Google Scholar]
- Hurley C. K. Electrophoresis of histones: a modified Panyim and Chalkley system for slab gels. Anal Biochem. 1977 Jun;80(2):624–626. doi: 10.1016/0003-2697(77)90687-x. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K., Strumwasser F. Neurotransmitter modulation, phosphodiesterase inhibitor effects, and cyclic AMP correlates of afterdischarge in peptidergic neurites. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5200–5204. doi: 10.1073/pnas.75.10.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Kupfermann I., Kandel E. R. Electrophysiological properties and functional interconnections of two symmetrical neurosecretory clusters (bag cells) in abdominal ganglion of Aplysia. J Neurophysiol. 1970 Nov;33(6):865–876. doi: 10.1152/jn.1970.33.6.865. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Stimulation of egg laying by extracts of neuroendocrine cells (bag cells) of abdominal ganglion of Aplysia. J Neurophysiol. 1970 Nov;33(6):877–881. doi: 10.1152/jn.1970.33.6.877. [DOI] [PubMed] [Google Scholar]
- Pinsker H. M., Dudek F. E. Bag cell control of egg laying in freely behaving aplysia. Science. 1977 Jul 29;197(4302):490–493. doi: 10.1126/science.197.4302.490. [DOI] [PubMed] [Google Scholar]
- Stuart D. K., Chiu A. Y., Strumwasser F. Neurosecretion of egg-laying hormone and other peptides from electrically active bag cell neurons of Aplysia. J Neurophysiol. 1980 Feb;43(2):488–498. doi: 10.1152/jn.1980.43.2.488. [DOI] [PubMed] [Google Scholar]
- Wallis M. The molecular evolution of pituitary hormones. Biol Rev Camb Philos Soc. 1975 Feb;50(1):35–98. doi: 10.1111/j.1469-185x.1975.tb00989.x. [DOI] [PubMed] [Google Scholar]



