Abstract
Objective
The aim of this study was to assess the diagnostic accuracy of imaging myocardial infarction with a two-dimensional (2D) single-shot inversion-recovery (IR)–gradient-echo (GE) sequence compared with a standard 2D segmented IR–GE sequence at 1.5 T using a dedicated cardiac coil.
Methods
22 patients with myocardial infarction documented in the past 3–12 months were examined at 1.5 T using a 5 channel cardiac coil. Imaging of delayed enhancement was performed 15 min after administration of 0.2 mmol of gadopentetate dimeglumine per kilogram of body weight. Immediately after completion of the single-shot sequence, which allows for coverage of the entire ventricle during a single breath-hold with nine slices, the segmented IR sequence was started. Infarct volumes, infarct transmurality and contrast-to-noise ratios (CNRs) of infarcted and healthy myocardium were compared between both techniques.
Results
Despite a moderate, non-significant loss of CNR (CNRsingle-shot IR=31.2±4.1; CNRsegmented IR=37.9±4.1; p=0.405), the 2D single-shot technique correctly determined infarct size when compared with the standard 2D segmented IR–GE sequence. Assessment of both infarct volume (r=0.95; p<0.0001) and transmurality (r=0.97; p<0.0001) is possible, with excellent correlation of both techniques.
Conclusion
Single-shot delayed enhancement imaging during a single breath-hold is feasible at 1.5 T with the use of a dedicated cardiac coil. Despite a moderately lower CNR, the single-shot technique allows for fast and accurate determination of infarct size with high spatial resolution and has the potential to reduce electrocardiogram and breathing artefacts.
Correct determination of the extent of dysfunctional yet viable myocardium is crucial for therapeutic decision-making, whether a patient receives coronary catheterisation and angioplasty or requires bypass surgery. Myocardial viability is the major determinant for patient survival and prognosis [1]. While nuclear imaging techniques such as positron emission tomography and single photon emission CT can correctly determine myocardial viability by assessing metabolic parameters of the myocardium, their spatial resolution is too low to report on the exact transmural extent of myocardial infarction as well as functional parameters with special regard to myocardial contractility [2-4]. Cardiac MRI is the only technique that currently allows both determination of cardiac function and myocardial viability [5-8]. By measuring the increase in extracellular space associated with death of cardiomyocytes, delayed enhancement imaging with different gadolinium chelates depicts the myocardial infarct with high accuracy and reproducibility when performed in the subacute or chronic phase [9,10]. Usually, delayed enhancement imaging is performed using segmented inversion-recovery (IR)–gradient-echo (GE) sequences—both two-dimensional (2D) and three-dimensional (3D) techniques [11-19]. A major drawback of the segmented approach is the long acquisition time: with one slice being acquired during a breath-hold period, when a segmented 2D IR–GE technique is used, the time for a single section is estimated to be about 10–14 heartbeats. To cover the entire ventricle on short-axis views, 9–10 breath-holds are required for complete data acquisition. Segmented techniques, both 2D and 3D, are sensitive to motion artefacts caused by arrhythmias or breathing artefacts [17,20,21]. Single-shot techniques that cover the entire ventricle during a single breath-hold can significantly reduce the acquisition time, thereby reducing electrocardiogram (ECG) and breathing artefacts. While segmented IR–GE sequences acquire the data for one slice over several R–R cycles (defined as the interval from the peak of one QRS complex to the peak of the next as shown on an ECG), only one R–R interval is needed for data acquisition of one slice in the single-shot mode [11,22,23]. This technique is therefore advantageous in patients with cardiac arrhythmias as well as respiratory diseases. However, single-shot techniques cause a loss of contrast-to-noise ratio (CNR), leading to potentially decreased diagnostic accuracy [23]. Therefore, until recently, single-shot techniques have been proven to work successfully only at 3 T [11,22] or with a steady-state free-precession (SSFP) readout technique, which has a mixed T1/T2 contrast. The mixed contrast in SSFP readout techniques can lead to overestimation of infarct volumes in subacute infarctions, in which the area or volume of oedema is larger than the area of contrast enhancement [24]. Recent development in coil design is promising to achieve higher signal-to-noise ratios (SNRs), and thus may allow for the use of the single-shot GE technique also at a field strength of 1.5 T.
The aim of this study, therefore, was to determine the diagnostic accuracy of a 2D single-shot IR–GE sequence using a dedicated cardiac coil at 1.5 T compared with a standard segmented 2D IR–GE sequence as a reference technique at the same field strength.
Methods and materials
Patient population
22 patients (16 male, 6 female) with a history of myocardial infarction in the past 3–12 months were prospectively enrolled in the study. Informed consent was obtained from each patient. The study was performed according to the guidelines of the latest revised version of the Declaration of Helsinki. The mean age was 61.3±9.9 years (range, 41–76 years). The patients did not suffer from unstable angina or heart failure graded at NYHA (New York Heart Association) III/IV. Patients with poor renal function with a glomerular filtration rate of <30 ml min−1 were not included in the study. Patients with contraindications to MRI were also not included. The diagnosis of myocardial infarction in the history of each patient was made based on typical ECG findings and biochemical markers.
MRI
MRI was performed on a 1.5 T imaging system (Achieva; Philips Healthcare, Best, Netherlands) with a dedicated five-channel cardiac coil. ECG monitoring was performed during the entire examination via an integrated MR-compatible monitoring system. After acquisition of scout views, a long-axis view, two- and four-chamber views and short-axis views of the left ventricle were obtained. Gadopentetate dimeglumine (Magnevist®; Bayer Schering Pharma AG, Berlin, Germany) was administered at 0.2 mmol per kilogram of body weight via an antecubital venous access. The administration of gadopentetate dimeglumine was followed by a 20 ml saline injection. Delayed enhancement imaging was started 15 min after injection of gadopentetate dimeglumine. No parallel imaging technique was used. Optimisation of inversion time (TI) was performed using a standard Look–Locker [turbo field echo–echo planar imaging (EPI)] sequence. This pulse sequence is used to determine the correct TI to null the signal intensity (SI) of normal myocardium. Sequence parameters of the Look–Locker sequence include field of view (FOV), 270×270 mm; matrix, 128×99; slice thickness, 10 mm; in-plane resolution, 2.1×2.2 mm; repetition time (TR)/echo time (TE), 40/5.7 ms; flip angle, 15°; and EPI factor 9. The k-space was read out with a centrically reordered technique.
All delayed enhancement imaging was performed using prospective ECG-triggering in the breath-hold technique. Data acquisition was performed during the mid diastole, which was estimated by a time window of minimal cardiac motion. 15 min after injection of gadopentetate dimeglumine, the 2D single-shot IR–GE sequence was performed. The single-shot technique allows for acquisition of the data of the entire left ventricle, 9–10 slices, in a single breath-hold. Data were acquired by imaging one slice in the mid diastole of every heartbeat. Thus, 9–10 cardiac cycles were necessary to cover the entire left ventricle. Immediately after the single-shot acquisition, the reference segmented 2D IR–GE sequence was started. For both IR sequences, a T1 GE technique was applied.
2D single-shot IR imaging was performed with the following parameters: TR/TE, 3.8/1.2 ms; bandwidth, 305 Hz per pixel; flip angle, 20°; a typical FOV of 320×400 mm (individually adapted); matrix, 160×160; and a half scan factor of 0.75. The acquired voxel size was typically 2×2.5×8 mm, reconstructed as 1.25×1.25×8 mm. As a reference sequence, a standard 2D segmented IR sequence was used, with the following parameters: TR/TE, 3.6/1.2 ms; bandwidth, 382 Hz per pixel; flip angle, 25°, a typical FOV of 320×320 mm (individually adapted); matrix, 160×160; and number of signal averages=2. The acquired voxel size was 2.0×2.0×8 mm, reconstructed as 1.25×1.25×8 mm. Breath-hold time was 18±1 s for the single-shot IR technique and 106±4 s for the segmented IR sequence.
Data analysis
Analysis of DICOM (Digital Imaging and Communications in Medicine)-encoded data sets was performed using OsiriX (OsiriX Foundation, Geneva, Switzerland). Regions of interests (ROIs) were defined for the hyperenhanced, infarcted areas and the normal myocardium. Regions of hyperenhancement were defined by threshold values two standard deviations (SDs) higher than the remote myocardium. SI was measured in infarcted and normal left ventricular myocardium. The ROI for mean SI was placed manually at the centre of the infarction, and another ROI for mean SI was placed in the normal remote myocardium. ROIs were drawn as large as possible to yield homogeneous signals from scars or remote myocardium without contamination from adjacent regions. Noise was measured as SD in an ROI placed outside the patient in front of the chest. For determination of SIs, identical ROIs were drawn for both techniques. SIs, SNRs and CNRs were calculated for both sequences. SNRs were defined as SIinfarct/SInoise, or SImyocardium/SInoise. CNRs were calculated as (SIinfarct−SImyocardium)/SDnoise. For comparison of SNRs and CNRs, an unpaired two-tailed Student's t-test was performed.
For the determination of transmurality, a standard centreline algorithm was used, with chords reaching from the endo- to the epicardial border drawn perpendicular to the centreline.
To compare the infarct volumes, hyperenhanced areas of the myocardium were determined on every single short-axis slice and multiplied by the slice thickness. The correlation coefficient and the equation of the regression were determined for the infarct for the single-shot IR sequence as compared with the segmented IR technique. A Bland–Altman plot was calculated for the infarct volume.
Additionally, the number of segments affected by the infarct was determined in both groups according to the American Heart Association (AHA) 17-segment model [25]. Numbers of infarcted segments were compared between both approaches.
The transmural extent of myocardial damage was determined by the ratio of the thickness of the entire myocardium divided by the thickness of the hyperenhanced myocardium. The maximum of the transmural extent of each individual infarction was used for further analysis. For comparison of the transmural extent of the infarction determined with the two different techniques, the linear correlation coefficient and the regression equation were determined and a Bland–Altman plot was calculated accordingly. A p-value of <0.05 was considered to demonstrate statistical significance.
Results
Detection of hyperenhanced myocardium
Subendocardial delayed enhancement, a characteristic of infarcted myocardium, was detected in all patients investigated.
Signal-to-noise ratio and contrast-to-noise ratio
The mean SNR of the single-shot IR sequence for both the infarcted and the normal myocardium (infarcted: 26.9±2.9; normal: 3.0±0.4) were slightly lower than the SNR of the segmented IR–GE sequence (infarcted: 29.2±6.3; normal: 3.9±1.1); however, the difference was not statistically significant (Table 1). The mean CNR of the single-shot IR sequence was 31.2±6.7, while the mean CNR of the segmented technique was 37.9±4.1. Thus, the single-shot approach yielded moderately lower CNR than the segmented technique, but the difference between both was not significantly different (p=0.405).
Table 1. SNR and CNR of infarcted and normal myocardium of the segmented IR–GE and single-shot IR–GE sequences.
| Parameter | 2D segmented IR | Single-shot IR | p-value |
| SNRnormal | 3.9±1.1 | 3.0±0.4 | 0.453 |
| SNRinfarct | 29.2±6.3 | 26.9±2.9 | 0.747 |
| CNR | 37.9±4.1 | 31.2±6.7 | 0.405 |
2D, two-dimensional; CNR, contrast-to-noise ratio; GE, gradient echo; IR, inversion recovery; SNR, signal-to-noise ratio.
Infarct volume
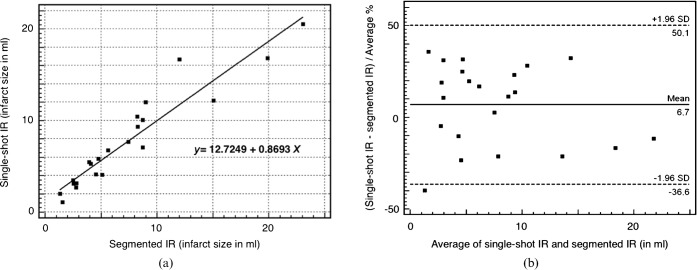
The determined infarct volumes were comparable between both techniques (Figure 1). The mean infarct volume as calculated by the single-shot IR sequence was 7.7±1.1 ml; when determined by the segmented IR sequence, the mean infarct volume was 7.4±1.2 ml. The assessment of the volume of myocardial infarction showed a high correlation between both techniques (r=0.9476; p<0.001).
Figure 1.
(a) Scatter diagram showing the infarct volumes of the segmented IR–GE and the single-shot IR–GE techniques. (b) Bland–Altman plot of infarct volumes (in millilitres) of the segmented IR–GE and the single-shot IR–GE techniques. GE, gradient echo; IR, inversion recovery; SD, standard deviation.
Number of affected segments
Segments affected by the myocardial infarction were graded according to the AHA 17-segment model. The number of affected segments was comparable between both techniques with no significant difference (p>0.05). However, as shown in Figure 2, the number of affected segments as determined using the single-shot IR technique may have been underestimated in some cases.
Figure 2.
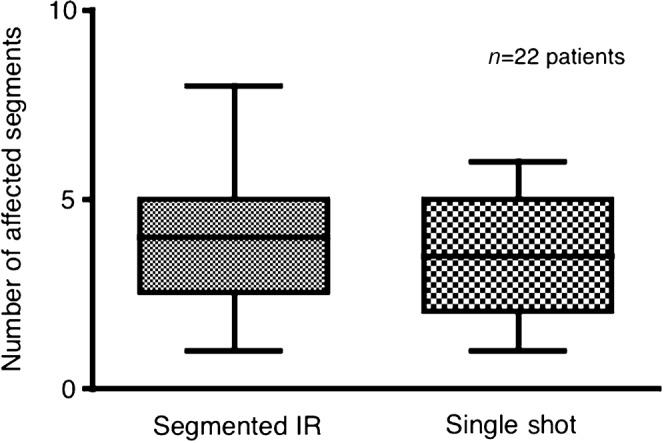
Box plot diagram showing the number of segments identified by delayed enhancement MRI with segmented vs single-shot IR–GE sequences. No significant difference is noted between the techniques; however, the single-shot technique may in some cases underestimate the extent of ischaemic injury, as depicted by the higher maximum of affected segments identified by the segmented 2D IR–GE technique. 2D, two-dimensional; GE, gradient echo; IR, inversion recovery.
Transmural extent of myocardial infarction
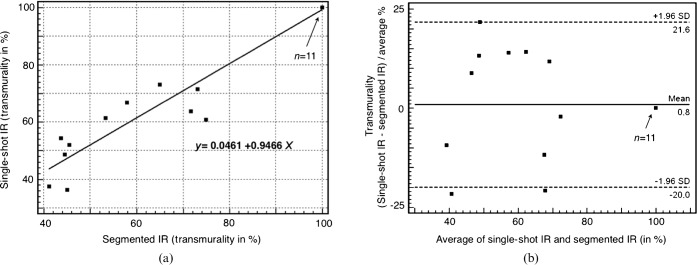
The transmurality of myocardial infarction was 78±5.2% when determined with the single-shot IR sequence, and 76±5.3% when measured with the segmented IR sequence. Both approaches (Figure 3) showed very good correlation for determining the transmural extent of the infarction (r=0.9701; p<0.001).
Figure 3.
(a) Scatter diagram showing the degree of transmurality of myocardial infarctions. The results of the segmented 2D IR–GE and the single-shot IR–GE sequences are compared. (b) Bland–Altman plot of infarct transmurality of the segmented 2D IR–GE and the single-shot IR–GE sequences. 2D, two-dimensional; GE, gradient echo; IR, inversion recovery; SD, standard deviation.
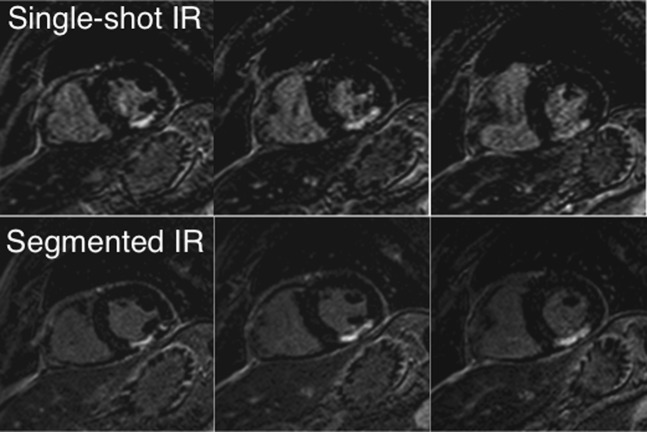
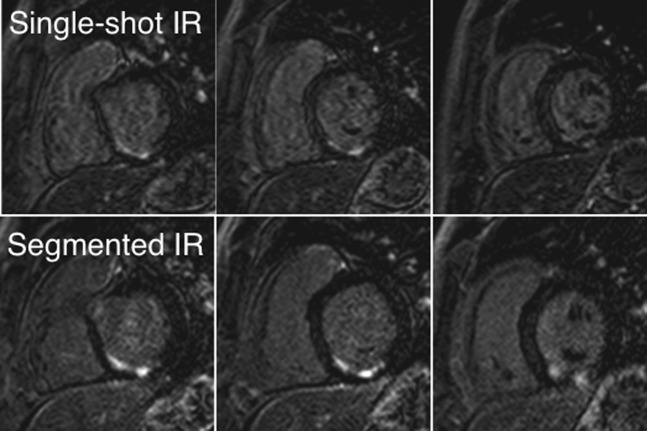
Figure 4 shows images of a 64-year-old male patient with predominantly transmural infarction of the inferior myocardium. A similar extent of myocardial infarction as determined with both techniques can be appreciated. Figure 5 shows images of a 40-year-old male patient with infarction of the inferior and inferior-septal myocardium. Both extent and transmurality of the infarct can be compared between both sequences.
Figure 4.
Short-axis view images of a 64-year-old patient after myocardial infarction with a predominantly transmural scar of the inferior wall. The images in the upper row were acquired with the single-shot IR–GE technique; the images in the lower row with the segmented 2D IR–GE technique served as a reference technique. 2D, two-dimensional; GE, gradient echo; IR, inversion recovery.
Figure 5.
A 40-year-old male patient with a non-transmural infarction of the inferior and inferior-septal segments. The upper images were acquired with the single-shot technique; the lower images were obtained with the segmented 2D IR–GE sequence. 2D, two-dimensional; GE, gradient echo; IR, inversion recovery.
Acquisition times
The acquisition time of the Look–Locker scout was ∼12–15 s. Breath-hold time was 18±1 s for the single-shot IR acquisition. The mere acquisition time of the segmented IR image stacks (reference sequence) was 106±4 s, divided into 9–10 breath-hold manoeuvers. Together with the breathing cycles in between the image acquisition periods, the overall time for the segmented IR sequence was 249±27 s.
Discussion
The accurate assessment of myocardial viability yields important information for the evaluation of further treatment options and the determination of prognosis after ischaemic injury [6,26]. It has been shown that viable myocardium has the potential to improve its function as long as the transmural extent of infarction is <50% [6,27]. In patients with a reduced left-ventricular ejection fraction in particular, the prediction of possible improvement of regional wall thickening is important for a positive outcome after surgical or interventional revascularisation. Owing to its higher spatial resolution, delayed enhancement MRI has proven superior in determining the transmural extent of the ischaemic myocardium to nuclear imaging techniques. The spatial resolution we applied in this study had an in-plane voxel size of 2.0×2.5 mm for the 2D single-shot technique (and 2.0×2.0 mm for the 2D segmented IR–GE technique), allowing for correct assessment of the transmurality in both sequences. The reconstructed voxel size was identical for both techniques to make CNR measurements comparable.
A variety of delayed enhancement sequences and techniques have been described for imaging myocardial infarction. Simonetti et al [23] compared 10 different pulse sequences for differentiating infarction from normal myocardium after contrast agent administration. The now widely-used and well-investigated breath-hold IR segmented GE sequence yielded the highest difference in SI between infarcted and normal myocardium. This sequence type is nowadays the most widely used technique for imaging myocardial infarction in clinical routine. To compare the breath-hold-based single-shot sequence with a segmented technique, we chose a standard 2D T1 GE as a reference sequence. T1 weighted GE sequences generally produce moderately lower signal than SSFP-based sequences; however, the latter contain some additional T2 weighting, which can lead to an overestimation of the myocardial scar owing to enhancement of adjacent oedema in subacute or acute myocardial infarctions.
In addition to segmented IR techniques, several single-shot sequences have been evaluated for delayed enhancement MRI. The single-shot technique allows for the acquisition of an entire slice during one heartbeat. The TI is set individually to null the signal intensity of the normal myocardium. The single-shot technique yields potentially lower SNRs and CNRs than the segmented techniques and therefore has substantial limitations in terms of diagnostic accuracy when used at low field strengths such as 1.5 T [23]. Bauner et al [11] reported successful implementation of a single-shot GE sequence at 3 T. They concluded that the loss of CNR in the single-shot mode was entirely compensated by the higher field strength and that determination of infarct size can be performed without a loss of diagnostic accuracy at 3 T. The short acquisition time of the single-shot technique has the advantage of reducing breathing and ECG artefacts. Elgeti et al [28] successfully applied a 2D single-shot phase-sensitive IR (PSIR) sequence technique for the detection of late gadolinium enhancement in non-ischaemic cardiomyopathies. The CNR in that study was ∼30% lower in the single-shot PSIR sequence than in a standard 2D IR sequence. However, the area of late gadolinium enhancement showed excellent correlation between both techniques and the image quality in both approaches was comparable. Until now, no successful application of single-shot IR imaging using a GE readout instead of an SSFP readout technique for imaging of myocardial infarction at 1.5 T has been reported.
In the present study, we investigated the use of a 2D single-shot IR–GE sequence at 1.5 T using a dedicated 5-channel cardiac coil and compared SNR, CNR, infarct size and transmural extent between both techniques. As expected, the single-shot sequence yielded a lower SNR and CNR than the segmented IR–GE sequence; however, the differences were not statistically significant. There was a good correlation of infarct size and transmurality between both techniques. The single-shot technique showed no relevant disadvantage in terms of reporting the extent of myocardial infarction. The decrease of CNR of 18% compared with the segmented technique can be regarded as acceptable. Scan time was reduced by half scan readout technique.
The use of 16 or even 32 channel receiver coils allows for the use of parallel imaging with high acceleration factors. However, these multi-element coils are characterised by a poorer geometry factor (g-factor) with a resulting loss of SNR/CNR within the centre of the images. The SNR loss is dependent on the acceleration factor, as first described by Pruessmann et al [29]. For only moderate acceleration factors, the SNR decreases approximately with the square root of scan time for favourable coil geometry. With acceleration factors increasing above 2–3, additional degradation of SNR occurs owing to noise amplification in the reconstruction process, characterised by the g-factor [29,30]. Thus, avoiding parallel imaging and using a dedicated cardiac coil with a low number of elements provides a higher SNR/CNR when a single-shot readout is applied.
The reasonable SNR and CNR values of the single-shot sequence can be explained by comparing the products of voxel size and the square root of the net measurement duration to which SNR is directly proportional. For the single-shot and the segmented data techniques, the number of k-space lines divided by bandwidth [(0.75×160/305) and (2×160/382), respectively] and computing the square root yields a ratio of 0.685. This loss is partly compensated by the voxel size ratio of 1.25. The product of 0.86 shows that the SNR of the single-shot sequence is reduced by only 14% compared with the segmented sequence. This is in good agreement with the results from the ROI-based CNR evaluation.
The single-shot sequence overcomes the disadvantage of the long acquisition times of the segmented IR–GE sequences. Using a segmented approach, approximately 10–14 heartbeats are necessary for the acquisition of a single section when a 2D readout technique is applied, which usually results in 9–10 breath-hold manoeuvres to cover the entire left ventricle. In contrast to segmented pulse sequence techniques, single-shot techniques allow for acquisition of 9–10 slices during a single breath-hold. This can be advantageous in critically ill patients with limited breath-hold capabilities, where incompliant breath-holding can lead to reduced image quality. In our study, the acquisition time was significantly shortened from 106 s (249 s including the rests between the breath holds) to 18 s. Similarly, the single-shot technique has the potential to reduce motion artefacts caused by cardiac arrhythmias that can also significantly impair image quality and interpretation when using segmented IR–GE sequences [31]. Compared with the single-shot technique at 3 T, imaging at 1.5 T is less susceptible to field inhomogeneities and dielectric resonance effects.
We chose not to use a phase-sensitive IR sequence such as that used by Elgeti et al [28], because our intention was to evaluate a stable technique suitable for patients with respiratory insufficiency and cardiac arrhythmias. The PSIR technique is rather sensitive to cardiac motion artefacts, especially ECG artefacts, given that two separate data sets are acquired in subsequent R–R intervals and further combined.
The present study was performed to evaluate the single-shot approach at 1.5 T vs a standard reference sequence. Therefore, no critically ill patients were investigated, as these patients would not be compliant enough for acquisition of the segmented IR sequence. The segmented IR technique combines different segments from different R–R cycles, increasing the possibility of image artefacts. This possibility is expected to be reduced by a limited data acquisition time. Further larger studies are required to provide evidence that the reduced acquisition time for a single-shot sequence really leads to a significant reduction of breathing and ECG artefacts.
The limitations of our study include the relatively small sample size and the weaker spatial resolution of the single-shot technique. The decreased spatial resolution of the single-shot IR sequence may have led to missing small infarcts and underestimating the extent of the myocardial scar in some cases, with fewer affected segments detected in the single-shot technique than in the segmented approach.
Despite the reasonable SNR/CNR performance, the use of a five-channel coil would limit to some degree extensive use of parallel imaging techniques, which have recently gained much attention in cardiac imaging.
In addition, it has been demonstrated that determination of SNRs is problematic when using multichannel phased-array coils when signal and noise are determined in separate regions [32]. However, when comparing the noise levels in various regions in the current study, we did not observe significant differences.
Conclusion
The results of our study demonstrate a successful application of 2D single-shot IR–GE delayed enhancement imaging of myocardial infarction at 1.5 T with a dedicated cardiac coil and appropriate sequence parameters. When compared with a segmented 2D GE technique, the single-shot sequence yields moderately lower SNR and CNR but still allows for accurate determination of the extent of myocardial infarction with a good spatial resolution. The dramatically shorter acquisition time can be advantageous in critically ill patients with impaired breath-holding capacities or cardiac arrhythmias.
References
- 1.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992–2002 [DOI] [PubMed] [Google Scholar]
- 2.Pirich C, Schwaiger M. The clinical role of positron emission tomography in management of the cardiac patient. Rev Port Cardiol 2000;19Suppl. 1:I89–100 [PubMed] [Google Scholar]
- 3.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003;361:374–9 [DOI] [PubMed] [Google Scholar]
- 4.Slart RH, Bax JJ, van Veldhuisen DJ, van derWall EE, Dierckx RA, Jager PL. Imaging techniques in nuclear cardiology for the assessment of myocardial viability. Int J Cardiovasc Imaging 2006;22:63–80 [DOI] [PubMed] [Google Scholar]
- 5.Fieno DS, Hillenbrand HB, Rehwald WG, Harris KR, Decker RS, Parker MA, et al. Infarct resorption, compensatory hypertrophy, and differing patterns of ventricular remodeling following myocardial infarctions of varying size. J Am Coll Cardiol 2004;43:2124–31 [DOI] [PubMed] [Google Scholar]
- 6.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–53 [DOI] [PubMed] [Google Scholar]
- 7.Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol 2009;54:1407–24 [DOI] [PubMed] [Google Scholar]
- 8.Arrighi JA. Integrated imaging of cardiac anatomy, physiology, and viability. Curr Cardiol Rep 2009;11:125–32 [DOI] [PubMed] [Google Scholar]
- 9.Rehwald WG, Fieno DS, Chen EL, Kim RJ, Judd RM. Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury. Circulation 2002;105:224–9 [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim T, Hackl T, Nekolla SG, Breuer M, Feldmair M, Schomig A, et al. Acute myocardial infarction: serial cardiac MR imaging shows a decrease in delayed enhancement of the myocardium during the 1st week after reperfusion. Radiology 2010;254:88–97 [DOI] [PubMed] [Google Scholar]
- 11.Bauner KU, Muehling O, Wintersperger BJ, Winnik E, Reiser MF, Huber A. Inversion recovery single-shot TurboFLASH for assessment of myocardial infarction at 3 Tesla. Invest Radiol 2007;42:361–71 [DOI] [PubMed] [Google Scholar]
- 12.Bekkers SC, Backes WH, Kim RJ, Snoep G, Gorgels AP, Passos VL, et al. Detection and characteristics of microvascular obstruction in reperfused acute myocardial infarction using an optimized protocol for contrast-enhanced cardiovascular magnetic resonance imaging. Eur Radiol 2009;19:2904–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewey M, Laule M, Taupitz M, Kaufels N, Hamm B, Kivelitz D. Myocardial viability: assessment with three-dimensional MR imaging in pigs and patients. Radiology 2006;239:703–9 [DOI] [PubMed] [Google Scholar]
- 14.Kino A, Zuehlsdorff S, Sheehan JJ, Weale PJ, Carroll TJ, Jerecic R, et al. Three-dimensional phase-sensitive inversion-recovery turbo FLASH sequence for the evaluation of left ventricular myocardial scar. AJR Am J Roentgenol 2009;193:W381–8 [DOI] [PubMed] [Google Scholar]
- 15.Klein C, Gebker R, Kokocinski T, Dreysse S, Schnackenburg B, Fleck E, et al. Combined magnetic resonance coronary artery imaging, myocardial perfusion and late gadolinium enhancement in patients with suspected coronary artery disease. J Cardiovasc Magn Reson 2008;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peukert D, Laule M, Taupitz M, Kaufels N, Hamm B, Dewey M. 3D and 2D delayed-enhancement magnetic resonance imaging for detection of myocardial infarction: preclinical and clinical results. Acad Radiol 2007;14:788–94 [DOI] [PubMed] [Google Scholar]
- 17.Saranathan M, Rochitte CE, Foo TK. Fast, three-dimensional free-breathing MR imaging of myocardial infarction: a feasibility study. Magn Reson Med 2004;51:1055–60 [DOI] [PubMed] [Google Scholar]
- 18.van denBosch HC, Westenberg JJ, Post JC, Yo G, Verwoerd J, Kroft LJ, et al. Free-breathing MRI for the assessment of myocardial infarction: clinical validation. AJR Am J Roentgenol 2009;192:W277–81 [DOI] [PubMed] [Google Scholar]
- 19.Warntjes MJ, Kihlberg J, Engvall J. Rapid T1 quantification based on 3D phase sensitive inversion recovery. BMC Med Imaging 2010;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellman P, Larson AC, Hsu LY, Chung YC, Simonetti OP, McVeigh ER, et al. Motion-corrected free-breathing delayed enhancement imaging of myocardial infarction. Magn Reson Med 2005;53:194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledesma-Carbayo MJ, Kellman P, Hsu LY, Arai AE, McVeigh ER. Motion corrected free-breathing delayed-enhancement imaging of myocardial infarction using nonrigid registration. J Magn Reson Imaging 2007;26:184–90 [DOI] [PubMed] [Google Scholar]
- 22.Huber A, Bauner K, Wintersperger BJ, Reeder SB, Stadie F, Mueller E, et al. Phase-sensitive inversion recovery (PSIR) single-shot TrueFISP for assessment of myocardial infarction at 3 tesla. Invest Radiol 2006;41:148–53 [DOI] [PubMed] [Google Scholar]
- 23.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology 2001;218:215–23 [DOI] [PubMed] [Google Scholar]
- 24.Detsky JS, Stainsby JA, Vijayaraghavan R, Graham JJ, Dick AJ, Wright GA. Inversion-recovery-prepared SSFP for cardiac-phase-resolved delayed-enhancement MRI. Magn Reson Med 2007;58:365–72 [DOI] [PubMed] [Google Scholar]
- 25.Alfakih K, Sparrow P, Plein S, Sivananthan MU, Walters K, Ridgway JP, et al. Delayed enhancement imaging: standardised segmental assessment of myocardial viability in patients with ST-elevation myocardial infarction. Eur J Radiol 2008;66:42–7 [DOI] [PubMed] [Google Scholar]
- 26.Bello D, Shah DJ, Farah GM, Di Luzio S, Parker M, Johnson MR, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation 2003;108:1945–53 [DOI] [PubMed] [Google Scholar]
- 27.Depre C, Vanoverschelde JL, Melin JA, Borgers M, Bol A, Ausma J, et al. Structural and metabolic correlates of the reversibility of chronic left ventricular ischemic dysfunction in humans. Am J Physiol 1995;268(3 Pt 2):H1265–75 [DOI] [PubMed] [Google Scholar]
- 28.Elgeti T, Abdel-Aty H, Wagner M, Busjahn A, Schulz-Menger J, Kivelitz D, et al. Assessment of late gadolinium enhancement in nonischemic cardiomyopathy: comparison of a fast Phase-Sensitive Inversion Recovery Sequence (PSIR) and a conventional segmented 2D gradient echo recall (GRE) sequence–preliminary findings. Invest Radiol 2007;42:671–5 [DOI] [PubMed] [Google Scholar]
- 29.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999;42:952–62 [PubMed] [Google Scholar]
- 30.Reeder SB, Wintersperger BJ, Dietrich O, Lanz T, Greiser A, Reiser MF, et al. Practical approaches to the evaluation of signal-to-noise ratio performance with parallel imaging: application with cardiac imaging and a 32-channel cardiac coil. Magn Reson Med 2005;54:748–54 [DOI] [PubMed] [Google Scholar]
- 31.Rosendahl L, Ahlander BM, Bjorklund PG, Blomstrand P, Brudin L, Engvall JE. Image quality and myocardial scar size determined with magnetic resonance imaging in patients with permanent atrial fibrillation: a comparison of two imaging protocols. Clin Physiol Funct Imaging 2010;30:122–9 [DOI] [PubMed] [Google Scholar]
- 32.Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging 2007;26:375–85 [DOI] [PubMed] [Google Scholar]