Abstract
Objective
The purpose of this study was to compare the proton-density (PD)-weighted fast spin-echo (FSE) and fast-recovery FSE (FRFSE) sequences for the evaluation of the anatomical structures of the knee.
Method
24 healthy volunteers who underwent MRI by both sagittal PD-weighted FSE and FRFSE sequences were evaluated. The signal-to-noise ratio, contrast-to-noise ratio (CNR) and anatomical detail visualisation were compared for the two techniques.
Results
The mean CNRs and reader ratings for both readers were significantly higher for the PD-weighted FRFSE images than for the PD-weighted FSE images in the cartilages/the femorotibial joint effusion and the cruciate ligaments/the effusion around the cruciate ligaments; however, the mean CNRs and reader ratings for both readers were significantly higher for the PD-weighted FSE sequences than for the PD-weighted FRFSE sequences in the cartilages/the menisci and the cruciate ligaments.
Conclusions
The main advantages of the PD-weighted FRFSE sequence are the increase in contrast between fluid and non-fluid tissues and the time saved by using the procedure. However, in the absence of joint effusion, the PD-weighted FRFSE sequence generates a poorer contrast between the cartilage and meniscus, the cruciate ligaments and fat of the intercondylar fossa.
MRI of the knee is traditionally done with multiple two-dimensional (2D) multislice acquisitions. Fast spin-echo (FSE) is commonly used to provide proton-density (PD) or T2 weighted images in a reasonable scanning time. These images are useful to look for internal derangements such as meniscal tears [1,2], ligamentous injury [3] or cartilage damage [4,5].
2D FSE has limitations in examinations of the knee. The voxels are not isotropic, with relatively thick slices compared with the in-plane resolution, leading to partial volume artefacts. In addition, because of the anisotropic nature of the voxels, these images do not lend themselves to reformations. Magnetisation transfer due to slice selection can decrease the signal in cartilage or muscle [6]. Finally, slice gaps do not permit accurate quantification of structures such as cartilage volume.
Using isotropic three-dimensional (3D) T2 or PD-weighted techniques may solve these problems and potentially optimise visualisation of knee abnormalities [7,8]. Isotropic voxels would allow reformations with different slice thicknesses in any imaging plane, reducing the imaging time by eliminating the need to acquire sequences in multiple imaging planes. The 3D FSE techniques were developed by several investigators. Mugler [9] and Park et al [10] first described a 3D FSE sequence with variable flip angles and long echo trains and established the technique for brain imaging. Subsequently, a new fast-recovery 3D FSE sequence with a longer echo train acquisition [3D XETA (eXtended Echo Train Acquisition)] was developed by Busse et al [11] for knee imaging and was evaluated by Gold et al [7] to optimise the echo time (TE) and echo train length (ETL) as well as to improve scanning efficiency. This sequence allowed acquisition of T2 or PD-weighted 3D FSE images with isotropic resolution within an acceptable data acquisition time with minimal blurring for clinical knee MRI at 1.5 T.
Certainly, the 3D FSE sequence is an excellent technique for evaluation of the knee, especially in terms of avoiding the partial volume artefacts and magnetisation transfer effects. However, it is difficult to completely replace the 2D FSE sequence with the 3D one. Because the 3D FSE sequence requires long acquisition times, there is an increased opportunity for patient motion. Owing to this constraint, shorter 2D sequences are often preferred, although they do not permit the free 3D multiplanar reformatting without loss of image quality in the three major anatomical planes that 3D sequences provide.
2D fast-recovery FSE (FRFSE) was developed to increase the fluid signal in short repetition time (TR) imaging [12]. This sequence, similar to driven-equilibrium imaging [12-15], tips magnetisation back to the z-axis after each TR. Otherwise, this sequence has limitations similar to 2D FSE with respect to anisotropic voxels and magnetisation transfer effects [7]. This technique can be used either for T2 weighted imaging (long TEs) with relatively short TRs or for T1 like imaging (short TEs) with an artificially increased signal intensity of free water. However, no investigator has explored the performance of the 2D PD-weighted FRFSE sequence for the evaluation of the knee.
The purpose of this study was to compare the 2D PD-weighted FSE sequence with the 2D PD-weighted FRFSE sequence for the evaluation of the knee. For technical assessment and visualisation of anatomical structures, a volunteer study was conducted. Technical assessment included a comparison of the signal-to-noise ratio (SNR) and the contrast-to-noise ratio (CNR) of relevant anatomical structures. Furthermore, the visualisation of anatomical details of both sequences was compared.
Methods and materials
Patients
The institutional review board gave its approval, and informed consent was obtained from all subjects included in this study. MRI was performed on 24 healthy volunteers (8 right knees, 16 left knees; 10 females, 14 males; age range 24–75 years). The volunteers had no history of knee pain or prior surgery in either knee.
MRI
All MRI scans were acquired on a 1.5 T whole-body MR system (Signa® Horizon; GE Medical Systems, Milwaukee, WI) using a dedicated eight-channel receive-only knee coil. A custom leg holder, with the knee in approximately 20° of flexion, was used to minimise motion and position the coil.
Sagittal PD-weighted FSE images were obtained using the following parameters: TR/TE=3500/28 ms, ETL=6, slice thickness=4 mm, 3.2 mm gap, field of view (FOV)=16 cm, matrix=256×192, number of excitations=2, bandwidth=±15.2 kHz and total acquisition time=3 min 51 s.
Sagittal PD-weighted FRFSE images were obtained using the following parameters: TR/TE=2400/24 ms, ETL=6, slice thickness=4 mm, 3.2 mm gap, FOV=16 cm, matrix=256×192, number of excitations=2, bandwidth=±15.2 kHz and total acquisition time=2 min 19 s.
Semi-quantitative imaging analysis
The sagittal PD-weighted FSE and FRFSE images were evaluated for SNR and CNR. Regions of interest (ROIs) for determining signal intensities were defined in the following tissue types: the anterior and posterior horn of the meniscus, the medial femoral and tibial cartilage, the lateral femoral and tibial cartilage, the anterior cruciate ligament (ACL), the posterior cruciate ligament (PCL), the fat of the intercondylar fossa, the medial head of the gastrocnemius muscle, the suprapatellar bursal effusion, the femorotibial effusion and background. The measurements were performed by one musculoskeletal radiologist (OT) in the interpretation of MRI of the knee who was not blinded to the sequence, using a standard console (Image VINS Pro; Yokogawa Electric Corporation, Tokyo, Japan). The standard deviation (SD) of the noise was measured from a single ROI placed in the background.
The size of each ROI within the anterior horn of the medial meniscus varied from 0.89 to 3.97 cm2 (mean 2.53 cm2), the posterior horn of the medial meniscus from 1.05 to 6.18 cm2 (mean 2.94 cm2), the anterior horn of the lateral meniscus from 0.97 to 4.65 cm2 (mean 2.43 cm2), the posterior horn of the lateral meniscus from 0.98 to 5.46 cm2 (mean 2.60 cm2), the medial femoral cartilage from 0.39 to 1.57 cm2 (mean 0.70 cm2), the medial tibial cartilage from 0.36 to 1.54 cm2 (mean 0.71 cm2), the lateral femoral cartilage from 0.36 to 1.94 cm2 (mean 0.85 cm2), the lateral tibial cartilage from 0.41 to 1.85 cm2 (mean 0.84 cm2), the ACL from 0.49 to 3.58 cm2 (mean 1.73 cm2), the PCL from 0.84 to 5.05 cm2 (mean 2.67 cm2), the medial head of the gastrocnemius muscle from 7.85 to 29.45 cm2 (mean 18.47 cm2), the suprapatellar bursal effusion from 0.45 to 16.7 cm2 (mean 5.87 cm2), the femorotibial effusion from 0.31 to 2.75 cm2 (mean 1.10 cm2) and the air near the lesion from 11.65 to 27.91 cm2 (mean 20.10 cm2).
The SNR of the regions was calculated as SNR=the mean signal intensity (SI) of the regions/SD of air near the regions. The SNR calculation was performed for the anterior and posterior horn of the meniscus, the medial femoral and tibial cartilage, the lateral femoral and tibial cartilage, the ACL, the PCL, the fat on the intercondylar fossa, the medial head of the gastrocnemius muscle, the suprapatellar bursal effusion and the femorotibial effusion.
The CNR of the regions was calculated as CNR=(|the mean SI of the region−the mean SI of the normal structure adjacent to the region|)/SD of air near the lesion. The CNR calculation was performed for the medial femoral cartilage/posterior horn of the medial meniscus, the medial tibial cartilage/posterior horn of the medial meniscus, the lateral femoral cartilage/posterior horn of the lateral meniscus, the lateral tibial cartilage/posterior horn of the lateral meniscus, the ACL/the fat of the intercondylar fossa, the PCL/the fat of the intercondylar fossa and the medial head of the gastrocnemius muscle/the fat of the intercondylar fossa.
Qualitative imaging analysis
An additional qualitative analysis was performed by two musculoskeletal radiologists (OT, with 20 years of experience; YH, with 9 years of experience). Both readers were blinded to the clinical data. First, both readers (OT, YH) separately reviewed sagittal PD-weighted FSE images. To reduce a potential learning bias, both readers separately reviewed sagittal PD-weighted FRFSE images after 2 weeks. The images were presented in random order to each of the readers at each session. Both readers were asked to grade the image quality of the menisci, cartilage, ligaments (ACL and PCL), muscle (medial head of the gastrocnemius muscle), suprapatellar bursal effusion and femorotibial joint effusion using the following criteria: edge sharpness, amount of blurring, artefacts, contrast between fluid and cartilage, contrast between fluid and soft tissue, delineation of ligamentous structures and muscle, and amount of noise. To assess the visualisation quality, the anatomical structures were assigned scores ranging from 1 to 4 (1=poor, 2=acceptable, 3=good, 4=excellent) by both readers.
Statistical analysis
The statistical analysis was performed by using the Statcel software program (v. 7.0; OMS Inc., Tokyo, Japan). p-values <0.05 were considered to indicate a statistically significant difference. First, the PD-weighted FSE and FRFSE images were compared pairwise with respect to the SNR and CNR of the anatomical structures using the Wilcoxon signed-ranked test. Second, the reader ratings of the visual assessment of the normal structures with a four-point scale for both imaging results were compared using the Wilcoxon signed-ranked test. Third, the κ value was calculated to assess interobserver variability in the assignment of an image quality of the normal structures by using the Excel software program (Excel Statistics 2008 for Windows; SSRI Inc., Tokyo, Japan).
The level of agreement was defined as follows: κ values of <0.00 indicated no agreement; κ values of 0.00–0.40 indicated a poor agreement; κ values of 0.41–0.75 represented a good agreement; and κ values of 0.76–1.00 represented an excellent agreement.
Results
SNR comparisons for the PD-weighted FSE vs FRFSE images of the normal structures of the knee are listed in Table 1. In the posterior horn of the medial meniscus (p<0.05), the anterior (p<0.05) and posterior horns of the lateral meniscus (p<0.001), the medial and lateral femoral cartilage (p<0.001), the medial and lateral tibial cartilage (p<0.001), the ACL (p<0.001), the PCL (p<0.001), the medial head of the gastrocnemius muscle (p<0.001), the fat of the intercondylar fossa (p<0.05), the fluid in the suprapatellar bursa (p<0.05) and the fluid in the femorotibial joint (p<0.05), the mean SNRs were significantly higher for the PD-weighted FSE images than for the PD-weighted FRFSE images.
Table 1. Signal-to-noise ratio comparison of fast spin-echo (FSE) and fast-recovery FSE (FRFSE) PD-weighted sequences of the knee.
| Anatomical structures of the knee | Proton-density-weighted FSE imaging | Proton-density-weighted FRFSE imaging | z score | p-value |
| Meniscus | ||||
| Anterior horn of the medial meniscus | 3.82 | 2.87 | −2.57 | <0.05 |
| Posterior horn of the medial meniscus | 4.13 | 3.6 | −3.29 | <0.05 |
| Anterior horn of the lateral meniscus | 3.78 | 3.38 | −2.8 | <0.05 |
| Anterior horn of the lateral meniscus | 3.48 | 2.93 | −3.43 | <0.001 |
| Cartilage | ||||
| Medial femoral cartilage | 17.4 | 14.77 | −4.26 | <0.001 |
| Medial tibial cartilage | 16.16 | 13.83 | −4.17 | <0.001 |
| Lateral femoral cartilage | 19.03 | 15.9 | −4.26 | <0.001 |
| Lateral tibial cartilage | 17.9 | 15.03 | −4.29 | <0.001 |
| Ligament | ||||
| Anterior cruciate ligament | 8.11 | 6.89 | −3.49 | <0.001 |
| Posterior cruciate ligament | 5.32 | 4.12 | −3.91 | <0.001 |
| Muscle | ||||
| Medial head of the gastrocnemius muscle | 17.08 | 13.25 | −4.26 | <0.001 |
| Others | ||||
| Fat of the intercondylar fossa | 42.17 | 40.71 | −2.17 | <0.05 |
| Fluid in the suprapatellar bursa | 37.25 | 39.41 | −2 | <0.05 |
| Fluid in the femorotibial joint | 31.2 | 33.7 | −2.2 | <0.05 |
CNR comparisons for the PD-weighted FSE vs FRFSE images of the normal structures of the knee are listed in Table 2. The mean CNRs were significantly higher for the PD-weighted FSE images than for the PD-weighted FRFSE images in the anterior horn of the medial meniscus as compared with the medial femoral cartilage (p<0.05), the posterior horn of the medial meniscus as compared with the medial femoral cartilage (p<0.001), the anterior horn of the lateral meniscus as compared with the lateral femoral cartilage (p<0.05), and the posterior horn of the lateral meniscus as compared with the lateral femoral cartilage (p<0.001) (Figure 1). In the medial femoral (Figure 2) and tibial cartilage as compared with fluid in the medial femorotibial joint (p<0.001), the lateral femoral cartilage as compared with fluid in the lateral femorotibial joint (p<0.001), the lateral tibial cartilage as compared with fluid in the lateral femorotibial joint (p<0.05), and the medial head of the gastrocnemius muscle as compared with fat of the intercondylar fossa (p<0.05), the mean CNRs were also significantly higher for the PD-weighted FSE images than for PD-weighted FRFSE images. However, there were no significant differences between the mean CNRs for the PD-weighted FSE images and those for the PD-weighted FRFSE images in comparison with the ACL/fat of the intercondylar fossa (p=0.91) and the PCL/fat of the intercondylar fossa (p=0.67).
Table 2. Contrast-to-noise ratio comparison of fast spin-echo (FSE) and fast-recovery FSE (FRFSE) PD-weighted sequences of the knee.
| Anatomical structures of the knee | Proton-density-weighted FSE imaging | Proton-density-weighted FRFSE imaging | z score | p-value |
| Cartilage | ||||
| Anterior horn of the medial meniscus/medial femoral cartilage | 3.82 | 2.86 | −2.57 | <0.05 |
| Posterior horn of the medial meniscus/medial femoral cartilage | 4.13 | 3.29 | −3.29 | <0.001 |
| Anterior horn of the lateral meniscus/lateral femoral cartilage | 3.78 | 3.38 | −2.8 | <0.05 |
| Posterior horn of the lateral meniscus/lateral femoral cartilage | 3.48 | 2.93 | −3.43 | <0.001 |
| Medial femoral cartilage/fluid in the medial femorotibial joint | 13.8 | 16.84 | −3.4 | <0.001 |
| Medial tibial cartilage/fluid in the medial femorotibial joint | 15.04 | 17.78 | −3.74 | <0.001 |
| Lateral femoral cartilage/fluid in the lateral femorotibial joint | 12.06 | 15.71 | −4.03 | <0.001 |
| Lateral tibial cartilage/fluid in the lateral femorotibial joint | 12.05 | 14.21 | −2 | <0.05 |
| Ligament | ||||
| Anterior cruciate ligament/fat of the intercondylar fossa | 34.01 | 33.97 | −0.11 | 0.91 |
| Posterior cruciate ligament/fat of the intercondylar fossa | 36.81 | 36.59 | −0.43 | 0.67 |
| Muscle | ||||
| Medial head of the gastrocnemius muscle/fat of the intercondylar fossa | 25.09 | 26.69 | −2.97 | <0.05 |
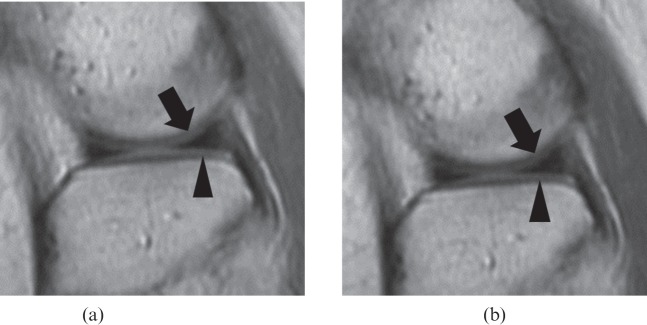
Figure 1.
A 44-year-old female volunteer. Visual comparison of proton density (PD)-weighted (a) fast spin-echo (FSE) and (b) fast-recovery FSE (FRFSE) images. Compared with the PD-weighted FSE image [repetition time (TR)/echo time (TE)=3500/28, echo train length=6], the PD-weighted FRFSE image (TR/TE=2400/24, echo train length=6) provides a poor distinction between the femoral (arrow) and tibial cartilage (arrow head) and the meniscus. The PD-weighted FRFSE image provides lower signal intensity for cartilage and lower contrast-to-noise ratio for cartilage/meniscus.
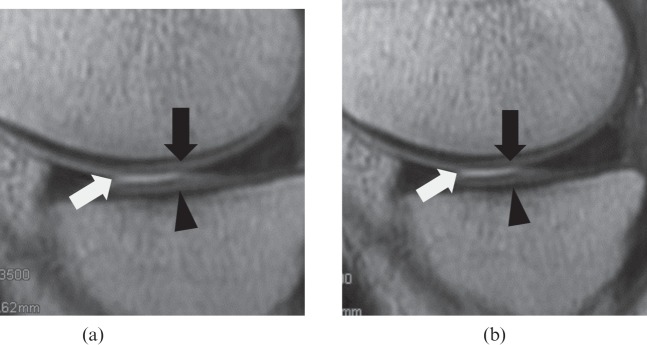
Figure 2.
A 41-year-old male volunteer. Visual comparison of proton density (PD)-weighted (a) fast spin-echo (FSE) and (b) fast-recovery FSE (FRFSE) images. Compared with the PD-weighted FSE image [repetition time (TR)/echo time (TE)=3500/28, echo train length=6], the PD-weighted FRFSE image (TR/TE=2400/24, echo train length=6) provides a better distinction between the femoral (black arrow) and tibial cartilage (arrow head), and femorotibial effusion (white arrow). The PD-weighted FRFSE image provides lower signal intensity for cartilage, higher signal intensity for joint effusion, and higher contrast-to-noise ratio for cartilage/joint effusion.
The reader ratings of subjective imaging contrast of the normal structures of the knee are listed in Tables 3 and 4. The mean ratings for the PD-weighted FSE images were significantly higher than those for the PD-weighted FRFSE images for Reader 1, for the anterior and posterior horn of the medial meniscus (p<0.05), the ACL (p<0.05), the PCL (p<0.05), the medial head of the gastrocnemius muscle (p<0.001), the fluid in the suprapatellar bursa (p<0.05) and the fluid in the femorotibial joint (p<0.05). Furthermore, in the medial femoral and tibial cartilage as compared with the posterior horn of the medial meniscus (p<0.001), the lateral femoral and tibial cartilage as compared with the posterior horn of the lateral meniscus (p<0.001), the medial femoral and tibial cartilage as compared with fluid in the medial femorotibial joint (p<0.001), and the lateral femoral and tibial cartilage as compared with fluid in the lateral femorotibial joint (p<0.001), the mean ratings for the PD-weighted FSE images were significantly higher than those for the PD-weighted FRFSE images for Reader 1. However, there were no significant differences between the mean ratings for PD-weighted FSE and FRFSE images for Reader 1, for the anterior (p=0.16) and posterior horn of the lateral meniscus (p=0.16). For Reader 2, the mean ratings for the PD-weighted FSE images were significantly higher than those for the PD-weighted FRFSE images, for the posterior horn of the medial meniscus (p<0.05), the ACL (p<0.05), the PCL (p<0.05) and the medial head of the gastrocnemius muscle (p<0.001). Furthermore, in the medial femoral and tibial cartilage as compared with the posterior horn of the medial meniscus (p<0.001), the lateral femoral cartilage as compared with the posterior horn of the lateral meniscus (p<0.001), the lateral tibial cartilage as compared with the posterior horn of the lateral meniscus (p<0.05), the medial femoral and tibial cartilage as compared with fluid in the medial femorotibial joint (p<0.001), the lateral femoral cartilage as compared with fluid in the lateral femorotibial joint (p<0.001), and the lateral tibial cartilage as compared with fluid in the lateral femorotibial joint (p<0.05), the mean ratings for the PD-weighted FSE images were significantly higher than those for the PD-weighted FRFSE images for Reader 2. However, there were no significant differences between the mean ratings for PD-weighted FSE and FRFSE images for Reader 2, for the anterior horn of the medial meniscus (p=0.71), the anterior (p=0.08) and posterior (p=0.65) horns of the lateral meniscus, the fluid in the suprapatellar bursa (p=0.06) and the fluid in the femorotibial joint (p=0.37).
Table 3. Ratings of fast spin-echo (FSE) and fast-recovery FSE (FRFSE) proton-density-weighted sequences of the knee for Reader 1.
| Anatomical structures of the knee | Proton-density weighted FSE imaging | Proton-density-weighted FRFSE imaging | z score | p-value |
| Meniscus | ||||
| Anterior horn of the medial meniscus | 3.79 | 3.67 | −1.73 | <0.05 |
| Posterior horn of the medial meniscus | 3.75 | 3.46 | −2.65 | <0.05 |
| Anterior horn of the lateral meniscus | 3.83 | 3.67 | −1.41 | 0.16 |
| Posterior horn of the lateral meniscus | 3.83 | 3.63 | −1.41 | 0.16 |
| Cartilage | ||||
| Medial femoral cartilage/posterior horn of the medial meniscus | 3.83 | 3.21 | −3.88 | <0.001 |
| Medial tibial cartilage/posterior horn of the medial meniscus | 3.42 | 2.67 | −4.24 | <0.001 |
| Lateral femoral cartilage/posterior horn of the lateral meniscus | 3.89 | 3.25 | −3.64 | <0.001 |
| Lateral tibial cartilage/posterior horn of the lateral meniscus | 3.83 | 3.21 | −3.64 | <0.001 |
| Medial femoral cartilage/fluid in the medial femorotibial joint | 2.79 | 3.58 | −4.36 | <0.001 |
| Medial tibial cartilage/fluid in the medial femorotibial joint | 2.79 | 3.38 | −3.74 | <0.001 |
| Lateral femoral cartilage/fluid in the lateral femorotibial joint | 2.96 | 3.67 | −4.12 | <0.001 |
| Lateral tibial cartilage/fluid in the lateral femorotibial joint | 2.96 | 3.71 | −4.24 | <0.001 |
| Anterior cruciate ligament | 2.87 | 3.17 | −2.71 | <0.05 |
| Posterior cruciate ligament | 3.33 | 3.71 | −3.00 | <0.05 |
| Muscle | ||||
| Medial head of the gastrocnemius muscle | 3.00 | 3.88 | −4.38 | <0.001 |
| Others | ||||
| Fluid in the suprapatellar bursa | 2.67 | 3.13 | −2.84 | <0.05 |
| Fluid in the femorotibial joint | 3.63 | 3.91 | −2.65 | <0.05 |
Table 4. Ratings of fast spin-echo (FSE) and fast-recovery FSE (FRFSE) proton-density-weighted sequences of the knee for Reader 2.
| Anatomical structures of the knee | Proton-density-weighted FSE imaging | Proton-density-weighted FRFSE imaging | z score | p-value |
| Meniscus | ||||
| Anterior horn of the medial meniscus | 3.79 | 3.83 | −0.38 | 0.71 |
| Posterior horn of the medial meniscus | 3.71 | 3.95 | −2.12 | <0.05 |
| Anterior horn of the lateral meniscus | 3.88 | 3.75 | −1.73 | 0.08 |
| Posterior horn of the lateral meniscus | 3.83 | 3.88 | −0.45 | 0.65 |
| Cartilage | ||||
| Medial femoral cartilage/posterior horn of the medial meniscus | 3.71 | 3.08 | −3.64 | <0.001 |
| Medial tibial cartilage/posterior horn of the medial meniscus | 3.38 | 2.79 | −3.74 | <0.001 |
| Lateral femoral cartilage/posterior horn of the lateral meniscus | 3.92 | 3.38 | −3.61 | <0.001 |
| Lateral tibial cartilage/posterior horn of the lateral meniscus | 3.83 | 3.58 | −2.12 | <0.05 |
| Medial femoral cartilage/fluid in the medial femorotibial joint | 2.83 | 3.75 | −4.49 | <0.001 |
| Medial tibial cartilage/fluid in the medial femorotibial joint | 2.79 | 3.63 | −4.47 | <0.001 |
| Lateral femoral cartilage/fluid in the lateral femorotibial joint | 2.96 | 3.67 | −3.49 | <0.001 |
| Lateral tibial cartilage/fluid in the lateral femorotibial joint | 3.17 | 3.67 | −3.21 | <0.05 |
| Anterior cruciate ligament | 2.67 | 3.17 | −3.00 | <0.05 |
| Posterior cruciate ligament | 3.42 | 3.88 | −3.05 | <0.05 |
| Muscle | ||||
| Medial head of the gastrocnemius muscle | 3.00 | 3.88 | −4.58 | <0.001 |
| Others | ||||
| Fluid in the suprapatellar bursa | 2.79 | 3.04 | −1.89 | 0.06 |
| Fluid in the femorotibial joint | 3.58 | 3.71 | −0.90 | 0.37 |
The κ values for the ratings of the subjective imaging contrast for the evaluation of the normal structures of the knee are listed in Table 5. For the analysis of the PD-weighted FSE images, the κ values were 0.37 for the ACL and 0.31 for the femorotibial joint effusion. These findings suggest that interobserver agreement for the ratings of the subjective imaging contrast for the ACL and the femorotibial joint effusion was poor. For the analysis of the PD-weighted FSE images, the κ values were 0.62 for the anterior horn of the medial meniscus, 0.61 for the posterior horn of the medial meniscus, 0.70 for the posterior horn of the lateral meniscus, 0.60 for the medial femoral cartilage, 0.64 for the lateral femoral cartilage, 0.67 for the lateral tibial cartilage, 0.72 for the PCL, 0.47 for the medial head of the gastrocnemius muscle and 0.58 for the suprapatellar bursal effusion. These findings suggest that interobserver agreement for the ratings of the subjective imaging contrast for the anterior and posterior horns of the medial meniscus, posterior horn of the lateral meniscus, the medial femoral cartilage, the lateral femoral and tibial cartilage, the PCL, the medial head of the gastrocnemius muscle and the suprapatellar bursal effusion was good. For the analysis of the PD-weighted FSE images, the κ values were 0.78 for the anterior horn of the lateral meniscus and 0.82 for the medial tibial cartilage. These findings suggest that the interobserver agreement for the ratings of the subjective imaging contrast for the anterior horn of the lateral meniscus and medial tibial cartilage was excellent.
Table 5. Interobserver agreement on the ratings of subjective image contrast.
| Anatomical structures of the knee | Proton-density-weighted FSE imaging |
Proton-density-weighted FRFSE imaging |
||
| κ value±SE | 95% CI | κ value±SE | 95% CI | |
| Meniscus | ||||
| Medial meniscus anterior horn | 0.62±0.20 | 0.23–0.99 | 0.67±0.14 | 0.39–0.96 |
| Medial meniscus posterior horn | 0.61±0.16 | 0.30–0.92 | 0.41±0.16 | 0.09–0.72 |
| Lateral meniscus anterior horn | 0.78±0.21 | 0.36–1.19 | 0.63±0.14 | 0.35–0.91 |
| Lateral meniscus posterior horn | 0.70±0.20 | 0.31–1.09 | 0.45±0.14 | 0.17–0.73 |
| Cartilage | ||||
| Medial femoral cartilage | 0.60±0.16 | 0.30–0.91 | 0.45±0.18 | 0.11–0.80 |
| Medial tibial cartilage | 0.82±0.11 | 0.60–1.04 | 0.38±0.16 | 0.06–0.69 |
| Lateral femoral cartilage | 0.64±0.15 | 0.35–0.93 | 0.25±0.20 | −0.15–0.65 |
| Lateral tibial cartilage | 0.67±0.14 | 0.39–0.96 | 0.32±0.20 | −0.08–0.72 |
| Ligament | ||||
| Anterior cruciate ligament | 0.37±0.15 | 0.08–0.65 | 0.26±0.14 | −0.01–0.54 |
| Posterior cruciate ligament | 0.72±0.12 | 0.48–0.96 | 0.61±0.19 | 0.23–0.99 |
| Muscle | ||||
| Medial head of the gastrocnemius muscle | 0.47±0.32 | −0.16–1.09 | 0.24±0.27 | −0.29–0.77 |
| Others | ||||
| Fluid in the suprapatellar bursa | 0.58±0.17 | 0.25–0.90 | 0.62±0.14 | 0.34–0.89 |
| Fluid in the femorotibial joint | 0.31±0.19 | −0.06–0.68 | 0.44±0.21 | 0.02–0.85 |
CI, confidence interval; SE, standard error.
For the analysis of the PD-weighted FRFSE images, the κ values were 0.38 in the medial tibial cartilage, 0.25 in the lateral femoral cartilage, 0.32 in the lateral tibial cartilage, 0.26 in the ACL and 0.24 in the medial head of the gastrocnemius muscle. These findings suggest that interobserver agreement for the ratings of the subjective imaging contrast in the medial tibial cartilage, the lateral femoral cartilage, the lateral tibial cartilage, the ACL and the medial head of the gastrocnemius muscle was poor. For the analysis of the PD-weighted FRFSE images, the κ values were 0.67 in the anterior horn of the medial meniscus, 0.41 in the posterior horn of the medial meniscus, 0.63 in the anterior horn of the lateral meniscus, 0.45 in the posterior horn of the lateral meniscus, 0.64 in the PCL, 0.62 in the suprapatellar bursal effusion and 0.44 in the femorotibial effusion. These findings suggest that interobserver agreement for the ratings of the subjective imaging contrast in the anterior horn of the medial meniscus, the posterior horn of the medial meniscus, the anterior horn of the lateral meniscus, the posterior horn of the lateral meniscus, the PCL, the suprapatellar bursal effusion and the femorotibial effusion was good.
Discussion
For the last 10 years, a number of pulse sequences have been proposed [7,12-14] which use a fast-recovery pulse to restore longitudinal magnetisation before the next excitation. The fast-recovery pulse shifts T1 weighting towards T2/T1 weighting [13]. The contrast between cartilage and joint fluid is generated by enhancing the signal from joint fluid rather than suppressing the cartilage signal as T2 weighted techniques do [13,16], thus preserving the contrast between cartilage and bone. Several investigators have indicated that the main advantage of incorporating a fast-recovery pulse is the increase in contrast between fluid and non-fluid tissues, which leads to an improved ability to delineate cartilage, ligaments, tendons and fluid compartments [12-15]. In the present study, the mean SNRs were significantly higher for the PD-weighted FRFSE images than for PD-weighted FSE images in the suprapatellar and femorotibial effusions, and the mean CNRs were significantly higher for PD-weighted FRFSE images than for PD-weighted FSE images in the cartilages of all the compartments compared with the femorotibial joint effusion. In addition, reader ratings for both readers were significantly higher for PD-weighted FRFSE images than for PD-weighted FSE images in the cartilages of all the compartments compared with the femorotibial joint effusion, ACL and PCL. These results of the present study therefore concur with those of other investigators, indicating that the technique can provide better contrast between cartilage, ligaments, tendons and fluid compartments, although the present study evaluated PD-weighted imaging, whereas the other studies evaluated T1 weighted imaging techniques.
By contrast, in the present study, the mean SNRs were significantly higher for PD-weighted FSE images than for PD-weighted FRFSE images in the medial and lateral menisci, the cartilage in all the compartments, the ACL, the PCL, medial head of the gastrocnemius muscle and the fat of the intercondylar fossa. These findings may be attributable to the disadvantages of the fast-recovery technique. Similar to other sequences, the fast-recovery methods attempt to recover as much of the magnetisation as possible before allowing for T1 recovery [13]. However, the disadvantage is that the shortened recovery time due to the additional spin echo may cause cartilage signal loss. According to the present study, the mean CNRs and reader ratings for both readers were significantly higher for PD-weighted FSE images than for PD-weighted FRFSE images in the meniscus compared with the cartilage of all the compartments. These findings may also be explained by the inevitable disadvantage due to the shortened recovery time for the FRFSE imaging.
Several investigators have reported that a 2D multislice FSE sequence combined with a driven equilibrium pulse provides a bright signal for the joint fluid with otherwise unchanged signal intensities as compared with a normal T1 weighted FSE sequence at high spatial resolution and with short scan times [12,15]. We recognise the usefulness of the incorporation of a driven equilibrium pulse (or fast-recovery pulse) into a standard T1 weighted FSE sequence that generates contrast between the cartilage and joint fluid. However, radiologists should keep in mind that the PD-weighted FRFSE sequence generates poor contrast between the cartilage and meniscus. Hence, it may be difficult to evaluate the cartilage and meniscal lesions with the PD-weighted FRFSE sequence when there is limited effusion in the femorotibial joint.
In the present study, no significant differences were observed in the mean CNRs of the ACL and PCL between PD-weighted FSE images and FRFSE images. These findings may be attributable to the lower SNRs of both the cruciate ligaments and the fat of the intercondylar fossa. However, the mean ratings for the PD-weighted FRFSE images were significantly higher than those for the PD-weighted FSE images in the ACL and PCL for both readers. This may be explained by the higher SNRs of the joint effusion around the ACL and PCL in the PD-weighted FRFSE images. In the presence of the joint effusion around both of the cruciate ligaments, the technique can provide higher contrast between the cruciate ligaments and the joint effusion.
The κ values in the cartilage of all of the compartments included in the analysis of the PD-weighted FSE sequence tended to be higher than those of the PD-weighted FRFSE sequence. These findings may also be explained by the cartilage signal loss due to the shortened recovery time for the FRFSE imaging. For the analysis of both the PD-weighted FSE and FRFSE sequences, interobserver agreement for the ratings of the subjective imaging contrast in the ACL was poor; in contrast, agreement in the PCL was good. On MR images, the PCL appeared as a homogeneous structure with parallel, well-delineated borders [17]; conversely, the ACL had a more complex appearance, with less homogeneous signal intensity and less well-defined borders because of the differences in gross architecture [18]. These findings may explain the poor interobserver agreement for the ACL and good interobserver agreement for the PCL in the present study.
There are several limitations to this study. First, our study included only normal volunteers. An additional analysis of various pathological conditions using patients with derangements of the knee is required. Second, we evaluated the anatomical structures only in the sagittal images. In our study, the patellofemoral joint in the axial image and the medial and lateral collateral ligaments in the coronal images were not included, but we believe that almost all anatomical structures can be evaluated in the sagittal images. Third, the semi-quantitative analysis portion of the present study was conducted solely by one radiologist. Because of the application of ROIs to very small structures, second measurements by other radiologists would have reduced the bias. In summary, we found the main advantages of the PD-weighted FRFSE sequence to be the increase in contrast between fluid and non-fluid tissues and the relatively short acquisition time. However, the PD-weighted FRFSE sequence generates poor contrast between the cartilage and meniscus, and the cruciate ligaments and the fat of the intercondylar fossa, because of the signal loss of the anatomical structures except for the joint effusion due to the shortened recovery time. Further studies including patients with derangement of the knee and additional structures are needed to evaluate the diagnostic value of using the PD-weighted FRFSE sequence in comparison with the PD-weighted FSE sequence.
References
- 1.Escobedo EM, Hunter JC, Zink-Brody GC, Wilson AJ, Harrison SD, Fisher DJ. Usefulness of turbo spin-echo MR imaging in the evaluation of meniscal tears: comparison with a conventional spin-echo sequence. AJR Am J Roentgenol 1996;167:1223–7 [DOI] [PubMed] [Google Scholar]
- 2.Jee WH, McCauley TR, Kim JM, Jun DJ, Lee YJ, Choi BG, et al. Meniscal tear configurations: categorization with MR imaging. AJR Am J Roentgenol 2003;180:93–7 [DOI] [PubMed] [Google Scholar]
- 3.Schaefer FK, Schaefer PJ, Brossmann J, Frahm C, Muhle C, Hilgert RE, et al. Value of fat-suppressed PD-weighted TSE-sequences for detection of anterior and posterior cruciate ligament lesions: comparison to arthroscopy. Eur J Radiol 2006;58:411–15 [DOI] [PubMed] [Google Scholar]
- 4.Sonin AH, Pensy RA, Mulligan ME, Hatem S. Grading articular cartilage of the knee using fast spin-echo proton density–weighted MR imaging without fat suppression. AJR Am J Roentgenol 2002;179:1159–66 [DOI] [PubMed] [Google Scholar]
- 5.Bredella MA, Tirman PF, Peterfy CG, Zarlingo M, Feller JF, Bost FW, et al. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. AJR Am J Roentgenol 1999;172:1073–80 [DOI] [PubMed] [Google Scholar]
- 6.Wolff SD, Chesnick S, Frank JA, Lim KO, Balaban RS. Magnetization transfer contrast: MR imaging of the knee. Radiology 1991;179:623–8 [DOI] [PubMed] [Google Scholar]
- 7.Gold GE, Busse RF, Beehler C, Han E, Brau AC, Beatty PJ, et al. Isotropic MRI of the knee with 3D fast spin-echo extended echo-train acquisition (XETA): initial experience. AJR Am J Roentgenol 2007;188:1287–93 [DOI] [PubMed] [Google Scholar]
- 8.Notohampiprodjo M, Horng A, Pietschmann MF, Müller PE, Horger W, Park J, et al. MRI of the knee at 3T: first clinical results with an isotropic PDfs-weighted 3D-TSE-sequence. Invest Radiol 2009;44:585–97 [DOI] [PubMed] [Google Scholar]
- 9.Mugler JP., 3rd Overview of MR imaging pulse sequences. Magn Reson Imaging Clin N Am 1999;7:661–97 [PubMed] [Google Scholar]
- 10.Park J, Mugler JP, 3rd, Horger W, Kiefer B. Optimized T1-weighted contrast for single-slab 3D turbo spin-echo imaging with long echo trains: application to whole-brain imaging. Magn Reson Med 2007;58:982–92 [DOI] [PubMed] [Google Scholar]
- 11.Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med 2006;55:1030–7 [DOI] [PubMed] [Google Scholar]
- 12.Woertler K, Rummeny EJ, Settles M. A fast high-resolution multislice T1-weighted turbo spin-echo (TSE) sequence with a DRIVen equilibrium (DRIVE) pulse for native arthrographic contrast. AJR Am J Roentgenol 2005;185:1468–70 [DOI] [PubMed] [Google Scholar]
- 13.Hargreaves BA, Gold GE, Lang PK, Conolly SM, Pauly JM, Bergman G, et al. MR imaging of articular cartilage using driven equilibrium. Magn Reson Med 1999;42:695–703 [DOI] [PubMed] [Google Scholar]
- 14.Gold GE, Fuller SE, Hargreaves BA, Stevens KJ, Beaulieu CF. Driven equilibrium magnetic resonance imaging of articular cartilage: initial clinical experience. J Magn Reson Imaging 2005;21:476–81 [DOI] [PubMed] [Google Scholar]
- 15.Radlbauer R, Lomoschitz F, Salomonowitz E, Eberhardt K, Stadlbauer A. MR imaging of the knee: Improvement of signal and contrast efficiency of T1-weighted turbo spin echo sequences by applying a driven equilibrium (DRIVE) pulse. Eur J Radiol 2010;75:e82–7 [DOI] [PubMed] [Google Scholar]
- 16.Recht MP, Resnick D. MR imaging of articular cartilage: current status and future directions. AJR Am J Roentgenol 1994;163:283–90 [DOI] [PubMed] [Google Scholar]
- 17.Sonin AH, Fitzgerald SW, Hoff FL, Friedman H, Bresler ME. MR imaging of the posterior cruciate ligament: normal, abnormal, and associated injury patterns. Radiographics 1995;15:551–61 [DOI] [PubMed] [Google Scholar]
- 18.Starman JS, Vanbeek C, Armfield DR, Sahasrabudhe A, Baker CL, 3rd, Irrgang JJ, et al. Assessment of normal ACL double bundle anatomy in standard viewing planes by magnetic resonance imaging. Knee Surg Sports Traumatol Arthrosc 2007;15:493–9 [DOI] [PubMed] [Google Scholar]