Abstract
Objective
To investigate the relative performance of T2 weighted short tau inversion–recovery (STIR) and fat-suppressed T1 weighted gadolinium contrast-enhanced sequences in depicting active inflammatory lesions in ankylosing spondylitis (AS).
Methods
Whole-spine MRI was performed on 32 patients with AS, who participated in a clinical trial of infliximab treatment, by STIR and contrast-enhanced sequences at baseline and after 30 weeks. The AS spine MRI-activity (ASspiMRI-a) scoring method was used. The images from these two imaging techniques were evaluated separately by two independent readers.
Results
For the pre-treatment lesion status, the intraclass correlation coefficients comparing STIR readings and contrast-enhanced readings were 0.69±0.23 for Reader 1 and 0.65±0.21 for Reader 2. At baseline, the mean ASspiMRI-a score was 15.4% and 17.7% higher for contrast-enhanced images than for STIR images for Reader 1 and Reader 2, respectively. After infliximab treatment, Reader 1 rated an ASspiMRI-a score reduction of 50.8±33.6% and 25.3±35.3% for STIR images and contrast-enhanced images, respectively, whereas Reader 2 rated an ASspiMRI-a score reduction of 42.4±50.4% and 32.9±35.6% for STIR images and contrast-enhanced images, respectively.
Conclusion
While both contrast-enhanced and STIR sequences showed sensitivity to change over a short period of time after infliximab treatment, these two sequences may reflect different disease mechanisms.
Ankylosing spondylitis (AS) is a chronic inflammatory enthesopathy which results in pain and stiffness leading to progressive spinal immobility, fusion and deformity [1]. The disease especially affects males aged around 20–30 years and often goes unrecognised in the early stages of the disease [2]. Radiography is limited in the earlier stages of AS given its low sensitivity for detecting early inflammatory AS spinal lesions [3]. Two new developments are changing the management of AS, namely effective new therapy such as the antitumour necrosis factor alpha (TNFα) agent infliximab, and the application of imaging modalities such as MRI to recognise early disease and to assess inflammatory activity and the response to therapy. Following its approval for use in rheumatoid arthritis, infliximab, which is a potent inhibitor of the major pro-inflammatory cytokine TNFα, has been used successfully in AS. Active AS patients receiving infliximab demonstrate significant improvement in signs and symptoms [4-7].
MRI is the most sensitive imaging modality for detection of spinal inflammatory disease [8-10]. MRI is able to detect acute spinal lesions even at an early stage, and can demonstrate changes over time in AS patients treated with TNFα targeting therapy [11,12]. MRI scoring methods for quantifying the level of spinal inflammatory activity have been developed and clinically tested [12-15]. These MRI-based methods rely on (a) the detection of bone marrow oedema on T2 weighted fat-suppressed sequences and (b) hyperdiffusion of gadolinium-based molecules into the interstitium of inflamed tissues being visible on fat-suppressed T1 weighted contrast-enhanced sequences. The three most commonly applied scoring systems, i.e. the AS spine MRI-activity (ASspiMRI-a) score [12], the Berlin modification of the ASspiMRI-a score [13] and the Spondyloarthritis Research Consortium of Canada score [14], have been used as outcome measures in clinical trials [15-17]. The ASspiMRI-a scoring system is a reliable method shown to be sensitive to change in the level of spinal inflammation over a relatively short period of 3 months [12]. Using this system, a good correlation between the MRI inflammatory activity score and clinical outcome was found with improvement in the MRI activity score closely paralleling improvement in the clinical score [12].
The major disadvantage of contrast-enhanced techniques is the need for injection of contrast medium, making MRI invasive, more time consuming, more expensive and incurring a low risk of contrast agent complications. On the other hand, T2 weighted fat-suppressed techniques such as the short tau inversion–recovery (STIR) sequence are easier and faster to perform with no added risk. The question has been raised for the assessment of clinical therapeutic trials, whether contrast enhancement adds relevant information to STIR imaging in the detection of active spinal lesions in AS. Hermann et al [18] proposed adopting spinal MRI as an outcome parameter for randomised clinical trials; STIR imaging alone would suffice for detection of serial change in acute lesions. In this study we performed a direct comparison of STIR imaging and T1 weighted gadolinium contrast enhancement in monitoring AS treatment.
Methods and materials
Patients
This study was approved by the research ethics committee of the Chinese University of Hong Kong, Hong Kong, China, and all participating patients provided written informed consent. Participants eligible for the study were recruited from the rheumatology clinic and fulfilled the modified New York criteria for AS [3]. All participants were adults and had clinically active spinal disease. In total, 32 patients were recruited, comprising 29 males and 3 females. The participants' mean age was 35.3±10.2 years (range 19–56 years), and they were randomly divided into two groups. Half of the subjects received infliximab treatment while the other half received infliximab plus methotrexate treatment for 22 weeks. There was no difference in demographic or baseline characteristics for both groups of patients. The infusion of infliximab (5 mg kg−1 in 250 ml 0.9% sodium chloride) was administered at weeks 16, 18 and 22. Methotrexate was started at a weekly dose of 7.5 mg with an incremental increase in dose of 2.5 mg every 2 weeks to a total dosage of 15 mg by week 6, and thereafter continued to week 22. The clinical response to treatment was evaluated chiefly on the basis of response criteria recommended by the ASAS (Assessments in Ankylosing Spondylitis) Working Group [19] and these results have been separately reported [20]. No additional improvement with the addition of methotrexate to infliximab in AS treatment was observed in the study, both clinically and radiologically [20].
MRI
MRI of the spine was performed at baseline and at 30 weeks on a 1.5 T imaging unit (Sonata; Siemens AG, Erlangen, Germany), using a synergy spine coil and with the patient supine. Coverage extended from the skull base to the lower border of S2 in two sections, namely the skull base to T10 followed by T8 to the lower border of S2. Sagittal T1 weighted turbo spin echo [repetition time (TR) 500 ms, echo time (TE) 19 ms, 3 mm thickness, field-of-view 380 mm, matrix 512×512], T2 weighted STIR fat-suppressed (inversion time 160 ms, TR 3240 ms, TE 77 ms, 3 mm thickness, field-of-view 380 mm, matrix 256×256) and contrast-enhanced sagittal T1 weighted spectral pre-saturation with inversion–recovery fat-suppressed sequences (TR 570 ms, TE 9.6 ms, 3 mm thickness, field-of-view 380 mm, matrix 256×512) were performed. For contrast enhancement, a bolus of gadoteric acid (Dotarem®; Guerbet Group, Villepinte, France) at a concentration of 0.15 mmol kg−1 of body weight was injected intravenously into a forearm vein.
MR image reading
Non-annotated MR images were scored independently at the end of the study by two readers blinded to the patient's name and date of examination. Reader 1 is a general radiologist with an interest in musculoskeletal imaging who undertook additional self-training in MRI of AS through literature reading and studying atlases before scoring the images. Reader 2 is a specialised musculoskeletal radiologist with over 20 years' experience in spinal MRI. Pre- and post-treatment examinations were mixed to ensure that both readers were unaware of whether the MR examinations being evaluated were of pre- or post-treatment status. STIR images and contrast-enhanced images were assessed separately with at least 3 days' interval time. MR images were analysed using the ASspiMRI-a score system [12]. This scoring system separately grades active disease change (erosions, oedema and inflammation) at each vertebral unit. A vertebral unit is defined as that area between the mid-points of two adjacent vertebral bodies. A score of 1–3 was assigned for active spinal inflammation (oedema or contrast enhancement) involving: 1, up to 25% of a vertebral unit; 2, up to 50% of a vertebral unit: or 3, >50% of a vertebral unit. In addition, a score of 4–6 was assigned if these lesions also showed bony erosion with: 4, being minor bony erosion; 5, moderate bony erosion; and 6, severe bone erosion. Summation of the individual vertebral units yielded a global activity score for each patient's spine examination. With 23 vertebral units (from the middle of the second cervical vertebra to the middle of the first sacral vertebra) being assessed, the total ASspiMRI-a score for the spine from C2 to S1 could potentially range from 0 to 138 [12].
Statistical analysis
Statistical analysis of cohorts comprised descriptive measures such as mean sum activity scores and number of affected vertebral units. To investigate agreement between scores based on marrow oedema and contrast enhancement, intraclass correlation coefficients were calculated for pre-treatment scores. Overall correlation between the two assessment methods was calculated using Spearman rank correlation. To analyse agreement patterns between both readers for each MR data set, Bland and Altman plots were constructed [21], and the “smallest detectable change” calculated [22]. “Smallest detectable change” is defined as 1.96×(standard deviation of inter-reader difference)/ 2−0.5 [18]. Inter-reader agreement for each sequence data set was compared using the “smallest detectable change”, favouring the sequence with the lower smallest detectable change value. All computations were performed using SAS® software (SAS Institute, Cary, NC) or GraphPad (Prism 4, GraphPad Software, San Diego, CA). Data are presented as the mean±standard deviation. All p-values were two-sided, with p<0.05 being considered statistically significant.
Results
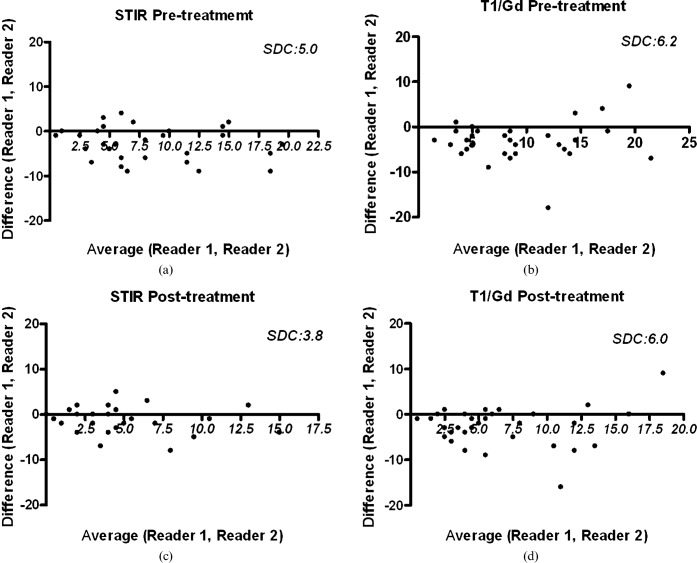
At baseline, all patients had at least one active inflammatory lesion present on the MRI examination before treatment. Infliximab or infliximab plus methotrexate treatment decreased AS lesion activity scores (Table 1). On average, Reader 1 tended to score higher than Reader 2 in both sequences and Reader 1 also reported a higher number of affected vertebral units. Bland and Altman analysis showed that for both sequences inter-reader variability was maintained across the spectrum of activity scores, for both pre-and post-treatment scoring, with a consistent difference which was less apparent when corner oedema was evaluated and compared with contrast-enhanced lesions (Figure 1). Analysis of corner oedema on STIR sequences led to a smaller “smallest detectable change” than analysis of contrast-enhanced lesions on T1 weighted sequences for both readers.
Table 1. Comparison of Reader 1 and Reader 2 for pre-treatment MRI assessment (ASspiMRI-a score) and showing the number of affected VUs per patient and the number of erosions per vertebral unit. The ICC between readings is shown, as well as the overall correlation (Spearman Rank correlation) and p-value.
| Reader 1 |
Reader 2 |
||||
| Assessment | STIR | T1W/Gd | STIR | T1W/Gd | |
| Pre-treatment | |||||
| ASspiMRI-a | 9.53 (±5.75) | 11.00 (±5.52) | 6.72 (±5.11) | 7.91 (±6.19) | |
| No. of affected VU | 6.9 (±4.3) | 8.3 (±3.4) | 5.0 (±3.5) | 5.7 (±3.6) | |
| No. of affected VUs with erosions | 0.6 (±0.9) | 0.8 (±1.0) | 0.5 (±0.8) | 0.65 (±1.2) | |
| ICC | 0.69 (±0.23) | 0.65 (±0.21) | |||
| Spearman's r | 0.85 (p<0.0001) | 0.88 (p<0.0001) | |||
| p-value | 0.019 | 0.03 | |||
| Post-treatment | |||||
| ASspiMRI-a | 4.56 (±4.28) | 7.97 (±4.82) | 3.59 (± 3.71) | 5.31 (±5.17) | |
| Spearman's r | 0.85 (p<0.0001) | 0.88 (p<0.0001) | |||
| p-value | 0.001 | <0.0001 | |||
| ASspiMRI-a score reduction | 4.97 (±4.36) | 3.03 (±3.49) | 3.12 (± 3.58) | 2.59 (±2.85) | |
| ASspiMRI-a score reduction in % | 50.8 (±33.6) | 25.3 (±35.3) | 42.4 (±50.4) | 32.9 (±35.6) | |
| p-value for score reduction | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
ASspiMRI-a, ankylosing spondylitis spine MRI-activity; ICC, intraclass correlation coefficient; No., number; STIR, short tau inversion–recovery; T1W, T1 weighted gadolinium contrast-enhanced technique; VU, vertebral units.
Figure 1.
Bland and Altman plots. (a) Distribution of pre-treatment ankylosing spondylitis (AS) lesion scores for the short tau inversion–recovery (STIR) technique. (b) Distribution of pre-treatment AS lesion scores for the T1 weighted gadolinium contrast-enhanced (T1/Gd) technique. (c) Distribution of post-treatment AS lesion scores for the STIR technique. (d) Distribution of post-treatment AS lesion scores for the contrast-enhanced T1/Gd technique. SDC, smallest detectable change.
The Spearman correlation between corner oedema and contrast-enhanced lesion readings had a ρ-value between 0.85 and 0.88 (Table 1, p<0.0001, Figure 2). The intraclass correlation coefficient comparing corner oedema and contrast-enhanced lesion readings was fair for both readers (Table 1). For both readers on both pre- and post-treatment MR assessment, scores based on corner oedema change were lower than those based on contrast enhancement. On contrast-enhanced data sets, both readers also detected a higher number of affected vertebral units per patient and a higher number of affected vertebral units with erosion per patient (Figures 3 and 4). STIR images seemed more responsive to therapeutic response than contrast-enhanced images (Table 1).
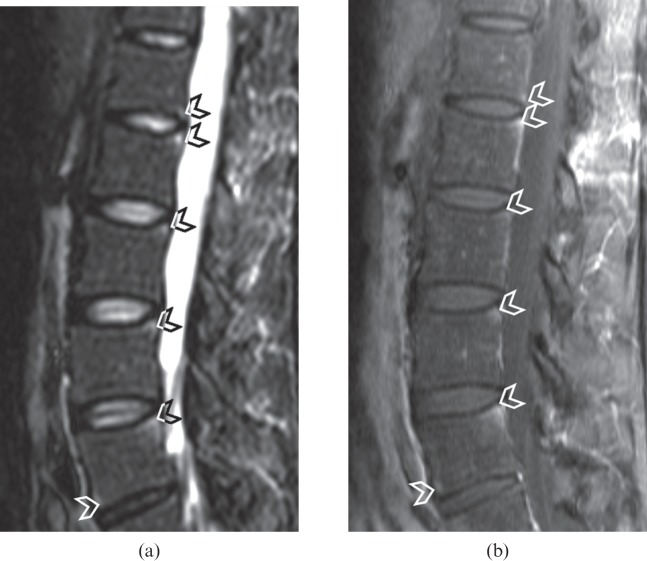
Figure 2.
(a) Short tau inversion–recovery (STIR) sagittal image of a lumbar spine showing oedema at the posteroinferior corner of the L1 vertebral body and the posterosuperior corner of the L2, L3, L4 and L5 vertebral bodies as well as the anteroinferior corner of the L5 vertebral body (arrowheads). These lesions are also depicted on (b) the contrast-enhanced T1 weighted fat-suppressed image (arrowheads), with good agreement between the two images. With the spine units L1–L5, Reader 1 had an ankylosing spondylitis spine MRI-activity score of 5 for STIR images and contrast-enhanced images, whereas Reader 2 had a score of 4 for STIR images and contrast-enhanced images.
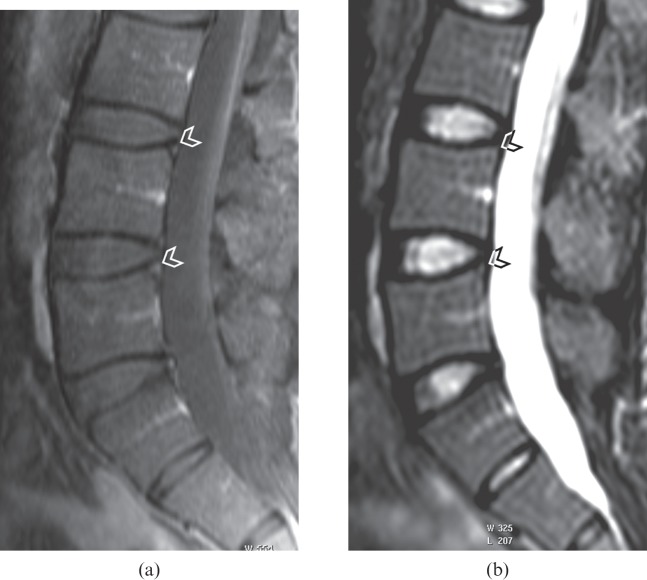
Figure 3.
(a) Contrast-enhanced T1 weighted fat-suppressed sagittal image of a lumbar spine showing enhancement at the posterosuperior corner of the L4 and L5 vertebral bodies (arrowheads). These active lesions are not evident on (b) the short tau inversion–recovery (STIR) imaging (arrowheads), with poor agreement between the two images. With the spine units L3–S1, Reader 1 had an ankylosing spondylitis spine MRI-activity score of 2 for contrast-enhanced images and 0 for STIR images, whereas Reader 2 had a score of 1 for contrast-enhanced images and 0 for STIR images.
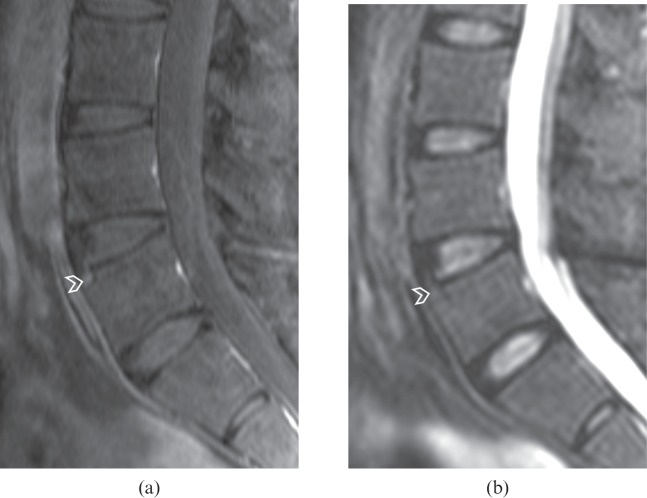
Figure 4.
(a) Contrast-enhanced T1 weighted fat-suppressed sagittal image of a lumbar spine showing enhancement at the anterosuperior corner of the L5 vertebral body (arrowhead). This lesion is not evident on (b) the short tau inversion–recovery (STIR) image (arrowhead), with poor agreement between the two images. With the spine units L3–S1, both readers had an ankylosing spondylitis spine MRI-activity score of 1 for contrast-enhanced images and 0 for STIR images.
Discussion
The first MRI scoring system proposed to evaluate active AS inflammation was the ASspiMRI-a system, which includes both STIR and gadolinium contrast-enhanced sequences to evaluate corner oedema and contrast enhancement, respectively [12]. The reliability and discrimination of scoring systems for spinal inflammation using MRI have been validated in clinical trials of new anti-inflammatory therapeutic agents with a fair correlation existing between MR parameters and the histological grading of inflammation [23]. Infliximab reduces spinal inflammation and neovascularisation, which contribute to both the corner oedema and contrast enhancement seen in MRI. Applying this ASspiMRI-a scoring system, it was reported that both STIR and contrast-enhanced sequences led to similar scores, and both techniques indicated change after infliximab treatment [12,18,24]. Hermann et al [18] reported that, for monitoring of therapeutic effect, very few differences existed between both these two MRI sequences with regard to inter-reader variability and smallest detectable change and, more importantly, reported that the addition of contrast-enhanced sequences does not seem to add significant information.
The MR scanner parameters used in previous studies have been for the most part similar to the one applied in this study, although the current MR image resolution was higher than some other previous studies [24,25]. As lower signal-to-noise ratio is a feature of STIR imaging compared with T1 weighted imaging, it was necessary to use a larger voxel size for STIR imaging than the T1 weighted contrast-enhanced sequence. A lower spatial resolution for the STIR sequence than the T1 weighted sequence has also been used in other studies [24]. Sagittal images were analysed in the current study similar to previous MRI-based AS studies [12,24,25].
Patients in the current study appeared to have more active AS lesions than patients in the study carried out by Hermann et al [18] with higher overall ASspiMRI-a scores and a greater number of vertebral units affected (Table 1). Comparing inter-reader differences, the smallest detectable changes for oedema of 5.0 for pre-treatment and 3.8 for post-treatment in this study are better than the 6.7 value found by Hermann et al [18], while the smallest detectable changes for contrast enhancement of 6.2 for pre-treatment and 6.0 for post-treatment in this study are comparable to the 6.2 value found by Hermann et al [18]. These results are also shown in Bland and Altman plots (Figure 1) [18]. This is at odds with previous reports in which both intra- and inter-reader reliability in assessing oedema change faired worse than that for enhancement [24]. The intraclass correlation coefficient between pre-treatment STIR readings and contrast-enhanced readings in our study was 0.69 for Reader 1 and 0.65 for Reader 2, which is lower than the results of Hermann et al [18] and may indicate greater disagreements between contrast enhancement readings and corner oedema readings in our study.
Despite rigorous attempts to standardise lesion definition, considerable variation remains in reporting active inflammatory lesions on MRI [18,26]. Most AS lesions are small, comprising only a few pixels of increased signal intensity. The large field of view necessary to cover the spine can result in suboptimal resolution. MRI is also subject to artefacts, including swallowing artefacts in the cervical region, breathing artefacts in the thoracic region, incomplete fat suppression and partial-volume averaging effects. While 3 T imaging will improve image quality, most clinical trials have been performed on 1.0 T or 1.5 T MR units. It was recommended that assessment of inflammatory lesions for studies of diagnostic and prognostic utility should be conducted by two calibrated readers evaluating images independently [26]. A computer-aided detection or quantification system is likely to make the scoring process more robust, particularly for contrast-enhanced images in which baseline signal can be subtracted from post-enhancement images.
While AS inflammation manifests on MRI as areas of oedema or gadolinium enhancement, it is apparent that these changes do not necessarily occur in parallel. For example, 10.1% of the vertebral units that showed oedema on STIR imaging were normal on T1 weighted contrast-enhanced images while vertebral body corner enhancement was present in 6.4% of vertebral units that were considered normal on STIR imaging [24]. It was noteworthy that oedema-like changes are also apparent in up to 26% of healthy individuals with no clinical evidence of AS [25]. As such, it is questionable whether a corner oedema on MRI always equates to a true inflammatory lesion and it is possible that STIR imaging may be overestimating the level of AS disease activity present. Whether such a discrepancy exists for T1 weighted contrast-enhanced imaging has not been determined because no study has evaluated the prevalence of corner contrast enhancement in normal subjects. However, as opposed to previous reports, which were mainly from a single institution [12,18,24], our study demonstrated that contrast-enhanced MRI yielded a higher lesion score than STIR imaging although STIR imaging had better intrareader agreement. This is at odds with some earlier studies in which both readers had higher scores for oedema lesions than contrast enhancement lesions [24]. It is not clear whether this is a feature of the apparent higher disease activity at baseline in our patients. Our results indicate that not all lesions showing contrast enhancement are associated with a detectable oedema.
While our results indicate that STIR imaging demonstrates fewer lesions, these oedema-type lesions do seem to show a higher therapeutic response than on contrast enhancement. This point has been previously noted by Hermann et al [18] and also Braun et al [12]. Braun et al reported that STIR sequences yielded a higher apparent therapeutic response, while contrast-enhanced images had the lower intrarater variance. The observation that oedema changes respond more than contrast enhancement changes may reflect a specific anti-TNFα effect or a true difference between the lesions [27]. Anti-TNFα reduces circulating levels of vascular endothelial growth factor, which is an angiogenic peptide affecting both neovascularisation and vascular permeability. As such, a more detailed analysis of both the occurrence and response of corner oedema and contrast enhancement changes in AS may provide clues to the pathogenesis and treatment response of AS spinal enthesopathy.
Conclusion
Our study confirms previous reports that both vertebral body corner oedema and contrast enhancement changes are sensitive to change during treatment although oedema appears to be more responsive to particular treatment interventions. STIR imaging and contrast-enhanced imaging seem to be complementary rather than mutually exclusive in both assessment of disease activity and treatment response. Further research should also focus on clarifying the prevalence of corner oedema and contrast enhancement changes in normal subjects, and on providing more robust methods of quantifying these changes [28].
References
- 1.Braun J, Bollow M, Remlinger G, Eggens U, Rudwaleit M, Distler A, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998;41:58–67 [DOI] [PubMed] [Google Scholar]
- 2.Zink A, Listing J, Klindworth C, Zeidler H. The national database of the German Collaborative Arthritis Centres. I. Structure, aims, and patients. Ann Rheum Dis 2001;60:199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van derLinden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8 [DOI] [PubMed] [Google Scholar]
- 4.Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359:1187–93 [DOI] [PubMed] [Google Scholar]
- 5.Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, et al. Two year maintenance of efficacy and safety of infliximab in the treatment of ankylosing spondylitis. Ann Rheum Dis 2005;64:229–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, et al. Long-term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum 2003;48:2224–33 [DOI] [PubMed] [Google Scholar]
- 7.Braun J, Baraliakos X, Brandt J, Listing J, Zink A, Alten R, et al. Persistent clinical response to the anti-TNFalpha antibody infliximab in patients with ankylosing spondylitis over 3 years. Rheumatology (Oxford) 2005;44:670–6 [DOI] [PubMed] [Google Scholar]
- 8.Maksymowych WP, Lambert RG. Magnetic resonance imaging for spondyloarthritis: avoiding the minefield. J Rheumatol 2007;34:259–65 [PubMed] [Google Scholar]
- 9.Lambert RG, Salonen D, Rahman P, Inman RD, Wong RL, Einstein SG, et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2007;56:4005–14 [DOI] [PubMed] [Google Scholar]
- 10.Weber U, Kissling RO, Hodler J. Advances in musculoskeletal imaging and their clinical utility in the early diagnosis of spondyloarthritis. Curr Rheumatol Rep 2007;9:353–60 [DOI] [PubMed] [Google Scholar]
- 11.Marzo-Ortega H, McGonagle D, O'Connor P, Emery P. Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study. Arthritis Rheum 2001;44:2112–17 [DOI] [PubMed] [Google Scholar]
- 12.Braun J, Baraliakos X, Golder W, Brandt J, Rudwaleit M, Listing J, et al. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum 2003;48:1126–36 [DOI] [PubMed] [Google Scholar]
- 13.Haibel H, Rudwaleit M, Listing J, Heldmann F, Wong RL, Kupper H, et al. Efficacy of adalimumab in the treatment of axial spondyloarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum 2008;58:1981–91 [DOI] [PubMed] [Google Scholar]
- 14.Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Krishnananthan R, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:502–9 [DOI] [PubMed] [Google Scholar]
- 15.Ostergaard M, Poggenborg RP, Axelsen MB, Pedersen SJ. Magnetic resonance imaging in spondyloarthritis—how to quantify findings and measure response. Best Pract Res Clin Rheumatol. 2010;24:637–57 [DOI] [PubMed] [Google Scholar]
- 16.Lukas C, Braun J, van derHeijde D, Hermann KG, Rudwaleit M, Østergaard M, et al. Scoring inflammatory activity of the spine by magnetic resonance imaging in ankylosing spondylitis: a multireader experiment. J Rheumatol 2007;34:862–70 [PubMed] [Google Scholar]
- 17.van derHeijde D, Landewe R, Hermann KG, Rudwaleit M, Østergaard M, Oostveen A, et al. Is there a preferred method for scoring activity of the spine by magnetic resonance imaging in ankylosing spondylitis? J Rheumatol 2007;34:871–3 [PubMed] [Google Scholar]
- 18.Hermann KG, Landewe RB, Braun J, van derHeijde DM. Magnetic resonance imaging of inflammatory lesions in the spine in ankylosing spondylitis clinical trials: is paramagnetic contrast medium necessary? J Rheumatol 2005;32:2056–60 [PubMed] [Google Scholar]
- 19.Anderson JJ, Baron G, van derHeijde D, Felson DT, Dougados M. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum 2001;44:1876–86 [DOI] [PubMed] [Google Scholar]
- 20.Li EK, Griffith JF, Lee VW, Wang YX, Li TK, Lee KK, et al. Short-term efficacy of combination methotrexate and infliximab in patients with ankylosing spondylitis: a clinical and magnetic resonance imaging correlation. Rheumatology (Oxford) 2008;47:1358–63 [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Measurement error. BMJ 1996;313:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruynesteyn K, Boers M, Kostense P, van derLinden S, van derHeijde D. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change? Ann Rheum Dis 2005;64:179–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bollow M, Fischer T, Reisshauer H, Backhaus M, Sieper J, Hamm B, et al. Quantitative analyses of sacroiliac biopsies in spondylarthropathies: T cells and macrophages predominate in early and active sacroiliitis-cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis 2000;59:135–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baraliakos X, Hermann KG, Landewe R, Listing J, Golder W, Brandt J, et al. Assessment of acute spinal inflammation in patients with ankylosing spondylitis by magnetic resonance imaging: a comparison between contrast enhanced T1 and short tau inversion recovery (STIR) sequences. Ann Rheum Dis 2005;64:1141–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber U, Hodler J, Kubik RA, Rufibach K, Lambert RG, Kissling RO, et al. Sensitivity and specificity of spinal inflammatory lesions assessed by whole-body magnetic resonance imaging in patients with ankylosing spondylitis or recent-onset inflammatory back pain. Arthritis Rheum 2009;61:900–8 [DOI] [PubMed] [Google Scholar]
- 26.Pedersen SJ, Ostergaard M, Chiowchanwisawakit P, Lambert RG, Maksymowych WP. Validation of definitions for active inflammatory lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis. J Rheumatol 2009;36Suppl. 84:35–8 [Google Scholar]
- 27.Lambert RG, Pedersen SJ, Maksymowych WP, Chiowchanwisawakit P, Ostergaard M. Active inflammatory lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis: definitions, assessment system, and reference image set. J Rheumatol 2009;36Suppl. 84:3–17 [Google Scholar]
- 28.Wang YX. Medical imaging in pharmaceutical clinical trials: what radiologists should know. Clin Radiol 2005;60:1051–7 [DOI] [PubMed] [Google Scholar]