Summary
The anti-CD52 antibody alemtuzumab has been explored as a novel targeted therapy in T-cell malignancies. To assess the suitability of alemtuzumab therapy we carried out a comprehensive study of CD52 expression using flow cytometry (FC) in 78 untreated patients diagnosed with mature T/NK cell neoplasms, including 34 adult T-cell leukemia /lymphomas (ATLL), two anaplastic large cell lymphomas (ALCL), three angioimmunoblastic T-cell lymphomas (AITL), 16 cutaneous T-cell lymphomas (CTCL), four extra-nodal T/NK cell lymphomas (ENT/NKCL), four hepatosplenic T-cell lymphomas (HSTCL), 13 peripheral T-cell lymphomas, unspecified (PTCL-NOS), and two T-prolymphocytic leukemia (T-PLL). The level of CD52 expression was quantitated using QuantiBRITE standard beads. The level of CD52 expression varied widely within each diagnostic category. All AITL, HSTCL, and T-PLL cases were CD52 positive and the frequency of CD52 expression was high in PTCL-NOS (92.3%), ATLL (94.1%) and CTCL (87.5%), implying a rational role for alemtuzumab in the treatment of these diseases; however, CD52 expression was low in ALCL (50%) and ENT/NKCL (25%). FC testing for cell surface expression of CD52 is indicated in patients with T/NK cell malignancies being considered for alemtuzumab therapy. Further studies are necessary to determine if the level of CD52 expression correlates with response to therapy.
Keywords: Alemtuzumab, CD52, flow cytometry, NK cell lymphoma, T cell lymphoma
Introduction
CD52 is a glycosylphosphatidylinositol (GPI) anchored low molecular weight glycoprotein (21–28 kDa)(Xia, et al 1993a, Xia MQ 1993) expressed on the surface of B and T lymphocytes, natural killer (NK) cells, monocytes, macrophages, and some dendritic cells, but not on plasma cells, granulocytes, erythrocytes, platelets, or hematopoietic progenitor cells (Hale, et al 1990, Hernandez-Campo, et al 2007, Waldmann and Hale 2005). CD52 exists in two forms, CD52-I and CD52-II. Both forms are recognized by alemtuzumab (Campath-1H), a “humanized” rat IgG1 antibody developed by transferring the antigen-specific, complementary determining regions of the rat monoclonal antibody onto a human framework (Treumann, et al 1995, Waldmann and Hale 2005). Although CD52 has little diagnostic value, the antigen has been shown to be a valuable target for antibody therapy in lymphoid neoplasia because of its abundant cell surface expression, close apposition to the cell membrane, and lack of modulation after antibody binding (Bindon, et al 1988, Treumann, et al 1995). Upon binding to the cell surface CD52, alemtuzumab induces cell destruction via activation of complement dependent cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC), and induction of apoptosis (Nuckel, et al 2005, Project. 1997, Xia, et al 1993b). While all three activities have been demonstrated in vitro, the mechanism of in vivo cell killing remains unclear.
Mature T and natural killer (NK)-cell neoplasms are uncommon and comprise less than 10% of non-Hodgkin’s lymphomas (Project. 1997). Except for ALK-positive anaplastic large cell lymphoma, which is curable in the majority of patients with standard chemotherapy and T cell large granular lymphocytic leukemia (T-LGL), which is commonly indolent, most T and NK cell neoplasms are clinically aggressive and show disappointingly short responses to conventional chemotherapy compared to their B cell counterparts (Jaffe 2001).
Alemtuzumab has demonstrated significant activity against a number of B-cell malignancies, particularly in refractory and relapsed chronic lymphocytic leukemia, as well as other non-malignant hematopoietic disorders (Faulkner, et al 2004, Gupta, et al 2004, Keating, et al 2002). Several clinical trials have explored the role of alemtuzumab in the treatment of T-cell disorders, including peripheral T cell lymphoma-not otherwise specified (PTCL-NOS), T cell prolymphocytic leukemia (T-PLL), cutaneous T cell lymphoma(CTCL), and adult T-cell lymphoma/leukemia (ATLL)(Dearden 2006, Enblad, et al 2004, Gallamini A 2007, Kim JG 2007, Lundin, et al 2003, Pawson, et al 1997, Zhang, et al 2003). Most of these studies have demonstrated antitumor activity; however, in these trials CD52 expression by the malignant cells was not established prior to initiation of therapy. Alemtuzumab can also result in substantial toxicity due to attendant immunosuppression associated with its use, particularly increased risk of viral and other opportunistic infections (Alinari, et al 2007, Dearden and Matutes 2006, Enblad, et al 2004). Pre-treatment evaluation for expression of CD52 may aid in guiding patient management and limit unnecessary exposure to alemtuzumab’s potentially toxic effects.
Although several groups have investigated CD52 expression on malignant lymphocytes in selected mature T and NK cell lymphomas, these studies involved a limited variety and small number of cases. Moreover, most samples were evaluated using immunohistochemical methods on archived material (Piccaluga, et al 2007, Rodig, et al 2006). One retrospective study utilizing flow cytometry analyzed cryopreserved blood specimens (Ginaldi, et al 1998). Immunohistochemical studies (IHC) examining CD52 expression have limitations. Many cases of T and NK cell lymphomas demonstrate minimal to mild cytological atypia that is difficult to appreciate on IHC slides. These lymphomas are often associated with a prominent reactive background of normal B and T cells associated with fibrosis that may obscure visual identification of the neoplastic cells and limit interpretation. Since CD52 is ubiquitously expressed by all mature lymphocytes, it is often difficult to distinguish expression by the small to medium sized malignant lymphoid cells from the surrounding reactive lymphocytes on IHC slides. In addition, a fibrotic background may interfere with antibody’s ability to bind to its target antigen on the fixed embedded tumor cells in. Additionally, there is the possibility of antigen loss through formalin fixation and paraffin embedding, which may diminish the sensitivity of the study. Furthermore, CD52 must be expressed on the cell surface for alemtuzumab to be effective and IHC can not determine if a membrane associated antigen is on the external or internal cell membrane. In addition low level CD52 expression may not be detected by IHC whereas flow cytometry can readily detect these cell populations. Therefore, we believe that flow cytometry analysis on fresh specimens offers clear advantages in evaluating CD52 expression for patient’s being considered for alemtuzumab therapy.
To our knowledge, a comprehensive study of the presence of cell surface CD52 expression by flow cytometry (FC) among a broad spectrum of T and NK cell neoplasms has not been reported. We therefore conducted a prospective study FC study to determine cell surface CD52 expression in patients diagnosed with mature T cell and NK cell neoplasms prior to initiation of alemtuzumab therapy. The purpose of the study was to determine if alemtuzumab was a rational therapeutic choice in specific T cell and NK cell neoplasms based on its expression by the neoplastic clone.
Methods
Case selection
Specimens from seventy-eight patients with a confirmed diagnosis of a mature T cell or NK cell lymphoma based upon the WHO classification (Jaffe 2001) were submitted to the Flow Cytometry Unit, Laboratory of Pathology, National Cancer Institute (Bethesda, MD, USA) for evaluation of cell surface expression of CD52 by FC. Patients were undergoing eligibility evaluation for research protocols studying the efficacy of alemtuzumab alone or with chemotherapeutic regimens in various T cell lymphoproliferative disorders. All patients signed IRB-approved informed consent to be screened for eligibility. Clinical data were obtained through medical record review and by contacting the patients’ NIH staff physicians. All patients had a confirmed diagnosis of T cell or NK cell neoplasia with a distinctively abnormal FC immunophenotype that could be employed to distinguish malignant cells from normal lymphoid cell populations. The cases included 34 patients with HTLV-I associated adult T-cell leukemia/lymphoma (ATLL), 2 patients with anaplastic large cell lymphoma (ALCL), 3 angioimmunoblastic T-cell lymphomas (AITL), 16 cutaneous T-cell lymphomas (CTCL), 4 extra-nodal T/NK cell lymphomas (ENT/NKCL), 4 hepatosplenic T-cell lymphomas (HSTCL) including 3 of γδ and one of αβ T cell origin, 13 peripheral T-cell lymphomas, not otherwise specified (PTCL-NOS), and 2 T cell prolymphocytic leukemia (T-PLL) patients. Two cases of acute myeloid leukemia were selected as negative controls.
Tumor subclassification was based on the WHO criteria for hematologic malignancies using a combination of morphologic, immunohistochemical and flow cytometric immunophenotypic studies. When necessary, molecular studies for T cell receptor gamma gene rearrangement by polymerase chain reaction (PCR) and HTLV-1 serology (performed by ELISA and confirmed by western blot) and blood HTLV-1 viral load by real-time PCR were utilized. The diagnoses were confirmed by review of the original pathology reports, FC immunophenotype, IHC immunophenotype, and cytology or histological evaluation by three hematopathologists (L. Jiang, C. Yuan, and M. Stetler-Stevenson). All the specimens, including peripheral blood, bone marrow, body fluid, and fine needle aspiration from lymph node, skin nodules, and soft tissue mass, were collected and analyzed prior to initiation of alemtuzumab.
Immunophenotyping
All specimens were stained within 24 hour of collection with a panel of antibodies. Specimens were washed with phosphate buffered saline (PBS) to remove cytophilic antibodies before determining cell number. Cellularity was manually determined using a hemocytometer and viability was determined by trypan blue uptake. Specimens were stained for 30 min at room temperature with a cocktail of four antibodies (antibody concentration according to manufacturer’s recommendations). If red cells were present, erythrocytes were lysed by incubating with lysing solution (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) for 10 minutes at room temperature at a ratio of 1:9 (sample volume: lysing solution volume. After incubation, cells were pelleted by centrifugation (500 ×g for 15 minutes at room temperature), the media was aspirated, and the cells washed twice in a PBS solution containing 0.1% NaN3. The antibody panels were chosen based on the number of cells and diagnosis. The panel included antibodies against CD2, CD3, CD5, CD4, CD8, CD7, gamma/delta receptor, alpha/beta receptor, CD45 and CD52 was studied in all the cases. Moreover, depending on individual cases, antibodies targeting specific antigens characteristically expressed in the patient’s lymphomas were also used, including CD10 for AITL, CD25 for ATLL, and CD30 for ALCL. All cells were fixed in 1.0% paraformaldehyde and stored at 4°C for up to 12 h before FC acquisition. Normal lymphoid cells within specimens served as internal controls. Normal T cells were evaluated in 20 control specimens.
Four-color cytometry was performed using a BD Biosciences FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). The sensitivity of fluorescent detectors was monitored using Calibrite beads (BD Biosciences) according to the manufacturer’s recommendations. Data, collected in list mode, were analyzed with CellQuest Pro software (BD Bioscience) and FCSExpress (De Novo Software, Los Angeles, CA). At least 5,000 lymphocytes were acquired per tube. For analysis, relevant cell populations were analyzed by gating on forward scatter (FSC), side scatter (SSC), CD45, CD3, and characteristic markers for each specific entity (i.e. CD25 for ATLL), and the cell population of interest subsequently examined for staining with anti-CD52 (Southern Biotech, Birmingham, AL).
The antibody binding capacity (ABC) per cell of the malignant lymphoid cells was determined for anti-CD52 clone CF1D12 (Southern Biotech, Birmingham, AL) using the BD Biosciences QuantiBRITE system for fluorescence quantitation. The CD52 ABC value is the measurement of the mean value of the maximum capacity of each cell to bind the anti-CD52 antibody. QuantiBRITE PE Beads (BD Biosciences) are pre-calibrated standard beads containing known levels of PE molecules. QuantiBRITE beads were run through a FACSCalibur flow cytometer on the same day at the same instrument settings as the individual patient specimens. A standard curve comparing the geometric mean of fluorescence to known PE content of the QuantiBRITE beads was constructed using QunatiCALC software (BD Biosciences). The regression analysis, slope, intercept and correlation coefficient were determined. Analysis gates were drawn based upon immunophenotype and cell size to include only the malignant cells for determination of the geometric mean fluorescence of CD52 staining. The ABC values were generated from the measured geometric mean fluorescence of only the malignant cells using the QuantiBRITE standard curve. As a negative control, CD4 ABC values were determined in normal CD4 negative T cells.
Results
Seventy-eight cases of mature T cell and NK cell neoplasms, encompassing eight WHO classification diagnostic categories were included in the study. These specimens were obtained as part of protocol eligibility screenings or indicated diagnostic procedures. The specimens included 52 peripheral blood samples, three bone marrow aspirates, three body fluid samples, 18 fine needle aspirates, and two tissue (nasal) biopsy specimens.
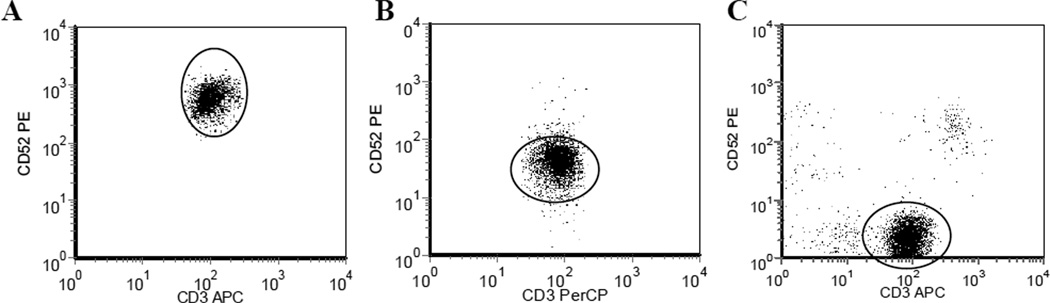
Cell surface expression of CD52 on the tumor cells was demonstrated in all (100%) cases of AITL, HSTCL, and T-PLL studied (Table 1). There was a high frequency of CD52 expression by neoplastic cells in the PTCL-NOS (92.3%) and ATLL (94.1%) cases studied (Table 1). However, in 1 PTCL-NOS and 2 ATLL cases the neoplastic cells failed to express CD52 on the cell surface (Table 1, Figure 1), indicating that alemtuzumab therapy would be unlikely to be active in these patients due to lack of expression of the antibody target.
Table1.
Summary of patients, specimens and frequency of CD52 expression.
| WHO Classification | No. of Cases (N= 78) |
CD52 expression (%) |
Specimen type |
|---|---|---|---|
| Angioimmunoblastic T- cell lymphoma (AITL) |
3 | 3 (100) | 2 PB, 1 LN FNA |
| Hepatosplenic T-cell lymphoma (HSTCL) |
4 | 4 (100) | 2 PB, 2 BM |
| T-Prolymphocytic leukemia(T-PLL) |
2 | 2 (100) | 2 PB |
| Peripheral T-cell lymphoma, unspecified (PTCL-NOS) |
13 | 12 (92.3) | 6 PB, 5 LN FNA, 1 BM, 1 CSF |
| Adult T-cell leukemia/lymphoma (ATLL) |
34 | 32 (94.1) | 23 PB, 1 CSF, 1 Ascites, 9 FNA (7 LN, 1 liver, 1 subcutaneous mass) |
| Cutaneous T-cell lymphoma (CTCL) |
16 | 14 (87.5) | 16 PB |
| Anaplastic T-cell lymphoma (ALCL) |
2 | 1 (50) | 2 FNA (1LN, 1 abdominal mass) |
| Extranodal T/NK cell lymphoma(ENT/NKCL) |
4 | 1 (25) | 2 Nasal BX, 1PB, 1 FNA (subcutaneous mass) |
PB, peripheral blood; LN, lymph node; FNA, fine needle aspirate; BM, bone marrow
Figure 1.
CD52 Expression in T Cell Malignancies. CD52 expression as measured by anti-CD52-PE antibody. A. High level CD52 expression (ABC-24,894). B. Low level CD52 expression (ABC-1,317). C. Negative for CD52. X axis- CD3, Y-axis CD52, oval indicates malignant cells (based upon complete immunophenotypic data)
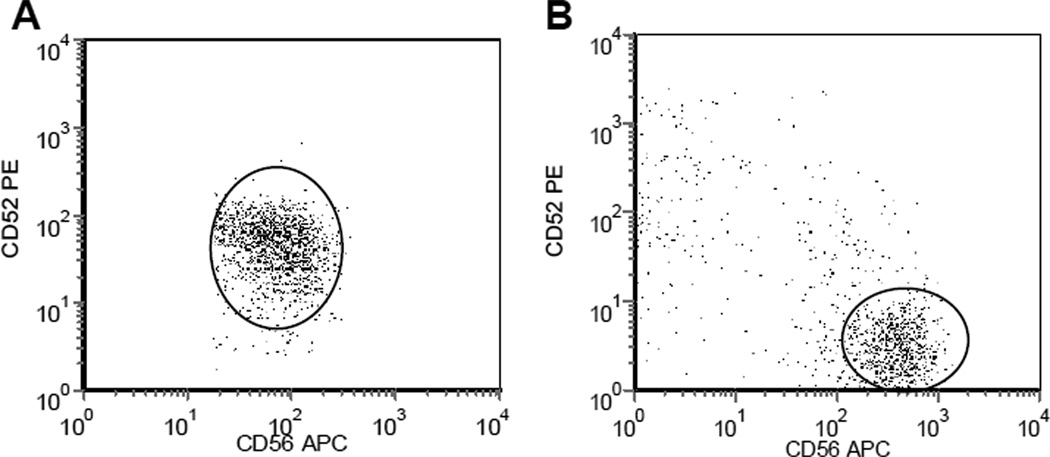
All of the sixteen CTCL cases studied were mycosis fungoides/Sézary syndrome. Fourteen of these cases (87.5%) demonstrated cell surface expression of CD52 (Table 1). One of two ALCL (50%) showed expression of CD52 on the CD30+ tumor cells. Only one of 4 (25%) of the extranodal T/NK cell lymphomas was positive for CD52 (Table 1, Figure 2). CD30 expression was studied on a limited number of cases. It is of interest to note that many of the cases of CD52 negative lymphomas expressed CD30. All of the CD52 negative ATLL and PTCL-NOS cases and 1 out of 3 CD52 negative extranodal T/NK cell lymphoma were CD30 positive (2/3 were CD30 negative). Of the ALCL cases, both were CD30 positive, with one CD52 positive and one negative case. CD30 expression was not studied in the CTCL cases as they were all demonstrated to be mycosis fungoides/Sezary syndrome.
Figure 2.
CD52 Expression in NK/T Cell Malignancies. CD52 expression as measured by anti-CD52-PE antibody. A. Positive for CD52 expression (ABC-1,336). B. Negative for CD52. X axis- CD56, Y-axis CD52, oval indicates malignant cells (based upon complete immunophenotypic data)
The level of CD52 expression varied widely (Table 2, Figure 1 & 2). There was no significant difference in the mean anti-CD52 antibody binding capacity (ABC) of the neoplastic T cells in ATLL AITL, HSTCL and PTCL-NOS. Anti-CD52 ABC values were lower in the single ALCL and ENT/NKCL cases positive for CD52 and in the 2 T-PLL cases in comparison to the mean ABC values in the other diagnostic categories. Since normal T cells are CD52 positive, the mean negative ABC value was determined using anti-CD4 and examining the CD4 negative T cells. The mean ABC value in a negative population was 82.15 +/− 13.7 (mean +/− S.D.).
Table2.
CD52 cell surface antibody binding capacity (ABC) by tumor cells in CD52 positive cases
| Diagnosis | Number of Cases |
Mean CD52 ABC |
Standard Deviation |
Range |
|---|---|---|---|---|
| Angioimmunoblastic T- cell lymphoma (AITL) |
3 | 6,225 | 3,165 | 3,113–9,440 |
| Hepatosplenic T-cell lymphoma (HSTCL) |
3* | 5,641 | 5,044 | 2,631– 11,464 |
| T-Prolymphocytic leukemia(T-PLL) |
2 | 827 | 455 | 505–1,149 |
| Peripheral T-cell lymphoma, unspecified (PTCL-NOS) |
12 | 4,590 | 4,075 | 1,114– 13,539 |
| Adult T-cell leukemia/lymphoma (ATLL) |
32 | 5,427 | 4,437 | 314–18,450 |
| Cutaneous T-cell lymphoma (CTCL) |
14 | 6,484 | 6,919 | 1,276– 24,894 |
| Anaplastic T-cell lymphoma (ALCL) |
1 | 597 | - | 597 |
| Extranodal T/NK cell lymphoma(ENT/NKCL) |
1 | 1,336 | - | 1,336 |
CD52 ABC value is the mean capacity per malignant cell to bind anti-CD52 antibody. ABC value is not available on 1 gamma delta HSTCL.
Discussion
Mature T-cell and NK cell leukemias and lymphomas are uncommon, comprising a small minority of non-Hodgkin’s lymphomas. With the exception of ALK positive anaplastic T cell lymphoma, early stage mycosis fungoides, and T-cell large granular lymphocyte leukemia, the majority of T and NK cell neoplasms are clinically aggressive and resistant to conventional chemotherapy. Therefore the promising outcomes attained in several clinical trials including alemtuzumab as a single agent or in combination with other chemotherapeutic agents in treatment of T cell neoplasia have generated great interest (Dearden and Matutes 2006). The general applicability of these studies, however, depends in part upon the frequency of CD52 expression across the T-cell lymphoproliferative diagnostic categories. Unfortunately, as these studies failed to establish CD52 expression on the neoplastic cells prior to initiation of therapy, no inference could be drawn between the presence of the therapeutic target, CD52, on the neoplastic cells and the response to alemtuzumab.
Our study attempts to bridge this gap, by examining CD52 expression on patient tumor specimens from eight WHO diagnostic categories of mature T and NK cell neoplasms. We observed a high frequency of CD52 expression by neoplastic cells in AITCL, HSTCL, T-PLL, PTCL-NOS, and ATLL. This is in contrast to previous smaller studies that reported a significantly lower frequency of CD52 expression among these entities (Piccaluga, et al 2007, Rodig, et al 2006). Based on our data, alemtuzumab therapy is likely to be more effective in these patients than previously suspected. The percentage of cases with expression of CD52 in AITL, HSTCL and PTCL-NOS in our study is much higher than that reported in other studies utilizing IHC techniques. Piccaluga et al reported that only 41% PTCL expressed CD52; Rodig et al detected expression of CD52 in only 40% of AITCL, 33% of HSTCL and 35% of PTCL-NOS. The difference between our observations and previous reports may be explained by a higher sensitivity of detection of antigen expression by FC compared to IHC (Thakhi 1996). We found that CTCL, ALCL, and ENT/NKCL demonstrated a lower frequency of CD52 expression. This finding strongly implies that alemtuzumab therapy may be useful in a smaller percentage of patients with these neoplasms. We observed that CD30 expression was frequently associated with absence of expression of CD52; although this was not true in all cases it further highlights the importance of evaluating CD52 expression in all patients.
Since assessment of CD52 expression typically occurs when the patient undergoes initial diagnostic evaluation and fresh tissue from the original diagnostic biopsy is no longer available, one might argue that detection of CD52 by IHC on paraffin embedded tissue blocks is more convenient, feasible, and minimizes unnecessary duplication of procedures for the patient. However, in our study, the majority of cases had FC CD52 assessment performed on a fresh specimen obtained by non- or minimally-invasive techniques. Only two cases required surgical biopsy to obtain a specimen for flow cytometric immunophenotyping, a nasal biopsy in two patients with extra-nodal T/NK cell lymphoma. The other specimens included 52 peripheral bloods, three bone marrows, three body fluids, and 18 fine needle aspirations, all obtained using minimally invasive techniques and with no significant patient morbidity. The majority of these specimens also yielded additional important staging and immunophenotypic information. In view of the known lower sensitivity of IHC on paraffin tissue compared to FC in fresh tissue (Thakhi 1996) and the benefit of obtaining additional data concerning extent of disease, we believe that re-sampling to obtain a fresh specimen for flow cytometric immunophenotyping for CD52 assessment is warranted and will be important in clarifying the level of antigen expression and response to therapy. The frequent observation of circulating malignant cells in peripheral blood of patients with T and NK cell neoplasms highlights the importance of flow cytometry on peripheral blood as a simple non-invasive site for detecting T cell neoplasms.
A number of clinical trials have shown promising overall response rates with alemtuzumab in T-PLL, CTCL, and PTCL-NOS; however, the response rate reported among these studies varies significantly (Dearden and Matutes 2006, Dearden, et al 2002, Dearden 2001). For example, the overall response rate varies from 55% to 100% in CTCL, 50% to 100% in T-PLL, and 36% to 60% in PTCL-NOS in different clinical trials. In our study, we found a much higher rate of CD52 expression in these three lymphoma categories (see Table 1). There is clearly a discrepancy between the clinical response rate and expression rate of CD52. The reason for this discrepancy is unclear. Ginaldi et al have suggested that the level of CD52 expression on the surface of the tumor cells may determine the response rate to alemtuzumab therapy (Ginaldi, et al 1998). By using quantitative flow cytometric analyses, they demonstrated that CLL and T-PLL patients who failed to respond to alemtuzumab treatment had lower levels of CD52 cell surface expression, whereas the highest levels were present in patients with major responses to therapy. We found that levels of CD52 expression varied widely, from 314 to 24,894 anti-CD52 antibody binding capacity units. Thus, the variable response to alemtuzumab therapy of patients with mature T/NK cell lymphomas/leukemias may be due to varying levels of CD52 expression in positive cases, in addition to a subset of patients lacking CD52 cell surface expression by the tumor cells. IHC cannot accurately quantitate cell surface expression of antigens, further bolstering the need for flow cytometric immunophenotyping to provide objective and precise quantitation of antigen expression.
In conclusion, the frequency of CD52 expression by mature T cell and NK cell neoplasms varies according to the specific diagnostic category. Our data indicates that alemtuzumab therapy may be ineffective in a significant number of cases of ALCL and ENT/NKCL, where the frequency of CD52 expression is low. Furthermore, even in diagnostic categories exhibiting a high frequency of CD52 expression, individual cases where the neoplastic cells fail to express CD52 are encountered. This may significantly impact an individual’s response to alemtuzumab treatment. Flow cytometry is more sensitive than IHC in detecting CD52 expression, and can specifically detect expression on the cell surface, whereas IHC cannot. In addition, flow cytometry can accurately quantitate levels of CD52 expression. We therefore strongly recommend flow cytometric assessment of CD52 cell surface expression prior to the initiation of CD52 targeted therapy.
References
- Alinari L, et al. Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene. 2007;26:3644–3653. doi: 10.1038/sj.onc.1210380. [DOI] [PubMed] [Google Scholar]
- Bindon CI, et al. Importance of antigen specificity for complement-mediated lysis by monoclonal antibodies. Eur J Immunol. 1988;18:1507–1514. doi: 10.1002/eji.1830181006. [DOI] [PubMed] [Google Scholar]
- Dearden CE, Matutes E. Alemtuzumab in T-cell lymphoproliferative disorders. Best Pract Res Clin Haematol. 2006;19:795–810. doi: 10.1016/j.beha.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Dearden CE, et al. Alemtuzumab in T-cell malignancies. Med Oncol. 2002;19(Suppl):S27–S32. doi: 10.1385/mo:19:2s:s27. [DOI] [PubMed] [Google Scholar]
- Dearden CE, Matutes E, Cazin B, Tjonnfjord GE, Parreira A, Nomdedeu B, Leoni P, Clark FJ, Radia D, Rassam SMB, Roques T, Ketterer N, Brito-Babapulle V, Dyer MJS, Catovsky D. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood. 2001;98:1721–1726. doi: 10.1182/blood.v98.6.1721. [DOI] [PubMed] [Google Scholar]
- Dearden CME. Alemtuzumab in T-cell lymphoproliferative disorders. Best Pract Res Clin Haematol, 2006;19:795–810. doi: 10.1016/j.beha.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Enblad G, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103:2920–2924. doi: 10.1182/blood-2003-10-3389. [DOI] [PubMed] [Google Scholar]
- Faulkner RD, et al. BEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patients. Blood. 2004;103:428–434. doi: 10.1182/blood-2003-05-1406. [DOI] [PubMed] [Google Scholar]
- Gallamini A ZF, Patti C, Billio A, Specchia MR, Tucci A, Levis A, Manna A, Secondo V, Rigacci L, Pinto A, Iannitto E, Zoli V, Torchio P, Pileri S, Tarella C. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110:2316–2323. doi: 10.1182/blood-2007-02-074641. [DOI] [PubMed] [Google Scholar]
- Ginaldi L, et al. Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to Campath-1H. Leuk Res. 1998;22:185–191. doi: 10.1016/s0145-2126(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Gupta V, et al. Favorable effect on acute and chronic graft-versus-host disease with cyclophosphamide and in vivo anti-CD52 monoclonal antibodies for marrow transplantation from HLA-identical sibling donors for acquired aplastic anemia. Biol Blood Marrow Transplant. 2004;10:867–876. doi: 10.1016/j.bbmt.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hale G, et al. The CAMPATH-1 antigen (CDw52) Tissue Antigens. 1990;35:118–127. doi: 10.1111/j.1399-0039.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Campo PM, et al. Quantitative analysis of the expression of glycosylphosphatidylinositol-anchored proteins during the maturation of different hematopoietic cell compartments of normal bone marrow. Cytometry B Clin Cytom. 2007;72:34–42. doi: 10.1002/cyto.b.20143. [DOI] [PubMed] [Google Scholar]
- Jaffe E, Harris NL, Stein H, Vardiman JW. WHO Classification, Pathology and Genetics Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- Keating MJ, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- Kim JG SS, Chae YS, Cho YY, Yang DH, Lee JJ, Kim HJ, Shin HJ, Chung JS, Cho GJ, Lee WS, Joo YD, Sohn CH, Oh SJ. Alemtuzumab plus CHOP as front-line chemotherapy for patients with peripheral T-cell lymphomas: a phase II study. Cancer Chemother Pharmacol. 2007;60 doi: 10.1007/s00280-007-0469-9. [DOI] [PubMed] [Google Scholar]
- Lundin J, et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101:4267–4272. doi: 10.1182/blood-2002-09-2802. [DOI] [PubMed] [Google Scholar]
- Nuckel H, et al. Alemtuzumab induces enhanced apoptosis in vitro in B-cells from patients with chronic lymphocytic leukemia by antibody-dependent cellular cytotoxicity. Eur J Pharmacol. 2005;514:217–224. doi: 10.1016/j.ejphar.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Pawson R, et al. Treatment of T-cell prolymphocytic leukemia with human CD52 antibody. J Clin Oncol. 1997;15:2667–2672. doi: 10.1200/JCO.1997.15.7.2667. [DOI] [PubMed] [Google Scholar]
- Piccaluga PP, et al. Expression of CD52 in peripheral T-cell lymphoma. Haematologica. 2007;92:566–567. doi: 10.3324/haematol.10767. [DOI] [PubMed] [Google Scholar]
- Project N-HsLC. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- Rodig SJ, et al. Heterogeneous CD52 expression among hematologic neoplasms: implications for the use of alemtuzumab (CAMPATH-1H) Clin Cancer Res. 2006;12:7174–7179. doi: 10.1158/1078-0432.CCR-06-1275. [DOI] [PubMed] [Google Scholar]
- Thakhi A, Edinger M, Myles J, et al. Flow cytometric immunophenotyping of non-Hodgkin’s lymphoma and related disorders. Cytometry. 1996;25:113–124. doi: 10.1002/(SICI)1097-0320(19961001)25:2<113::AID-CYTO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Treumann A, et al. Primary structure of CD52. J Biol Chem. 1995;270:6088–6099. doi: 10.1074/jbc.270.11.6088. [DOI] [PubMed] [Google Scholar]
- Waldmann H, Hale G. CAMPATH: from concept to clinic. Philos Trans R Soc Lond B Biol Sci. 2005;360:1707–1711. doi: 10.1098/rstb.2005.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia MQ, et al. Structure of the CAMPATH-1 antigen, a glycosylphosphatidylinositol-anchored glycoprotein which is an exceptionally good target for complement lysis. Biochem J. 1993a;293(Pt 3):633–640. doi: 10.1042/bj2930633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia MQ, et al. Efficient complement-mediated lysis of cells containing the CAMPATH-1 (CDw52) antigen. Mol Immunol. 1993b;30:1089–1096. doi: 10.1016/0161-5890(93)90155-5. [DOI] [PubMed] [Google Scholar]
- Xia MQ HG, Lifely MR, Ferguson MAJ, Campbell D, Packman L, Waldmann H. STRUCTURE OF THE CAMPATH-1 ANTIGEN, A GLYCOSYLPHOSPHATIDYLINOSITOL-ANCHORED GLYCOPROTEIN WHICH IS AN EXCEPTIONALLY GOOD TARGET FOR COMPLEMENT LYSIS. BIOCHEMICAL JOURNAL. 1993;293:633–640. doi: 10.1042/bj2930633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, et al. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD52 monoclonal antibody, Campath-1H. Cancer Res. 2003;63:6453–6457. [PubMed] [Google Scholar]