Abstract
In this review we use images from an 11-year-old male to describe Proteus syndrome, a complex disorder with multisystem involvement and great clinical variability. Our aim is to enhance recognition of the typical imaging findings, which can aid diagnosis of this rare condition.
Proteus syndrome (PS) is a rare complex disorder with multisystem involvement and great clinical variability, first described in 1979 [1]. The name was coined by Wiedemann et al [2] in 1983 after the Greek god of the sea, Proteus, who could change his form at will in order to avoid capture. It is now thought that the “Elephant Man” suffered from PS rather than neurofibromatosis Type 1, as previously described [3]. Although the aetiology of PS still remains unclear, there is evidence that it occurs after mutation of a somatic dominant gene that is typically lethal, except in the setting of mosaicism [4].
PS typically manifests as asymmetrical partial gigantism and hemihypertrophy. Other clinical findings include macrodactyly, plantar or palmar hyperplasia, haemangioma, lipoma, lymphangioma, varicosity, epidermal and connective tissue naevi, cranial exostosis, macrocephaly and skeletal anomalies [5]. Given the lack of a specific genetic test for this condition, the diagnosis is based on the clinical and radiological evaluation. Familiarity and awareness of various imaging findings of this condition are essential for appropriate diagnosis. We describe, with examples, the various imaging manifestations in this unusual syndrome. Images are from an 11-year-old male who underwent serial imaging during his multiple hospital admissions.
Manifestations
Soft tissue manifestations
Asymmetrical subcutaneous fat overgrowth is one of the common findings and is usually seen over the torso but can be seen over the extremities (Figures 1 and 2). This may be associated with muscle overgrowth or may be complex with proliferating lymphatic channels and vascular malformations. Rarely, muscle atrophy can also be seen.
Figure 1.
Subcutaneous fat hypertrophy on the left anterior chest wall (arrow).
Figure 2.
Asymmetrical atrophy of the left gluteal muscles with fatty infiltration (arrow).
Skeletal manifestations
These are varied and include kyphoscoliosis abnormal vertebral bodies (asymmetrical vertebral body overgrowth, posterior scalloping), segmentation defects, focal calvarial thickening, limb overgrowth, premature degenerative changes, macrodactyly and sometimes non-development of peripheral bones [4,5] (Figures 3–6).
Figure 3.
Loss of T4 vertebral body height (arrow).
Figure 6.
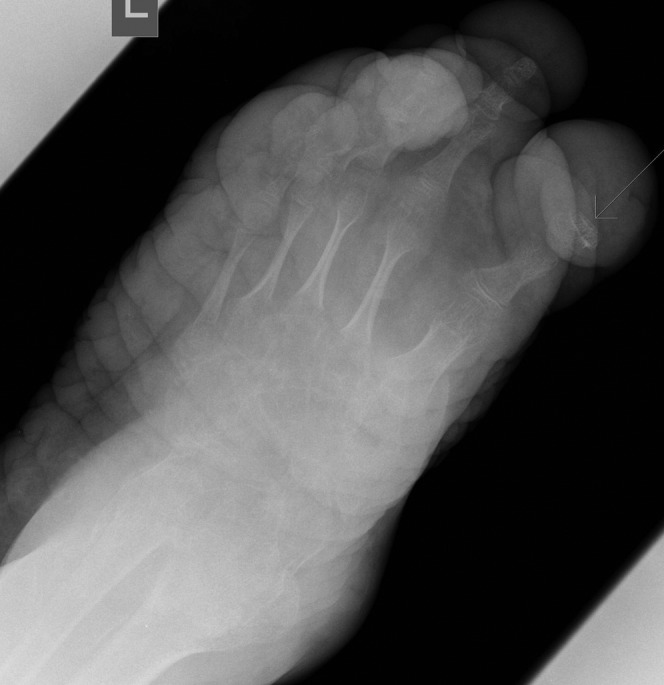
Anteroposterior view demonstrates absence of distal end of the first metatarsal distal phalanx (arrow).
Figure 4.
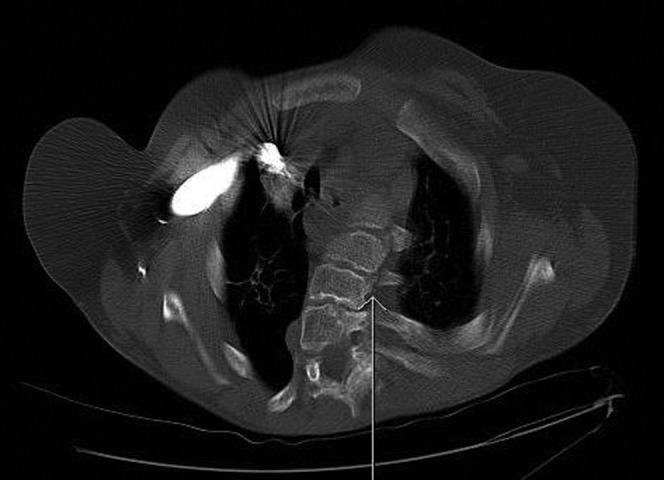
Kyphoscoliosis of the upper thoracic vertebrae (arrow) (axial CT bone windows).
Figure 5.
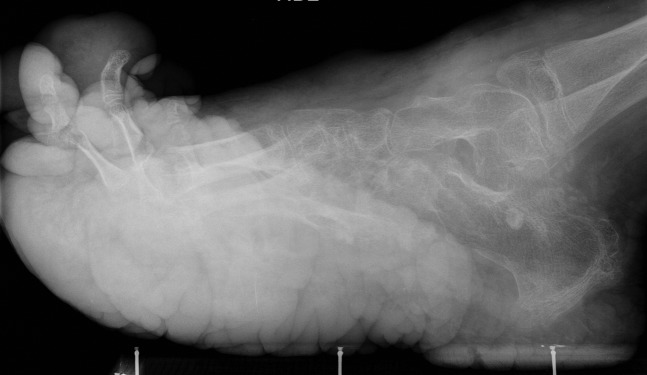
Lateral X-ray of the foot in an 11-year-old male, demonstrating soft tissue overgrowth in keeping with plantar cerebriform connective tissue naevus. The metatarsals are osteopenic, gracile and overtubulated with widened joint spaces. Degenerative changes from the gross deformities are noted in the tibiocalcaneal joint.
Vascular manifestations
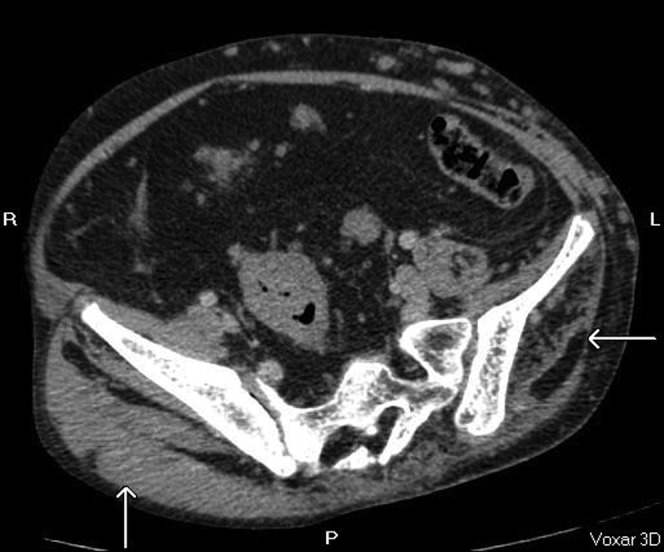
Vascular malformations can be profound and include vascular ectasia, haemangiomata, lymphangiomata and varicosities [6] (Figures 7–14). The vascular ectasia may be progressive (Figures 9–14). Deep-vein thrombosis leading to pulmonary embolism is the commonest cause of death in this group of patients, possibly related to venous stasis in grossly ectatic vessels.
Figure 7.
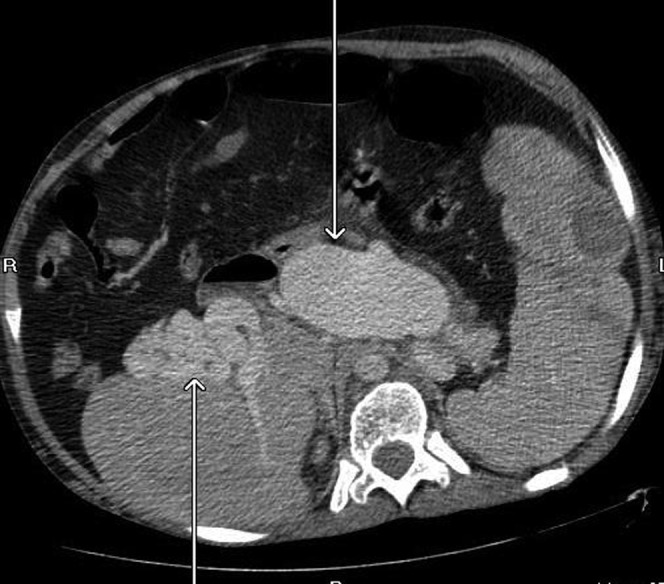
Ectactic confluence of splenic (central arrow) and portal vein (arrow to the left of the image) with varicosities at the hila (axial CT, post-contrast portal venous phase).
Figure 14.
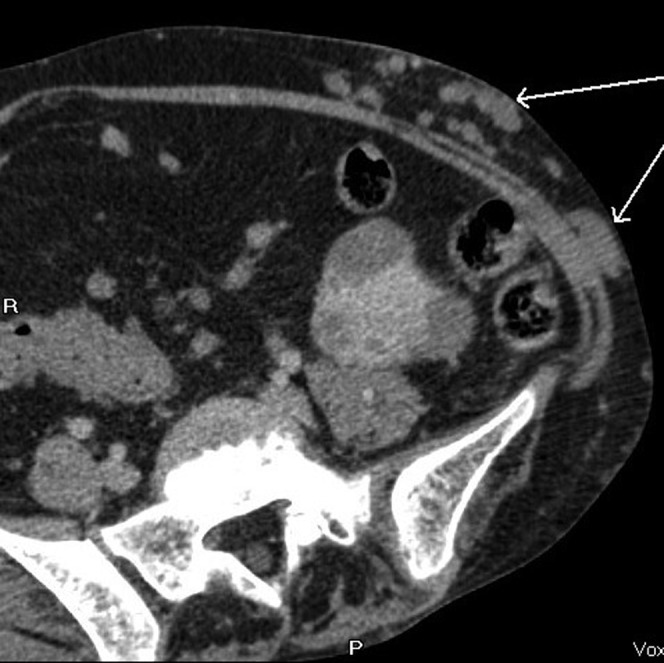
Enlarged subcutaneous collateral vessels (arrows).
Figure 9.
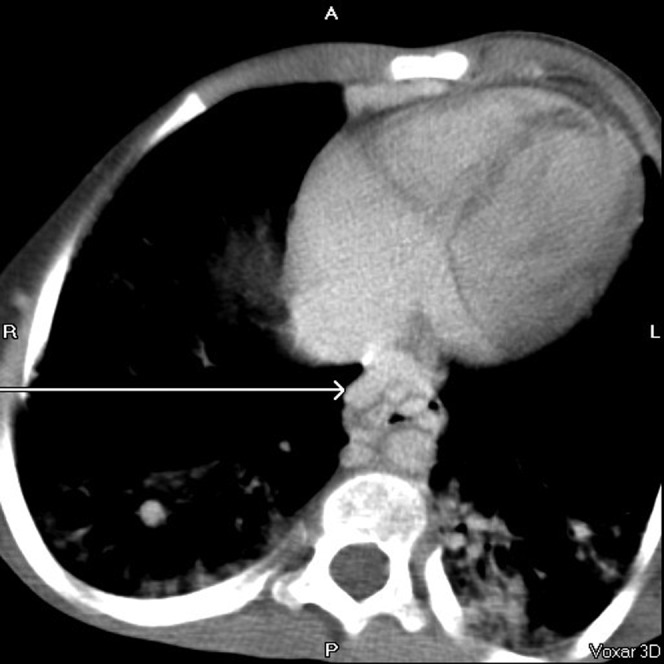
Inferior vena cava (arrow); Figures 10–14 show interval imaging on the same patient, demonstrating progression of the vascular abnormalities.
Figure 8.
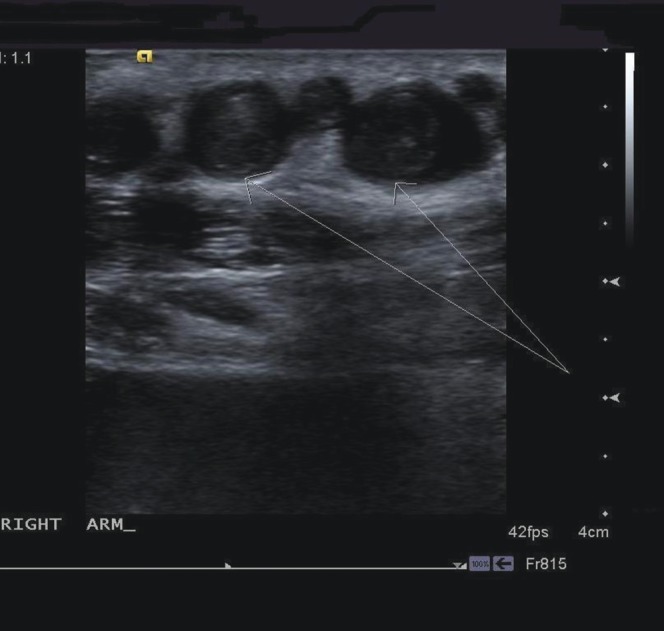
Transverse ultrasound images through right forearm demonstrating enlarged peripheral veins with thrombus (arrows) within.
Figure 10.
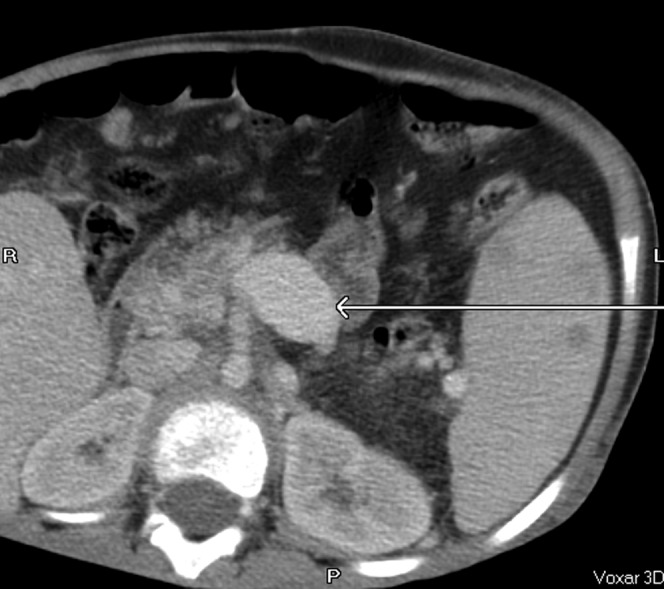
Dilated superior mesenteric vein (arrow).
Figure 11.
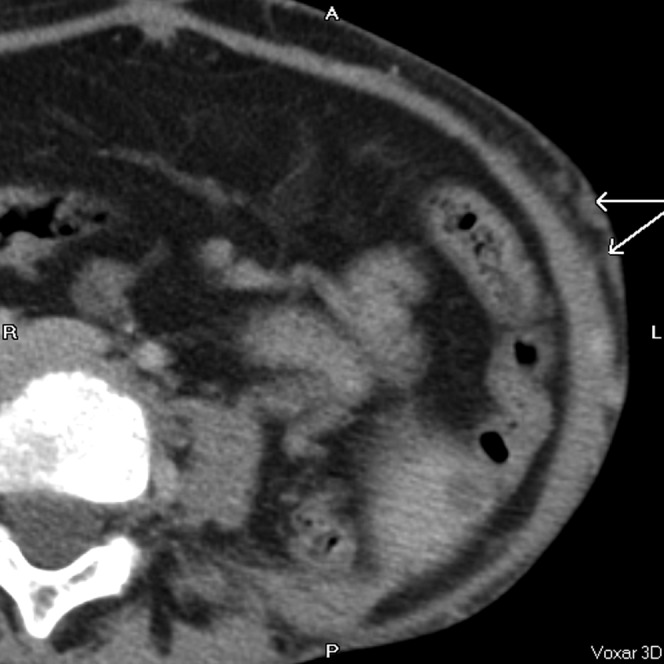
Subcutaneous collateral vessels (arrows).
Figure 12.
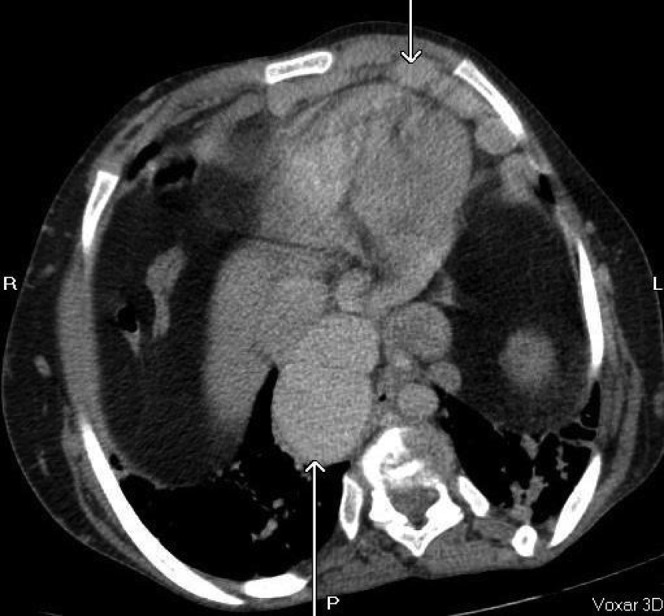
Gross aneurysmal enlargement of the inferior vena cava (lower arrow to the left of the image) and internal mammary vessels (upper arrow to the right of the image).
Figure 13.
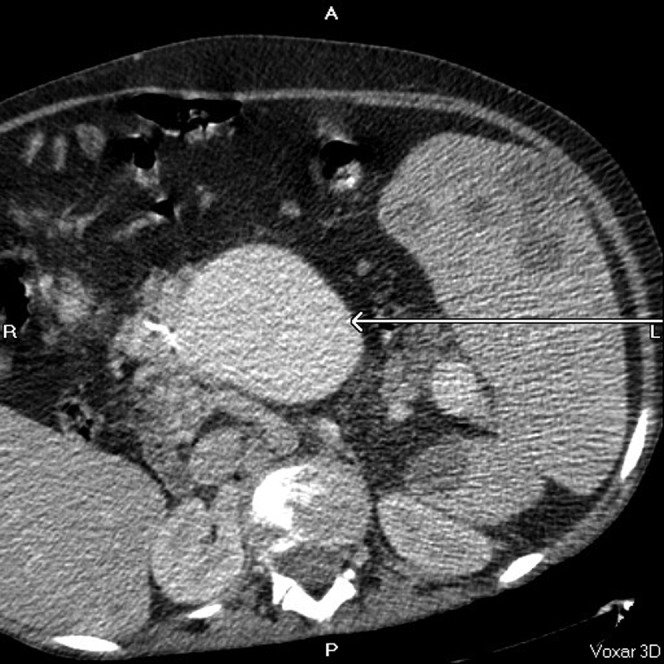
Superior mesenteric vein (arrow).
Visceral manifestations
Splenomegaly with or without cystic changes is usually seen. Nephromegaly, hydronephrosis, renal calculi, gastromegaly, colonic polyps, pancreatic lipomatosis, uterine leiomyomatas, hypoplastic uterus, cervical cysts and enlarged ovaries have been described [5,7] (Figures 15–17).
Figure 15.
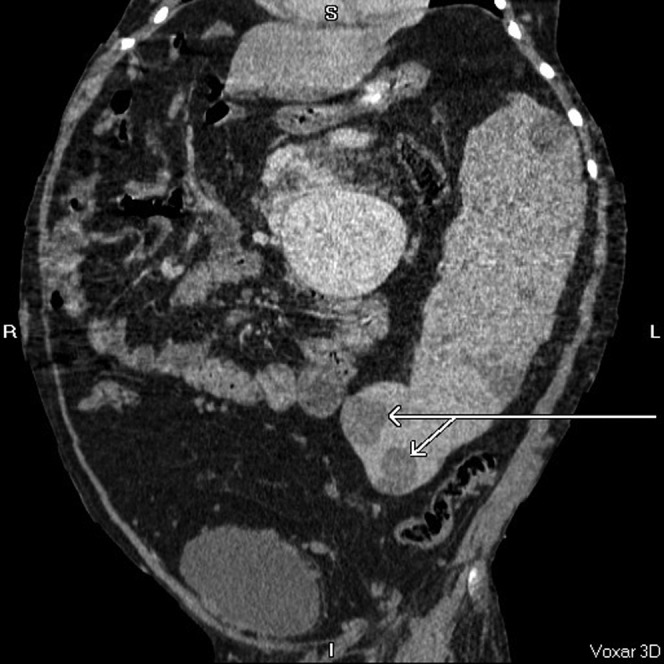
Massive splenomegaly (30 cm) with multiple splenic cysts (arrows), some of which have soft tissue components within. Coronal, axial and sagittal post-contrast CT images.
Figure 17.
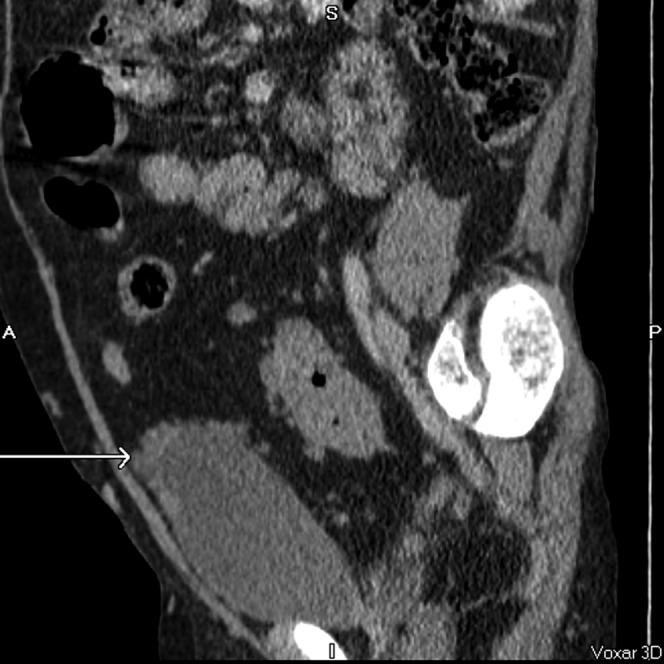
Multiple nodules around the bladder dome (arrow) creating spurious appearance of nodular bladder wall thickening. Coronal, axial and sagittal post-contrast CT images.
Figure 16.
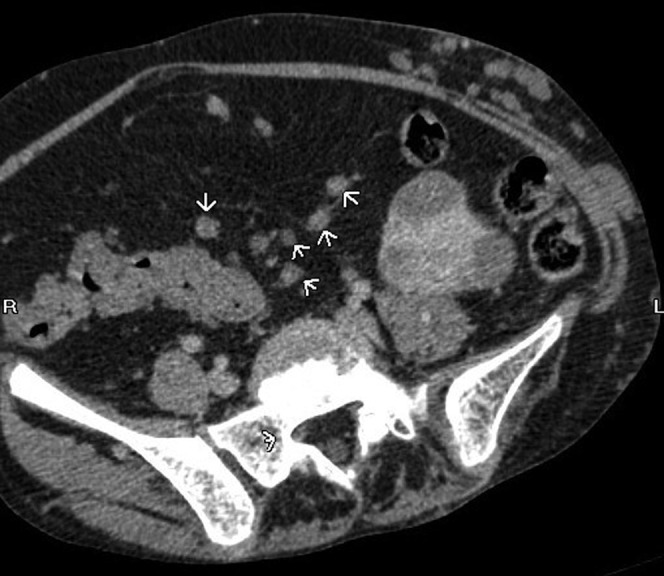
Peritoneal lymphatic nodules (arrowheads). Coronal, axial and sagittal post-contrast CT images.
Thoracic manifestations
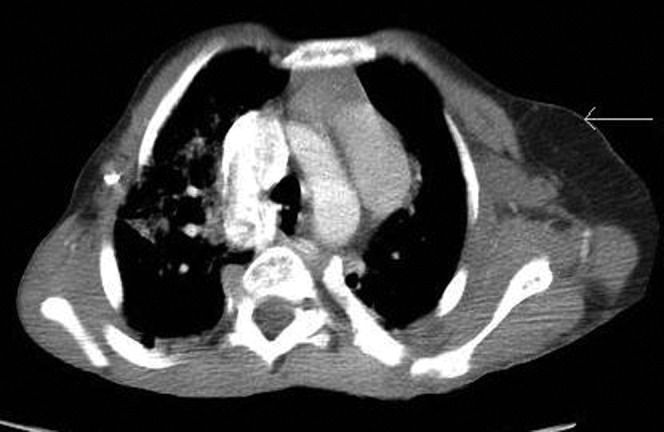
These are highly variable and include pulmonary emphysema, cystic changes, lung scarring and consolidation [5,7] (Figures 18, 19).
Figure 19.
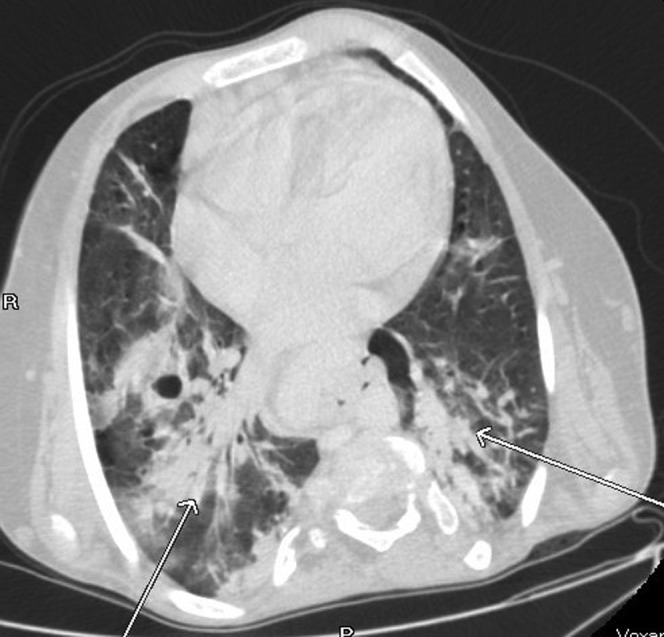
Extensive bibasal consolidation (arrows). This has not been reported as a common finding. The patient was admitted with non-specific abdominal pain and there were no clinical or biochemical features to suggest pneumonia. Axial CT, lung windows.
Figure 18.
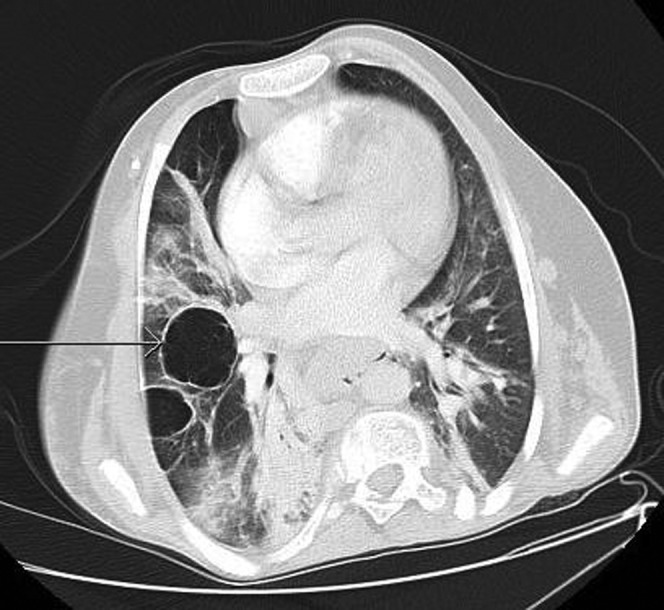
Emphysematous changes with bullae formation (arrow). Axial CT, lung windows.
Neurological manifestations
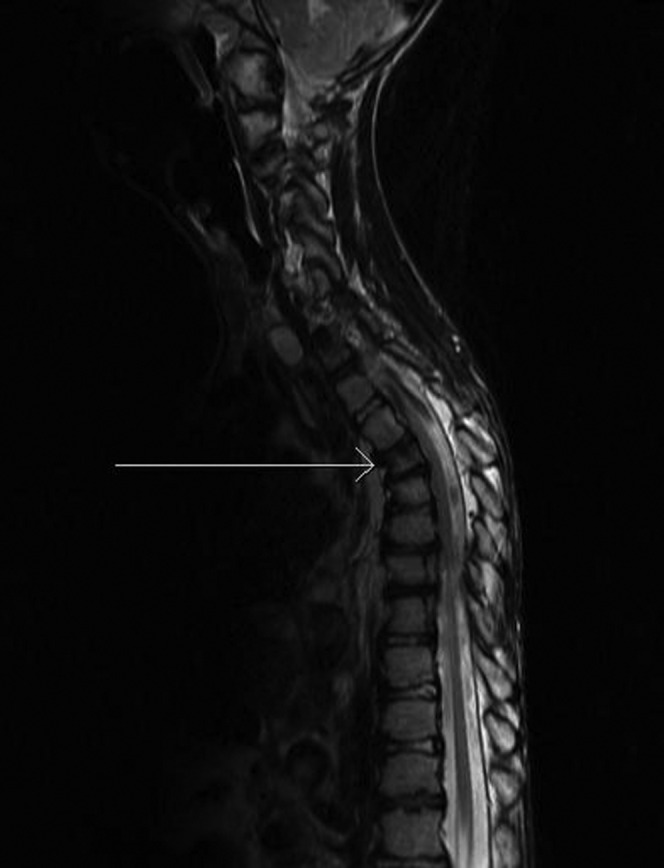
Cerebral arteriovenous malformations, abnormal grey–white matter differentiation and hydrocephalus are commonly seen. Schizencephaly, spinal lipomatosis and perineural cysts have also been described [8] (Figures 20 and 21).
Figure 20.
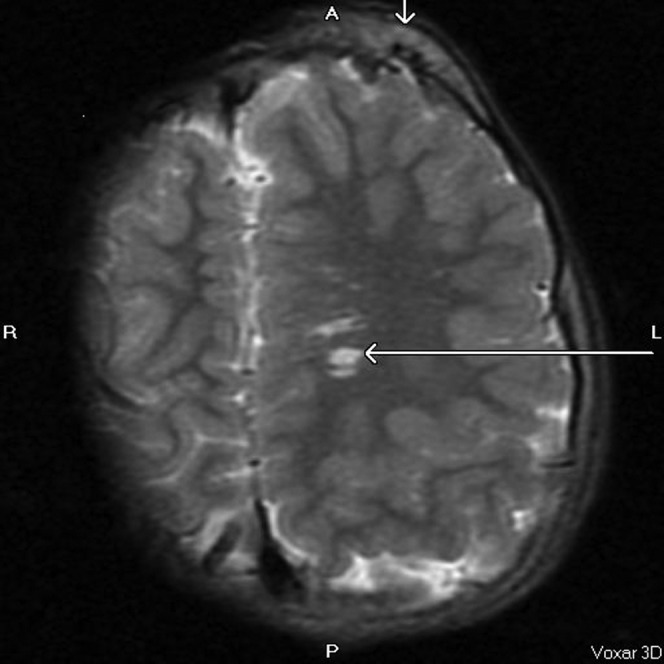
Asymmetrical calvarial thickening (vertical arrow) and arteriovenous malformation (horizontal arrow). MRI image, axial T2, repetition time 5700 ms, echo time 98 ms.
Figure 21.
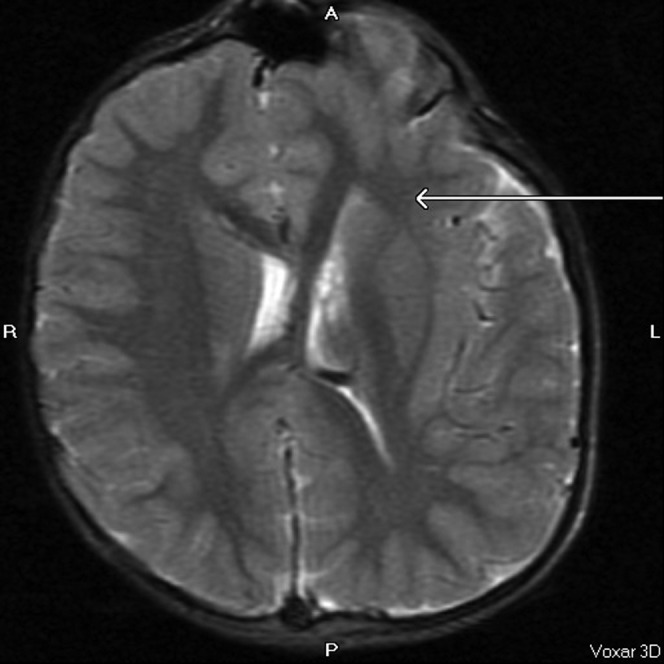
Asymmetry of the cerebral hemispheres and lateral ventricles with abnormal grey–white matter differentiation (arrow) and areas of macrogyria in the left cerebral hemisphere. MRI image, axial T2, repetition time 5700 ms, echo time 98 ms.
Diagnostic criteria
Owing to the unusual and highly variable manifestations, some radiologists follow diagnostic criteria developed in 1998 to help in the diagnosis [4,5]. For PS to be diagnosed, all the general criteria should be present, along with various specific criteria, including the presence of the Category A criterion, or two Category B criteria or three Category C criteria.
General criteria:
mosaic distribution of lesions
sporadic occurrence
progressive course.
Specific criteria:
Category A—cerebriform connective tissue naevus
Category B—epidermal nevus; asymmetric, disproportionate musculoskeletal or visceral overgrowth; and specific tumours that occur before the age of 30 years (ovarian cystadenoma, parotid monomorphic adenoma)
Category C—asymmetric adipose tissue deposition (lipomas, lymphoid hypoplasia), vascular or lymphatic malformations, lung cysts and facial phenotype.
Differential considerations
The two main differentials for this condition are Klippel–Trenaunay–Weber syndrome (KTWS) and neurofibromatosis Type 1.
In KTWS, the changes of soft tissue growth and hypertrophy are present at birth and are usually severe, whereas the changes are usually absent at birth in PS. The key distinction between the two conditions is presence of bone involvement and progressive hypertrophy in PS, which is absent in KTWS [5].
Neurofibromatosis is usually distinguished from PS clinically by the presence of the clinical triad of pigmented skin nodules (café-au-lait spots), Lisch nodules and axillary freckling.
Conclusion
PS is a rare and unusual multisystem disorder. Recognition of the typical imaging findings can aid diagnosis of this condition.
References
- 1.Cohen MM, Jr, Hayden PW. A newly recognized hamartomatous syndrome. Birth Defects Orig Artic Ser 1979;15:291–6 [PubMed] [Google Scholar]
- 2.Wiedemann H-R, Burgio GR, Aldenhoff P, Kunze J, Kaufmann HJ, Schirg E. The Proteus syndrome: partial gigantism of the hands and/or feet, nevi, hemihypertrophy, subcutaneous tumors, macrocephaly or other skull anomalies and possible accelerated growth and visceral affections. Eur J Pediatr 1983;140:5–12 [DOI] [PubMed] [Google Scholar]
- 3.Friedman LD. Medicine and the arts. The elephant man. Acad Med 2000;75:448–9 [DOI] [PubMed] [Google Scholar]
- 4.Biesecker LG, Happle R, Mulliken JB, Weksberg R, Graham JM, Jr, Viljoen DL, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet 1999;84:389–95 [DOI] [PubMed] [Google Scholar]
- 5.Jamis-Dow CA, Turner J, Biesecker LG, Choyke PL. Radiologic manifestations of Proteus syndrome. RadioGraphics 2004;24:1051–68 [DOI] [PubMed] [Google Scholar]
- 6.Hoeger PH, Martinez A, Maerker J, Harper JI. Vascular anomalies in Proteus syndrome. Clin Exp Dermatol 2004;29:222–30 [DOI] [PubMed] [Google Scholar]
- 7.Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet 2006;14:1151–7 [DOI] [PubMed] [Google Scholar]
- 8.Dietrich RB, Glidden DE, Roth GM, Martin RA, Demo DS. The Proteus syndrome: CNS manifestations. AJNR Am J Neuroradiol 1998;19:987–90 [PMC free article] [PubMed] [Google Scholar]