Abstract
Objective
To retrospectively evaluate the depiction of bronchial and non-bronchial systemic arteries with 64-detector row CT in patients undergoing endovascular treatment for life-threatening haemoptysis.
Methods
64-detector row helical CT and conventional angiography of the thorax were performed in 28 patients (25 males, 3 females; age range, 18–65 years; mean age, 40 years) with life-threatening haemoptysis. CT images were analysed to identify abnormal bronchial and non-bronchial systemic arteries and also to localise them in two planes.
Results
Using multidetector CT (MDCT), 43 bronchial arteries were identified on the right side and 46 on the left side. 89% of the right bronchial arteries originated from the right intercostobronchial arteries. A common trunk of origin of the right and left bronchial artery was noted in 46% of cases. 23 non-bronchial systemic arteries were noted on the right side and 41 on the left side. Pleural thickening >3 mm was confirmed to be a good predictor of non-bronchial systemic supply. An internal mammary artery diameter of >3 mm and an inferior phrenic artery diameter of >2 mm were sensitive indicators for non-bronchial systemic supply.
Conclusion
MDCT is a good investigation tool for evaluating life-threatening haemoptysis as it confirms the disease process, identifies the origin and ostial position of bronchial arteries, detects non-bronchial systemic arteries and acts as a roadmap for percutaneous transcatheter embolisation.
Haemoptysis is defined as bleeding that originates from the lower respiratory tract [1]. Various studies in the past have tried to give a fixed volume cut-off for haemoptysis beyond which it was called life threatening [2-8]. But it was later shown that life-threatening status is best defined clinically as any amount of haemoptysis which causes such a degree of impairment of respiratory function so as to threaten life [6,9,10]. Life-threatening haemoptysis has a mortality of 80%, mostly related to asphyxiation, which underlines the effort required to control the bleed [11].
In developing countries like India, the majority of cases are due to tuberculosis [12,13]. Bronchial artery embolisation is a good treatment modality for effective control of life-threatening haemoptysis in developed as well as developing countries and it has a low complication rate [14,15]. Depending on the underlying pathological condition, bronchial arteries, non-bronchial systemic arteries or pulmonary arteries can be a source of haemoptysis [16-21].
The current management protocol for life-threatening haemoptysis varies from place to place and, in general, the initial evaluation is with a chest radiograph. After haemodynamic stabilisation, patients undergo conventional angiography in which an aortogram is produced, followed by a search for bronchial arteries. After the bronchial arteries are located, the non-bronchial systemic arteries are searched for and selective angiographies are performed to detect and embolise them. The role of bronchoscopy is controversial—some considering it to be unnecessary in the management of life-threatening haemoptysis [9].
Methods and materials
Patient population
28 consecutive patients (25 males, 3 females; mean age, 40.2 years; range, 18–65 years) referred to our institution in an 8 month period (February 2008 to October 2008) for endovascular treatment of haemoptysis underwent multidetector row helical CT (MDCT) angiography as part of the pre-therapeutic evaluation. Even though the study was retrospective, ethics committee approval was obtained according to the policy of our institution. All of the patients were cases of primary haemoptysis with no history of embolisation.
Care was taken to avoid administration of contrast material if contraindications existed, such as renal insufficiency (creatinine level >150 mmol l−1), iodine intolerance, or use of biguanide in cases of diabetes mellitus. The present study is a retrospective review of the imaging studies performed in these patients.
A history of antituberculous therapy (ATT) was present in 23 patients. 16 patients had had 6 months of ATT, while another 4 patients had had more prolonged treatment since they were previously treated patients. Three patients were treatment defaulters, failing to complete the ATT course. One patient was sputum positive for tuberculosis during evaluation. Bronchiectasis was the cause of haemoptysis in the other patients. Mean haemoglobin of patients was 10.7 g per 100 ml (range, 4.6–17.0 g per 100 ml).
Multidetector CT angiography technique
CT angiography was performed with a 64-detector row scanner (Brilliance 64; Philips, Amsterdam, Netherlands) for all patients (120 kV, 60–100 mAs, rotation time of 0.5 s, 0.75-mm collimation and pitch of 1.5). The respective mean height of the volume scanned and the mean duration of data acquisition were 350 mm and 10 s.
Patients received 80–100 ml of contrast material (iohexol, Omnipaque™ 350; Amersham Health, Carrigtohill, Ireland) with 350 mg of iodine per millilitre and an injection rate of 4 ml s−1. The automatic bolus-triggering software program was systematically applied, with a circular region of interest positioned at the level of the ascending aorta and a threshold for triggering data acquisition preset at 100 HU.
From each data set, three series of images were systematically reconstructed as follows: contiguous 1-mm-thick transverse CT scans viewed at mediastinal and lung window settings, oblique coronal and sagittal maximum intensity projections (MIPs), and three-dimensional volume-rendered images of the thoracic vascular structures.
CT analysis
CT analysis was carried out in the following steps for cases of life-threatening haemoptysis.
Step 1: study lung parenchyma for disease process
The lung parenchyma was studied in detail, mainly looking for cavities, bronchiectasis and any mass lesion communicating with the bronchi. Aspergilloma within an old tuberculous cavity is an important cause of recurrent haemoptysis and hence was specially searched for, especially in patients with diabetes. Air-space disease with ground-glass changes or consolidation suggests active tuberculous infection. The analysis of lung parenchyma helps to lateralise the disease process when lesions are present in both lungs on chest radiography. It may also locate the probable lesion causing the bleed.
Step 2: measure pleural thickening
As previous studies have related pleural thickening with non-bronchial systemic artery supply and recurrent haemoptysis, pleural thickening was measured in every case of life-threatening haemoptysis [15]. Pleural thickening was measured as the maximum distance between the visceral pleura–lung interface and the parietal pleura–rib interface in the area of pathology (Figure 1).
Figure 1.
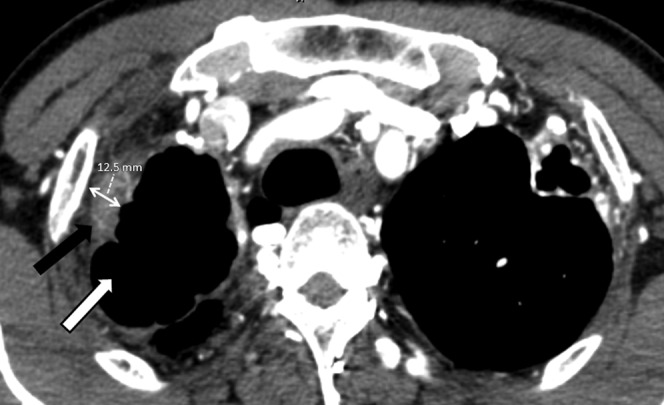
Maximal pleural thickening is measured in the region of pathology. A pleural thickness >3 mm is significant with increased risk of non-bronchial systemic artery supply. This patient has a pleural thickness of 12.5 mm and hence a diligent search was made for these arteries. Pleural thickening (black arrow) is associated with a tuberculous cavity (white arrow) in this patient.
Step 3: determine the bleeding bronchial arteries
Signs of a bleeding vessel on MDCT include
Hypertrophied vessel—diameter >1.5 mm.
Tortuosity of the vessel.
Parenchymal enhancement.
Bronchopulmonary shunt.
An active leak from the vessel.
Step 4: localise the origin of the bronchial artery in three planes
Next, the origin of the bleeding bronchial artery has to be localised in three planes. In the z-axis (craniocaudal), the carina, the vertebral body or the left main bronchus can be used as a reference (Figure 2). During angiography, although the carina and left main bronchus were easily identified, their position varied with respiration compared with the relatively immobile origin of the bronchial and non-bronchial systemic arteries. The vertebral body level was chosen since it is immobile and is the conventional reference. However, a few radiologists prefer to use the carina as the reference owing to the ease of identifying it under fluoroscopy. In the x- and y-axes (axial), the origin of the bronchial artery is plotted with reference to a clock face.
Figure 2.
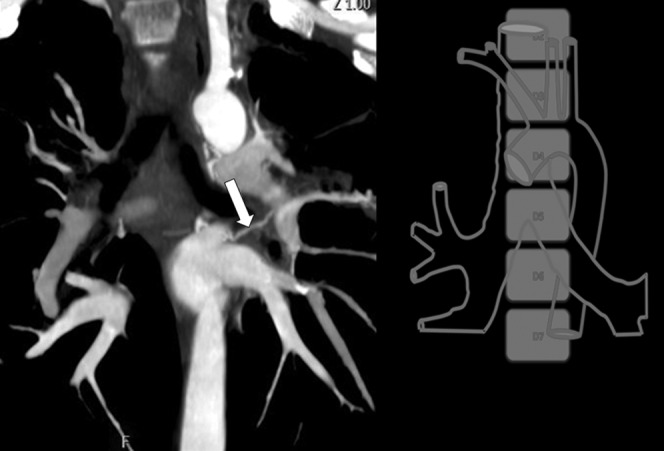
Usage of the z-axis reference level in coronal maximum intensity projection reconstruction. The carina, left main bronchus or the vertebral body level may be used as references for the bronchial artery (white arrow) in the z-axis.
Step 5: map the proximal course of the bronchial artery
The proximal course of the pathological bronchial or intercostobronchial artery was then mapped until the target superselective level using MIP, curved multiplanar reconstruction and three-dimensional shaded surface display techniques (Figure 3). Because an intercostobronchial artery origin of the right bronchial artery is common, it is important to note the division of the intercostals and bronchial trunks to avoid intercostal artery embolisation, which can give rise to pain and other complications. It is important to note the level of origin of any prominent radicular artery or an ectopic artery of Adamkiewicz from the bronchial or intercostobronchial artery. Rarely, coronary or phrenic branches can arise from the bleeding vessel and the origin of these vessels must also be mapped in order to avoid them by super-selective embolisation.
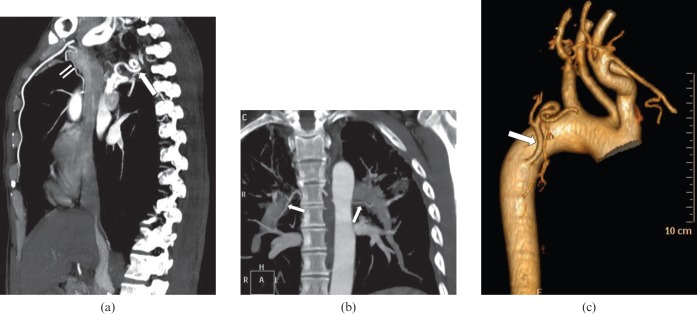
Figure 3.
Multidetector CT evaluation of bronchial arteries. (a) The tortuous course of a hypertrophied right bronchial artery (large arrow) is shown well in this sagittal maximum intensity projection (MIP) image. A non-bronchial systemic artery (small arrows) arising from the internal mammary artery can also be seen in this patient. (b) Coronal MIP image depicts the typical course of bilateral bronchial arteries (arrows) along the superolateral wall of the bronchi. (c) Three-dimensional shaded surface display takes more time for image processing, but is very useful to show the origin, angulations and proximal course in three dimensions. The arrow points to a hypertrophied right bronchial artery.
Step 6: search for and localise non-bronchial systemic arteries and pulmonary vessels causing haemoptysis
Various non-bronchial systemic arteries have been described as potential causes for primary and recurrent haemoptysis and it was necessary to identify these vessels after identifying the bronchial arteries. These vessels assume importance especially when the bronchial arteries are of normal size and when there is marked pleural thickening. When there is no hypertrophied non-bronchial systemic vessel, the pulmonary vessels are finally analysed, as they are rarely a source of life-threatening haemoptysis. Rasmussen aneurysms from the pulmonary vasculature can be seen in the wall of tuberculous cavities and can bleed profusely.
Results
The mean quantity of haemoptysis in the last 3 days prior to admission in patients with cavity was 422 ml whereas it was 248 ml in patients without cavity. The mean quantity of haemoptysis in the last 3 days prior to admission in patients with an aspergilloma was 451 ml, whereas it was 324 ml in patients without an aspergilloma.
It was found that the coronal MIP of the lungs was useful in depicting disease and disease, process capable of producing haemoptysis was noted in all 28 cases. Regarding the causative factors for haemoptysis, it was noted that cavitary disease due to tuberculosis was the most common (64%) followed by bronchiectasis (57%). A combination of cavity with bronchiectasis was noted in 29% of cases. An aspergilloma was present in 29% of cases. An ipsilateral non-bronchial systemic artery supply was seen in 75% of patients with significant right pleural thickening (>3 mm) and 81% of patients with significant left pleural thickening.
Bronchial arteries
89 bronchial arteries were identified in 28 patients by MDCT. The morphological characteristics of bronchial arteries detected on CT are shown in Table 1. 39% of patients had multiple right bronchial arteries (including four cases with triple vessels) and 64% of patients had multiple left bronchial arteries.
Table 1. Morphology of bronchial arteries on multidetector CT.
| Morphology | Right bronchial artery | Left bronchial artery | Common origin of bronchial artery |
| Visualisation in patients | 100% | 100% | 46.4% |
| Total number of vessels identified | 43 | 46 | 15 |
| Patients with single vessel | 17 | 10 | 11 |
| Patients with double vessels | 7 | 18 | 2 |
| Patients with triple vessels | 4 | 0 | 0 |
| Mean diameter of vessel | 1.6 mm | 1.5 mm | 1.5 mm |
| Average number of vessels per case | 1.5 | 1.6 | 0.5 |
| Patients with vessel hypertrophy (>1.5 mm) | 60.7% | 67.9% | 25% |
The level of origin of the bronchial arteries in the z-plane (related to the vertebral body level) is given in Table 2. Orthotopic origin (between D5 and D6 vertebral body level) was found in 91% of right bronchial arteries and 85% of left bronchial arteries. In all cases, the bronchial arteries on both sides were noted to originate between the D4 and D7 vertebral body level.
Table 2. Level of origin of bronchial arteries in the z-axis.
| Artery | D4 | D5 | D6 | D7 | Orthotopic (D5, D6) origin n (%) | Total |
| Right bronchial artery (excluding common trunk origin) | 1 | 19 | 7 | 1 | 26 (92.9) | 28 |
| Left bronchial artery (excluding common trunk origin) | 2 | 12 | 14 | 3 | 26 (83.9) | 31 |
| Common trunk origin of bronchial artery | 1 | 7 | 6 | 1 | 13 (86.7) | 15 |
| Total | 4 | 38 | 27 | 5 | 65 (87.8) | 74 |
When the arteries with a common trunk of origin were excluded, 96.4% of right bronchial arteries were found to have their origin between the 9 and 10 o'clock positions in the axial plane (Figure 4a).
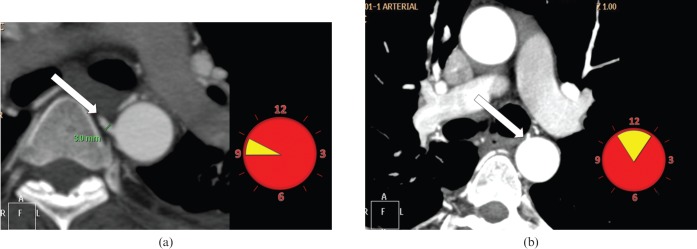
Figure 4.
Clock-face method of depiction of the ostial position of the bronchial artery in the axial plane. (a) A hypertrophied right bronchial artery (arrow) is noted to originate between the 9 and 10′o clock positions. Any bronchial artery with diameter >1.5 mm is supposed to be hypertrophied. The most common location of the right bronchial artery is shown in yellow on the clock face. (b) The left bronchial artery (arrow) is noted to originate between the 11 and 1′o clock positions. The left bronchial artery is slightly more variable in position and hence is more difficult to hook than the right bronchial artery. The most common location of the left bronchial artery is shown in yellow on the clock face.
Table 3. Ostial position of bronchial arteries in the axial plane.
| Artery | n (%)9 o'clock | 10 o'clock | 11 o'clock | 12 o'clock | 1 o'clock | 2 o'clock | 9–10 o'clock | 11–1 o'clock | Total |
| Right bronchial artery (excluding common trunk origin) | 25 | 2 | 0 | 0 | 1 | 0 | 27 (96.4) | 1 (3.6) | 28 |
| Left bronchial artery (excluding common trunk origin) | 2 | 1 | 7 | 12 | 8 | 1 (3.2) | 3 (9.7) | 27 (87.1) | 31 |
| Common trunk origin of bronchial artery | 3 | 0 | 2 | 8 | 2 | 0 | 3 (20) | 12 (80) | 15 |
| Total | 30 | 3 | 9 | 20 | 11 | 1 (1.4) | 33 (44.6) | 40 (54.1) | 74 |
An intercostobronchial origin of the right bronchial artery was noted in 89.3% of right bronchial arteries and they had their origin between the D5 and D6 level in 92% cases with their ostial position between the 9 and 10 o'clock positions in 96% of cases. The mean diameter of the intercostobronchial artery was 2.3 mm. None of the common trunks or the left bronchial arteries had an intercostobronchial origin.
The left bronchial arteries had their ostia between the 9 and 2 o'clock positions, with 87% of left bronchial arteries (excluding common trunk of origin) arising between the 11 and 1 o'clock positions (Figure 4b).
A common trunk of the origin of the bronchial arteries was noted in 46.4% of patients including two patients who had two common trunks in them. They were noted to have their origin between the D5 and D6 level in 87% and were found to have their ostia between the 11 and 1 o'clock positions in 80% of arteries.
Non-bronchial systemic arteries
A total of 64 arteries were identified, and the frequency of supply from various arteries is given in Table 4. The internal mammary artery, intercostal artery and inferior phrenic artery were the common arteries to supply pathological lesions in the lung (Figures 3a and 5a,b). Rare non-bronchial systemic arterial supply from gastric and hepatic arteries was also visualised with MDCT (Figure 5c,d).
Table 4. Frequency of non-bronchial systemic arteries.
| Artery | Right | Left | Total, n (%) |
| Internal mammary artery | 6 | 12 | 18 (28.1) |
| Intercostal artery | 8 | 10 | 18 (28.1) |
| Inferior phrenic artery | 2 | 6 | 8 (12.5) |
| Subclavian artery | 1 | 7 | 8 (12.5) |
| Thyrocervical trunk | 2 | 2 | 4 (6.2) |
| Costocervical trunk | 2 | 1 | 3 (4.7) |
| Lateralthoracic artery | 0 | 1 | 1 (1.6) |
| Acromiothoracic artery | 1 | 0 | 1 (1.6) |
| Pericardiophrenic artery | 1 | 0 | 1 (1.6) |
| Gastric artery | 0 | 1 | 1 (1.6) |
| Hepatic artery | 0 | 1 | 1 (1.6) |
| Total, n (%) | 23 (35.9) | 41 (64.1) | 64 (100) |
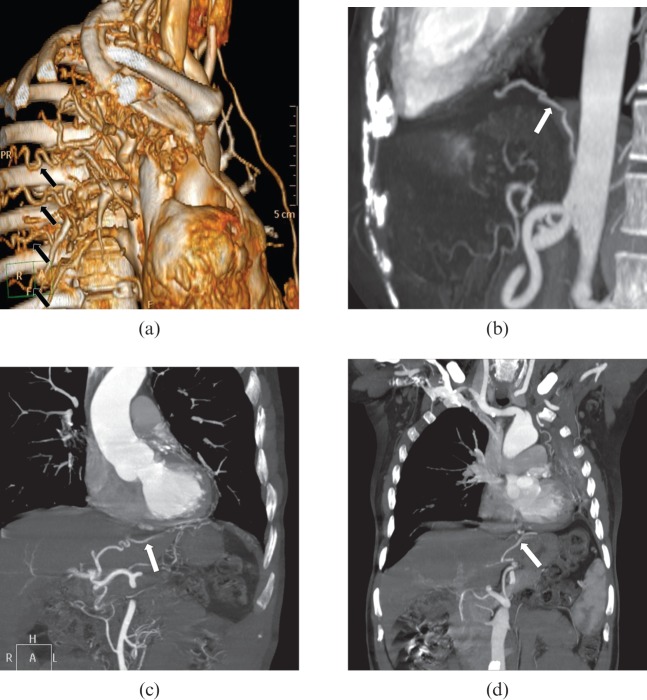
Figure 5.
Multidetector CT evaluation of non-bronchial systemic arteries. (a) Multiple tortuous intercostal arteries (arrows) are seen in this three-dimensional shaded surface display image in a patient with a tuberculous cavity and associated pleural thickening. (b) Oblique sagittal maximum intensity projection (MIP) image shows a hypertrophied inferior phrenic artery (arrow) in a patient with lung base pathology. (c) Branches arising from the left hepatic artery (arrow) supplying a lesion in the lower lobe of the left lung are noted in this oblique coronal MIP image. (d) Hypertrophied vessel (arrow) arising from the left gastric artery is noted to pass to the base of the left lung in a patient with long-standing tuberculosis of the left lung.
Significant pleural thickening (>3 mm) was noted to be 84.6% sensitive and 80% specific for the detection of a non-bronchial arterial supply and the correlation was found to be statistically significant (analysis: “n−1” χ2=22.85, p<0.0001).
The average size of the hypertrophied internal mammary arteries was found to be 3.8 mm. The sensitivity and specificity for a cut-off diameter of 3 mm for the internal mammary artery for non-bronchial systemic supply was noted to be 100%. Similarly, the inferior phrenic arteries were found to be hypertrophied and supplied lung lesions when a lower lobe lung lesion was associated with adjacent pleural thickening. A cut-off diameter of 2 mm for the inferior phrenic arteries had 75% sensitivity and 92% specificity for non-bronchial arterial supply and was also found to be statistically significant (analysis: “n−1” χ2=20.41, p<0.0001).
The origins of the intercostal arteries were found to be in a constant position. Between the D4 and D7 level, all right intercostal arteries were noted to originate from the descending thoracic aorta between the 9 and 10 o'clock positions and all left intercostal arteries between the 6 and 7 o'clock positions.
There was no evidence of active bleeding from either bronchial or non-bronchial systemic arteries on CT. Also, no spinal artery could be identified to originate from the bronchial arteries in the study.
Following MDCT, all patients underwent conventional angiography without the use of digital subtraction and aortography resulting in the successful embolisation of 38 pathological bronchial and 8 non-bronchial arteries. There was no recurrence of haemoptysis at 6 months' follow-up but 3 out of 28 patients (10.7%) had recurrence at 2 years. All three patients with recurrence had significant pleural thickening and multiple non-bronchial systemic arterial feeders, mainly from the intercostal arteries. Two of these patients underwent successful repeat embolisation, while one patient underwent surgery.
Discussion
Owing to the fact that the Cauldwell classification of bronchial arteries [22] omits mention of their common origin along with variations in bronchial arteries outside the 10 groups proposed by Uflacker et al [24], the authors feel that it is better not to classify them, but instead describe them with respect to their number, origin and course for every case. The previous reports had emphasised that >70% of bronchial arteries arise from the descending aorta [22-24]. In this study, all bronchial arteries on both sides were noted to originate between the D4 and D7 vertebral body level.
The fact that the majority of right bronchial arteries arise in the medial wall of the descending aorta whereas most left bronchial arteries arise in the anterior wall of the descending aorta is confirmed in this study [21,25,26]. The ostia of the right bronchial artery were commonly found to be between the 9 and 10 o'clock positions and those of the left bronchial artery between the 11 and 1 o'clock positions. The left bronchial artery is slightly more variable in position and hence it is more difficult to hook than the right bronchial artery. The intercostal arteries were also found to have a fixed ostial position. These data may be extremely useful for the interventional radiologist and help to avoid thoracic aortography. Catheters can also be selected earlier, since the angulation and diameter of the vessel are already known. There was a reduction in the quantity of contrast used during angiography, because there was no aortogram and the search for pathological vessels was much easier. However, this is countered by the contrast used for the CT angiogram and the total volume of contrast used is similar when conventional angiography is performed with or without a prior MDCT bronchial angiogram.
Significant pleural thickening (≥3 mm) and a hypertrophied internal mammary artery (≥3 mm) or inferior phrenic artery (≥2 mm) may be used as predictors for non-bronchial systemic arterial supply. Pleural thickening has already been shown to be associated with non-bronchial systemic supply, failure of identification of which may lead to recurrent haemoptysis [15,27,28]. Multiple non-bronchial systemic arteries were detected in this study with as many as 12 different arteries in a single patient and this indicates the need for a search for such arteries prior to embolotherapy in order to prevent recurrent haemoptysis.
These non-bronchial systemic arteries were found to be more common on the left side (41 on the left compared with 23 on the right). Because recurrent haemoptysis after a successful bronchial artery embolisation may be related to the presence of a non-bronchial systemic arterial supply, one should emphasise the usefulness of its depiction with CT angiography prior to the second embolisation session. Of particular importance are the subclavian artery and its branches (most commonly, the internal mammary artery) for upper lobe bleeding and the inferior phrenic artery for lower lobe bleeding [16,17,19-21,29,30]. However, numerous additional vessels may give rise to a non-bronchial systemic arterial supply to the lung, such as the branches of the axillary arteries, the intercostal arteries, or the hepatic and gastric arteries [15,31,32]. Hence MDCT angiography can help in the planning of a focused and efficient non-bronchial systemic artery embolisation, as was recently reported with single-detector row CT [25,33].
Because the most dreaded consequence of a bronchial artery embolisation is inadvertent occlusion of the spinal arteries, we included the search for an anterior spinal artery on curved MIPs of the cervicothoracic junction in all patients. The lack of identification of this vessel on CT and/or conventional angiograms in these patients precludes any conclusion regarding the accuracy of CT angiography in depicting this important collateral, which is present in about 5% of patients [25,34].
The main limitations of this study were the non-availability of digital subtraction in our institution and that the study was not blinded. The interventional radiologist already knew about the position of the bronchial arteries and selectively imaged only the pathological vessels. This may be the reason for the low detection rate of bronchial arteries with conventional angiography in this study.
By using MDCT angiography as the primary investigation after haemodynamic stabilisation, patients can proceed directly to conventional angiography and the pathological bronchial artery can be precisely located easily and can be embolised. There will be no need to perform an aortogram and the time and contrast required to search for bronchial and non-bronchial arteries will be saved.
In cases of haemodynamic instability when CT cannot be performed and patient directly undergoes angiography, the bronchial arteries must be searched for first in their most common location between the D4 and D7 levels on the z-axis with the right artery between the 9 and 10 o'clock positions and the left artery between the 11 and 1 o'clock positions in the axial plane. Whenever there is significant pleural thickening, a thorough search must be made for non-bronchial systemic arteries, as these may cause recurrent haemoptysis.
Conclusion
This study has shown that MDCT is an important diagnostic tool in the evaluation of life-threatening haemoptysis. It aids in the diagnosis of the disease process and detects pleural thickening, which indicates a high risk of recurrence due to non-bronchial arterial feeders. Axial localisation of the bronchial arteries by the clock method and z-axis (craniocaudal) localisation with reference to the vertebral body level were found to be very helpful in the selective catheterisation of bronchial arteries and aortography may no longer be required prior to embolisation.
64-detector row helical CT may be particularly more useful for detection of a common origin of the bronchial arteries and to detect non-bronchial systemic arteries, which is important to reduce recurrence of haemoptysis. Hypertrophied internal mammary and inferior phrenic arteries can be easily identified and size criteria may be used to predict non-bronchial systemic supply. The protocol for management of life-threatening haemoptysis may be changed with MDCT bronchial angiography being the primary investigation for life-threatening haemoptysis followed by treatment with embolisation.
References
- 1.Fraser RS, Pare P, Pare PD, eds. Diseases of the chest. Philadelphia, PA: Saunders; 1988. 394–6 [Google Scholar]
- 2.Marshall TJ, Jackson JE. Vascular intervention in the thorax: bronchial artery embolization for hemoptysis. Eur Radiol 1997;7:1221–7 [DOI] [PubMed] [Google Scholar]
- 3.Najarian KE, Morris CS. Arterial embolization in the chest. J Thorac Imaging 1998;13:93–104 [DOI] [PubMed] [Google Scholar]
- 4.Magilligan DJ, Jr, Ravipati S, Zayat P, Shetty PC, Bower G, Kvale P. Massive hemoptysis: control by transcatheter bronchial artery embolization. Ann Thorac Surg 1981;32:392–400 [DOI] [PubMed] [Google Scholar]
- 5.Fernando HC, Stein M, Benfield JR, Link DP. Role of bronchial artery embolization in the management of hemoptysis. Arch Surg 1998;133:862–6 [DOI] [PubMed] [Google Scholar]
- 6.Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000;28:1642–7 [DOI] [PubMed] [Google Scholar]
- 7.Andersen PE. Imaging and interventional radiological treatment of hemoptysis. Acta Radiol 2006;47:780–92 [DOI] [PubMed] [Google Scholar]
- 8.Koblízek V, Chovanec V, Krajina A, Salajka F, Lojik M, Raupach J, et al. The role of bronchial artery embolization in the treatment of hemoptysis. [In Czech.] Vnitr Lek 2006;52:1162–71 [PubMed] [Google Scholar]
- 9.Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001;177:861–7 [DOI] [PubMed] [Google Scholar]
- 10.Mal H, Rullon I, Mellot F, Brugière O, Sleiman C, Menu Y, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest 1999;115:996–1001 [DOI] [PubMed] [Google Scholar]
- 11.Uflacker R, Kaemmerer A, Neves C, Picon PD. Management of massive hemoptysis by bronchial artery embolization. Radiology 1983;146:627–34 [DOI] [PubMed] [Google Scholar]
- 12.Middleton JR, Sen P, Lange M, Salaki J, Kapila R, Louria DB. Death-producing hemoptysis in tuberculosis. Chest 1977;72:601–4 [DOI] [PubMed] [Google Scholar]
- 13.Davis CE, Jr, Carpenter JL, McAllister CK, Matthews J, Bush BA, Ognibene AJ. Tuberculosis. Cause of death in antibiotic era. Chest 1985;88:726–9 [DOI] [PubMed] [Google Scholar]
- 14.Bin SarwarZubairi A, Tanveer-ul-Haq , Fatima K, Azeemuddin M, Zubairi MA, Irfan M. Bronchial artery embolization in the treatment of massive hemoptysis. Saudi Med J 2007;28:1076–9 [PubMed] [Google Scholar]
- 15.Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life threatening hemoptysis: a comprehensive review. Radiographics 2002;22:1395–409 [DOI] [PubMed] [Google Scholar]
- 16.Vujic I, Pyle R, Parker E, Mithoefer J. Control of massive hemoptysis by embolization of intercostal arteries. Radiology 1980;137:617–20 [DOI] [PubMed] [Google Scholar]
- 17.Vujic I, Pyle R, Hungerford GD, Griffin CN. Angiography and the therapeutic blockade in the control of hemoptysis. Radiology 1982;143:19–23 [DOI] [PubMed] [Google Scholar]
- 18.Remy J, Lemaitre L, Lafitte JJ, Vilain MO, Saint Michel J, Steenhouwer F. Massive hemoptysis of pulmonary arterial origin: diagnosis and treatment. AJR Am J Roentgenol 1984;143:963–9 [DOI] [PubMed] [Google Scholar]
- 19.Rabkin JE, Astafjev VI, Gothman LN, Grigorjev YG. Transcatheter embolization in the management of pulmonary hemorrhage. Radiology 1987;163:361–5 [DOI] [PubMed] [Google Scholar]
- 20.Keller FS, Rosch F, Loflin TG, Nath PH, McElvein RB. Nonbronchial systemic collateral arteries: significance in percutaneous embolotherapy for hemoptysis. Radiology 1987;164:687–92 [DOI] [PubMed] [Google Scholar]
- 21.Jardin M, Remy J. Control of hemoptysis: systemic angiography and anastomoses of the internal mammary artery. Radiology 1988;168:377–83 [DOI] [PubMed] [Google Scholar]
- 22.Cauldwell E, Siekert R, Lininger R. The bronchial arteries: an anatomic study of 150 human cadavers. Surg Gynecol Obstet 1948;86:395–412 [PubMed] [Google Scholar]
- 23.Botenga AS. Selective bronchial and intercostals arteriography. Leiden, the Netherlands: Stenfert Kroese; 1970 [Google Scholar]
- 24.Uflacker R, Kaemmerer A, Picon PD, Rizzon CF, Neves CM, Oliveira ES, et al. Bronchial artery embolization in the management of hemoptysis: technical aspects and long-term results. Radiology 1985;157:637–44 [DOI] [PubMed] [Google Scholar]
- 25.Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 2004;233:741–9 [DOI] [PubMed] [Google Scholar]
- 26.Furuse M, Saito K, Kunieda E, Aihara T, Touei H, Ohara T, et al. Bronchial arteries: CT demonstration with arteriographic correlation. Radiology 1987;162:393–8 [DOI] [PubMed] [Google Scholar]
- 27.Tamura S, Kodama T, Otsuka N, Kihara Y, Nisikawa K, Yuki Y, et al. Embolotherapy for persistent hemoptysis: The significance of pleural thickening. Cardiovasc Intervent Radiol 1993;16:85–8 [DOI] [PubMed] [Google Scholar]
- 28.Wong ML, Szkup P, Hopley MJ. Percutaneous embolotherapy for life-threatening hemoptysis. Chest 2002;121:95–102 [DOI] [PubMed] [Google Scholar]
- 29.Yu-Tang Goh P, Lin M, Teo N, En ShenWong D. Embolization for hemoptysis: a six-year review. Cardiovasc Intervent Radiol 2002;25:17–25 [DOI] [PubMed] [Google Scholar]
- 30.Moore LB, McWey RE, Vujic I. Massive hemoptysis: control by embolization of the thyrocervical trunk. Radiology 1986;161:173–4 [DOI] [PubMed] [Google Scholar]
- 31.Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest 2002;121:789–95 [DOI] [PubMed] [Google Scholar]
- 32.Sellars N, Belli AM. Nonbronchial collateral supply from the left gastric artery in massive hemoptysis. Eur Radiol 2001;11:76–9 [DOI] [PubMed] [Google Scholar]
- 33.Yoon W, Kim YH, Kim JK, Kim YC, Park JG, Kang HK. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology 2003;227:232–8 [DOI] [PubMed] [Google Scholar]
- 34.Bookstein JJ, Moser KM, Kalafer ME, Higgins CB, Davis GB, James WS. The role of bronchial arteriography and therapeutic embolization in hemoptysis. Chest 1977;72:658–61 [DOI] [PubMed] [Google Scholar]