Abstract
Background
We previously noted that among atomic bomb survivors (ABS), the relative frequency of cases of adult papillary thyroid cancer (PTC) with chromosomal rearrangements (mainly RET/PTC) was significantly greater in those with relatively higher radiation exposure than those with lower radiation exposure. In contrast, the frequency of PTC cases with point mutations (mainly BRAFV600E) was significantly lower in patients with relatively higher radiation exposure than those with lower radiation exposure. We also found that among ABS, the frequency of PTC cases with no detectable gene alterations in RET, neurotrophic tyrosine kinase receptor 1 (NTRK1), BRAF, or RAS was significantly higher in patients with relatively higher radiation exposure than those with lower radiation exposure. However, in ABS with PTC, the relationship between the presence of the anaplastic lymphoma kinase (ALK) gene fused with other gene partners and radiation exposure has received little study. In this study, we tested the hypothesis that the relative frequency of rearranged ALK in ABS with PTC, and with no detectable gene alterations in RET, NTRK1, BRAF, or RAS, would be greater in those having relatively higher radiation exposures.
Methods
The 105 subjects in the study were drawn from the Life Span Study cohort of ABS of Hiroshima and Nagasaki who were diagnosed with PTC between 1956 and 1993. Seventy-nine were exposed (>0 mGy), and 26 were not exposed to A-bomb radiation. In the 25 ABS with PTC, and with no detectable gene alterations in RET, NTRK1, BRAF, or RAS, we examined archival, formalin-fixed, paraffin-embedded PTC specimens for rearrangement of ALK using reverse transcription–polymerase chain reaction and 5′ rapid amplification of cDNA ends (5′ RACE).
Results
We found rearranged ALK in 10 of 19 radiation-exposed PTC cases, but none among 6 patients with PTC with no radiation exposure. In addition, solid/trabecular-like architecture in PTC was closely associated with ALK rearrangements, being observed in 6 of 10 PTC cases with ALK rearrangements versus 2 of 15 cases with no ALK rearrangements. The six radiation-exposed cases of PTC harboring both ALK rearrangements and solid/trabecular-like architecture were associated with higher radiation doses and younger ages at the time of the A-bombing and at diagnosis compared to the other 19 PTC with no detectable gene alterations.
Conclusion
Our findings suggest that ALK rearrangements are involved in the development of radiation-induced adult-onset PTC.
Introduction
Thyroid cancer is one of the malignancies most closely associated with exposure to ionizing radiation in humans (1), such as the atomic bombs in Hiroshima and Nagasaki and the Chernobyl nuclear power plant accident (2,3). Radiation Effects Research Foundation (RERF) epidemiology studies of atomic bomb survivors (ABS) have found that an excess relative risk for papillary thyroid cancer (PTC) per Gy is remarkably high among survivors (4,5). The data from the studies after the Chernobyl accident also indicate a strong relationship between thyroid cancer and radiation exposure (3).
Gene alterations that lead to constitutive activation of the mitogen-activated protein kinase (MAPK)-signaling pathway—such as alterations of RET, neurotrophic tyrosine kinase receptor 1 (NTRK1), BRAF, and RAS genes—are frequently found in PTC (6–8). These gene alterations can be detected in >70% of PTC cases, so the constitutive activation of the MAPK-signaling pathway appears to be a major early event in papillary thyroid carcinogenesis.
Our molecular analysis on rearrangements of RET, NTRK1, and BRAF genes, and also point mutations of BRAF and RAS genes in adult-onset PTC cases from the Life Span Study (LSS) cohort of ABS, found that the relative frequency of PTC cases with RET/PTC or NTRK1 rearrangements (mainly RET/PTC) in all available PTC cases was significantly increased with an increased radiation dose, whereas PTC cases with BRAF or RAS point mutations (mainly BRAFV600E) were significantly decreased (9,10). Apart from those PTC cases with known gene alterations, we found that the relative frequency of PTC cases with so-called nondetected gene alterations (i.e., no alterations in the RET, NTRK1, BRAF, or RAS gene) tended to increase with an increased radiation dose. The prevalence of the selected PTC cases peaked between 1956 and 1962, and rapidly decreased thereafter (10).
We postulated that some of the cases of PTC among ABS for which we had been unable to find gene alterations might have gene alterations that had previously not been looked for. Therefore, we initiated further molecular analyses of these cases by determining if some of them had rearrangements of anaplastic lymphoma kinase (ALK).
The ALK gene was first identified as a fusion partner of nucleophosmin in anaplastic large-cell lymphoma (ALCL) with the t(2:5) chromosomal rearrangement (11,12). Translocation of ALK with multiple fusion partner genes was subsequently identified in ALCL as well as in other inflammatory myofibroblastic tumors (13). One novel type of ALK rearrangement was an echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion gene, which was recently detected in nonsmall-cell lung cancer (14). Numerous EML4-ALK fusion variants have been identified to date (15–18). The EML4-ALK fusion variants were also detected in breast and colon cancers (19), but to date, there has been no report on ALK rearrangements in thyroid cancer.
In this study, we report for the first time the finding that ALK rearrangement selectively occurred in radiation-exposed PTC cases that carried no known gene alterations, and that about half of the PTC cases with rearranged ALK developed solid/trabecular-like architecture in the cancer tissue. The PTC cases harboring both ALK rearrangements and solid/trabecular-like architecture were related to higher radiation doses and younger ages at the time of the bombings and at diagnosis compared to the other cases, implying a key role of ALK rearrangements in the development of radiation-induced thyroid cancer.
Methods
Study subjects and tissue specimens
The study subjects were 105 adults with PTC who were members of the LSS cohort of ABS of Hiroshima and Nagasaki diagnosed in selected hospitals in the two cities between 1956 and 1993. Of these, 79 were exposed (>0 mGy) and 26 were not exposed to A-bomb radiation. Of these 105 patients, 71 had been a part of our previous study on RET/PTC rearrangements (10). The 26 nonradiation-exposed subjects were either those with a radiation dose estimated to be 0 mGy or those who were not in the city of Hiroshima or Nagasaki at the time of the bombing. Study subjects who were not in these cities at the time of the bombing were assigned to the nonexposed group in this study, consistent with our previous article (10). This study was conducted with approval of the Human Investigation Committee and the Ethics Committee for Genome Research at the RERF.
Histological examination
Examination of histology was done by one of the authors (Y.H.) according to histopathological typing established by the WHO (20). All study specimens were nonbuffered, formalin-fixed, and paraffin-embedded PTC tissue specimens surgically resected from 1956 to 1993. Since amounts of tissue materials were limited, histological examination was conducted on one hematoxylin and eosin-stained tissue section per case.
RNA preparation and cDNA synthesis
RNA was extracted from microdissected noncancer or cancer regions using the High Pure RNA Paraffin Kit (Roche Diagnostics GmbH), as described previously (21). Reverse transcription was performed with random primers (9-mer) using 100 ng total RNA as template, as described previously (21).
Reverse transcription–polymerase chain reaction
Reverse transcription–polymerase chain reaction (RT-PCR) was carried out with the Fast Start High Fidelity PCR system (Roche Diagnostics GmbH) for the ALK gene, with AccuSure DNA polymerase (BIOLINE) for the EML4 (exon 13)/ALK (exon 20) fusion and with Phusion Hot Start II (New England BioLabs) for the EML4 (exon 20)/ALK (exon 20) fusion, using primer sets shown in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/thy) and as previously described (22).
Screening of rearranged ALK
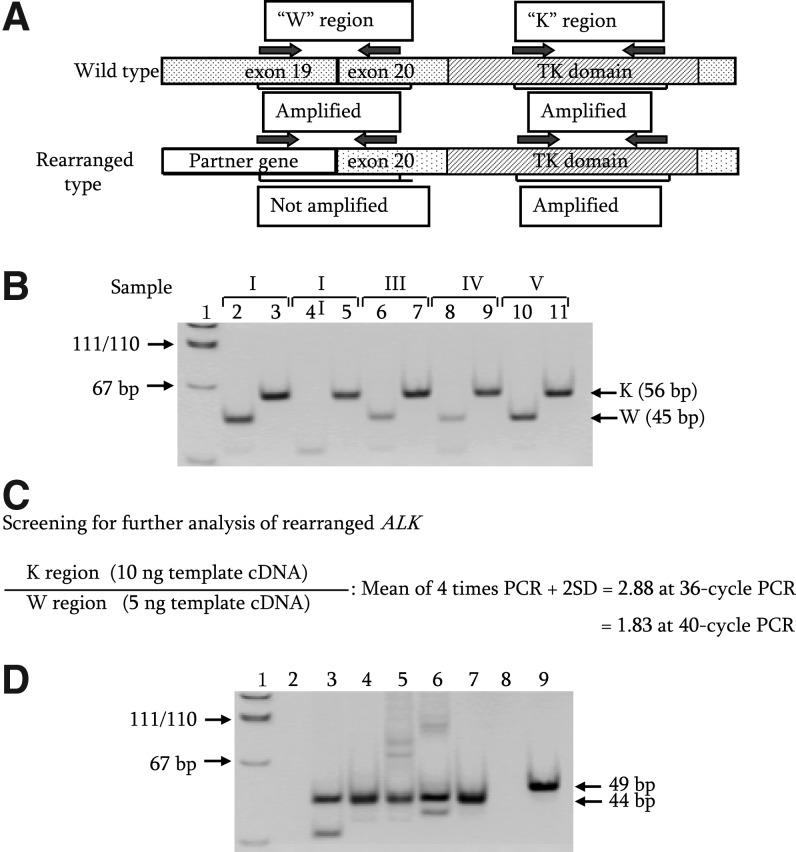
The primer sets were made against the ALK kinase domain (K region) and the region spanning the boundary of exons 19 and 20 of ALK (W region) as shown in Figure 1A. PCR amplifications, for the K region with cDNA derived from 10 ng of total RNA of in-house control PTC and for the W region with cDNA from 5 ng of the same total RNA, were conducted at 36 cycles and 40 cycles, respectively. These PCR amplifications were repeated four times. The K/W ratios of 1.9 at 40 cycles or 2.9 at 36 cycles were drawn from an average of four experiments plus 2× the standard deviation as shown in Figure 1C. When the intensity ratio of the K region to W region after a 40-cycle RT-PCR amplification was >1.9 (Fig. 1B; samples II, III, and IV) based on the calculation shown in Figure 1C, samples were further analyzed by 36-cycle PCR amplification to determine whether the samples were positive for ALK rearrangements. We assumed that the samples with an intensity ratio >2.9 at 36-cycle PCR amplification were positive for ALK rearrangements (Fig. 1B; samples II, III, and IV). We used only the K/W ratio for screening, and intensities of individual amplified DNA fragments were not taken into account for screening the candidates. This screening was conducted only when clear and detectable K-region bands were observed in the first PCR products. Subsequently, cDNA fragments of rearranged ALK genes were isolated and analyzed by the SMART rapid amplification of cDNA end (RACE) method, using primer sets shown in Supplementary Table S1 as described previously (22).
FIG. 1.
(A) Diagram of the rearranged ALK gene. W and K regions indicate the regions spanning boundary of exons 19 and 20, and of exons 26 and 27 (kinase domain) in ALK, respectively. (B) Expression levels of W and K regions in ALK. The 5 μL of 40-cycle PCR products were electrophoresed on an 8% acrylamide gel. Sample I (lanes 2 and 3) indicates RT-PCR products of in-house control PTC. Samples II–V (lanes 4–11) reveal RT-PCR products of four exposed PTC with nondetected gene alterations; lanes with even numbers are cDNA fragments derived from the W region, and those with odd numbers are fragments derived from the K region. Lane 1 indicates pUC19-MspI digest for DNA size marker. (C) Screening of the rearranged ALK gene. The intensity ratio for selection of rearranged ALK was calculated using RT-PCR amplification with cDNA derived from 10 ng of total RNA of in-house control PTC for the K region, and with cDNA derived from 5 ng of total RNA of the same PTC for the W region as template. (D) Detection of expression of EML4-ALK fusion genes by RT-PCR. Lanes 3–7 indicate cDNA fragments of the EML4-ALK fusion gene in PTC cases from which the 3′-end fragments of exon 13 of EML4 were isolated by the SMART RACE method. Lanes 6 and 7 correspond to PTC in samples IV and III of (B), respectively. Lane 9 indicates cDNA fragments (49 bp) derived from the chimera gene that was formed by fusion of exon 20 of EML4 and exon 20 of ALK. Lanes 2 and 8 show H2O for negative control, and Lane 1 shows pUC19-MspI digest for DNA size marker. ALK, anaplastic lymphoma kinase; PTC, papillary thyroid cancer; RT-PCR, reverse transcription–polymerase chain reaction; RACE, rapid amplification of cDNA ends.
Statistical analysis
The Fisher's exact test was used for categorical variables. The Mann–Whitney U test was used for nonparametric two-sample comparison of continuous variables. SPSS software (version 15.0) was use for the statistical analyses. p-value was calculated by using a two-sided test. The Cochran-Armitage test for nonparametric trend analysis was carried out with Excel Statistics 2006 software.
Radiation dose
A-bomb radiation dose used in the analyses were shielded-organ doses to the thyroid as estimated by the recently implemented DS02 system (23), except for those cohorts who were not in the city of Hiroshima or Nagasaki at the time of the bombing.
Results
Detection of ALK rearrangements in PTC cases with previously nondetected gene alterations
Molecular epidemiological characteristics of 105 PTC cases examined thus far are shown in Supplementary Table S2, with 34 PTC cases that were newly added after our previous article (10). When comparing 79 radiation-exposed subjects and 26 nonexposed ones, no significant differences were found in distribution of gender, city, age at the time of the bombing (ATB), age at diagnosis, or presence or absence of RET/PTC rearrangements, NTRK1 rearrangements, and BRAF or RAS point mutations. However, relative frequencies of RET/PTC rearrangements and BRAF mutation in radiation-exposed PTC cases showed significantly increasing or decreasing trends depending on the radiation dose (Ptrend=0.01 and 0.0006), as shown in Supplementary Table S2. Furthermore, when comparing nonexposed PTC cases with those exposed to relatively a high radiation dose (the 3rd tertile, >355 mGy) for RET/PTC rearrangements, the relative frequency was significantly higher in exposed PTC cases (>355 mGy) than in nonexposed PTC cases (1/26 cases vs. 9/26 cases, respectively; p=0.01). On the other hand, the relative frequency of the BRAF mutation was significantly lower in PTC cases exposed to the radiation dose (>355 mGy) than in nonexposed PTC cases (19/26 cases vs. 7/26 cases, respectively; p=0.002). Among 105 PTC cases examined thus far for RET, NTRK1, and BRAF rearrangements and BRAF and RAS point mutations, 25 PTC cases were found to have no alterations in these genes, which tended to increase with an increased radiation dose, though this is not statistically significant (Supplementary Table S2). It should be noted that these cases showed a temporal change with a peak a short time after exposure, which then rapidly decreased (i.e., median time after exposure was 23 years for this group vs. 32 years for cases with BRAF and RAS point mutations, p=0.014). These findings are consistent with those in our previous article (10).
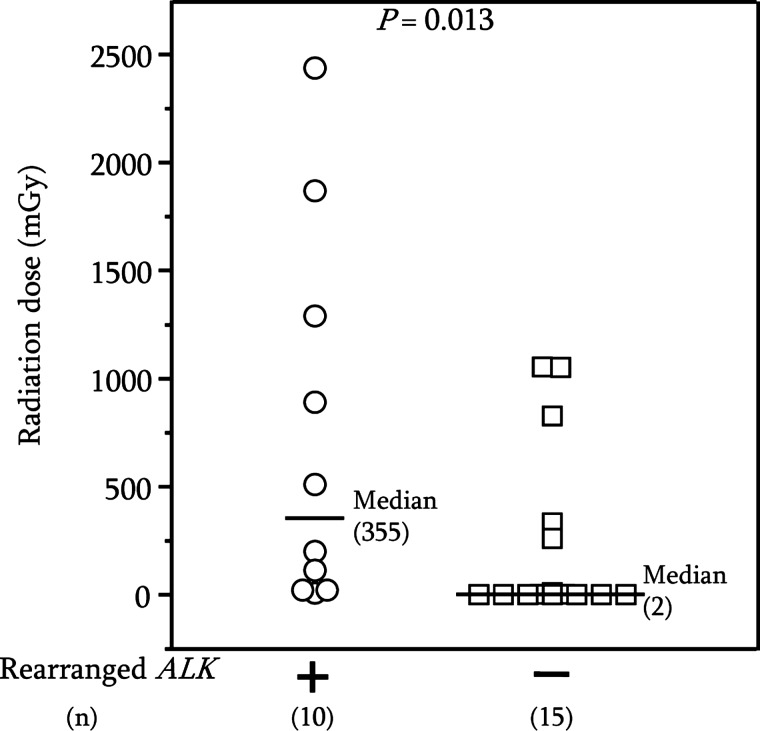
Of 25 PTC cases with previously nondetected gene alterations (also referred to as nondetected gene alterations) that carried no alterations in RET, NTRK1, BRAF, or RAS genes, we found ALK rearrangements in 10 of 19 radiation-exposed cases, but no ALK rearrangements in any of the six nonexposed cases (Table 1). The median dose in PTC cases with rearranged ALK was significantly higher than in grouped radiation-exposed and nonradiation-exposed PTC cases with no rearranged ALK (Fig. 2). However, there was no significant difference in the radiation dose between radiation-exposed PTC cases with rearranged ALK and with non-rearranged ALK. SMART 5′ RACE followed by sequencing analysis revealed that five PTC cases with rearranged ALK were a fusion of EML4 (exon 13) and ALK (exon 20), and that one case was a fusion of EML4 (exon 20) and ALK (exon 20) (Table 1). Expression of cDNA fragments of chimeric EML4- ALK in the PTC cases was confirmed by RT-PCR (Fig. 1D, lanes 3–7, and 9). The counterpart genes in the remaining four cases are currently being studied.
Table 1.
Rearranged ALK in Papillary Thyroid Cancer Cases with Nondetected Gene Alterations
| |
ALK rearrangements |
|
|
|---|---|---|---|
| PTC case | Present | Absent | pa |
| Nonexposed (n=6) | 0 | 6 | 0.051 |
| Exposed (>0 mGy, n=19) | 10b | 9 | |
Fisher's exact test.
Five cases were EML4(exon 13)/ALK(exon 20); one case was EML4(exon 20)/ALK(exon 20); ALK partner genes in four cases have not been determined yet.
PTC, papillary thyroid cancer.
FIG. 2.
Comparison of radiation dose distribution in PTC cases with (+, ◯) and without (−, □) rearranged ALK.
Morphological characteristics in PTC with rearranged ALK
Histological review of the10 PTC cases with rearranged ALK found that 6 of the10 had solid/trabecular-like architecture comprising either several small sites or several sites of greater total area within the cancer regions, but did not have insular architecture. Necrosis or mitotic figures were not observed in the single tissue section examined for each case. Supplementary Figure S1A and B show pathological photographs of two PTC cases with rearranged ALK, with solid/trabecular-like architecture in more than 50% of the cancers. Supplementary Figure S1C and D reveal a typical papillary structure for two exposed PTC cases with no rearranged ALK. In contrast, only 2 of 15 PTC cases with no rearranged ALK had such architecture. Analysis of 25 PTC cases with nondetected gene alterations revealed a close association between ALK rearrangement and solid/trabecular-like architecture (p=0.028, Table 2).
Table 2.
Association Between Rearranged ALK and Solid/Trabecular-Like Architecture
| |
Rearranged ALK |
||
|---|---|---|---|
| Present (n=10) | Absent (n=15) | pa | |
| Solid/trabecular-like architectureb | |||
| Present | 6c | 2 | 0.028 |
| Absent | 4 | 13 | |
Data represent 25 PTC cases with nondetected gene alterations.
Fisher's exact test.
The samples were counted as positive for solid/trabecular-like architecture when that architecture was found in several areas within cancer regions.
More than 50% of cancer regions revealed such architecture in two cases.
Association of PTC harboring both rearranged ALK and solid/trabecular-like architecture with radiation dose and age at A-bombing
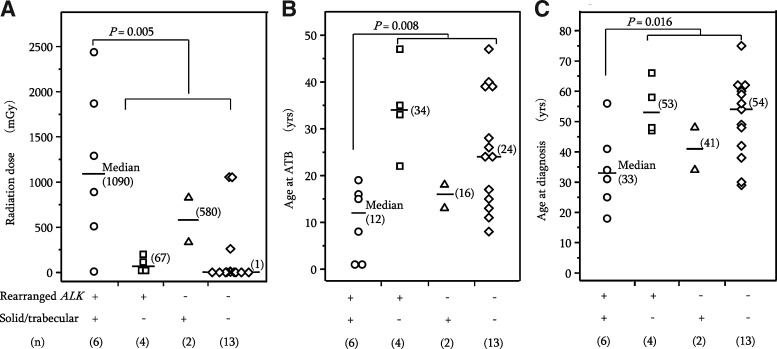
Since there were only two PTC cases with no rearranged ALK, but having solid/trabecular-like architecture, we compared six PTC cases harboring both rearranged ALK and solid/trabecular-like architecture with the remaining 19 PTC cases in terms of radiation dose and ages at A-bombing and diagnosis (Fig. 3). These six PTC cases received significantly greater radiation doses and were or younger age at the time of A-bombing and at the time of diagnosis compared to the other 19 PTC cases (Fig. 3A–C, p=0.005, 0.008, and 0.016, respectively). Furthermore, comparison of 6 PTC cases harboring both rearranged ALK and solid/trabecular-like architecture with the remaining exposed 13 PTC cases by Mann–Whitney test also showed significant differences for radiation (p=0.036), age at A-bombing (p=0.021), and age at diagnosis (p=0.020).
FIG. 3.
Epidemiological characteristics of PTC cases harboring both the rearranged ALK gene and solid/trabecular-like architecture, in relation to radiation dose (A), age at the time of atomic bombing (B), and age at the time of diagnosis (C).
Discussion
We report here the first evidence that rearrangements of ALK in adult-onset PTC cases develop in subjects exposed to radiation. Furthermore, the median radiation dose in PTC cases with rearranged ALK was significantly higher than in PTC cases with no rearranged ALK (Fig. 2), indicating involvement of rearranged ALK in radiation-induced papillary thyroid carcinogenesis. However, since only six nonexposed PTC cases were examined for rearranged ALK, it is necessary to examine a larger number of sporadic PTC cases to clarify possible presence of ALK rearrangements. We are thus unable at present to exclude the possibility that ALK rearrangements also occur in sporadic PTC. Although partner genes in four PTC cases have not yet been identified, PCR amplification in these cases was found in the K region, but not in the W region (Fig. 1), and therefore these PTC cases were judged to have rearranged ALK. We are attempting to identify the partner genes.
Some of the 10 PTC cases with rearranged ALK revealed weak, but observable, PCR-amplified fragments as shown in Figure 1C. Those bands may be in part due to amplified fragments derived from another allele with wild-type ALK. Another plausible interpretation is that the rearrangement did not occur in all tumor cells. It is important to clarify whether ALK rearrangement is a driver mutation or a passenger mutation to better understand radiation-related carcinogenesis of PTC. Furthermore, although rearrangements of ALK are involved in the pathogenesis of many malignancies, including lymphoma and lung cancer, few ALK rearrangements have been well defined relative to their pathogenic role in cancer. Recently, constitutive ALK activation in several cancers was reported to cause the activation of selected downstream pathways, including MAPK and STAT (24,25), but no reports are available regarding a putative pathogenic role of rearranged ALK in PTC. To assess biological significance of rearranged ALK in radiation-induced PTC, in vitro and in vivo comparative experiments with RET/PTC rearrangements and BRAF point mutation need to be performed to shed light on these issues.
The nonradiation-exposed subjects in this study were comprised of two distinct groups: subjects whose radiation dose levels were estimated to be zero by the DS02 system, and subjects who were not in Hiroshima or Nagasaki, and thus not a part of the DS02 dose estimation. Our analyses included two not-in-city subjects in the six nonexposed subjects with nondetected gene alterations, but even when we excluded these not-in-city subjects from the analysis, the results were substantially unchanged. Specifically, the six nonexposed subjects without rearranged ALK in Table 1 would become 4 (p=0.1 in this case); the median value of 2 mGy for 15 subjects with no rearranged ALK in Figure 2 would become 3 mGy for 13 subjects (p=0.034); and the 13 subjects (denoted by “◊”) in Figure 3 harboring neither rearranged ALK nor solid/trabecular-like architecture would become 11, and their median values of 1 mGy, 24 years, and 54 years in Figure 3A–C would be 2 mGy, 24 years, and 54 years (p=0.009, 0.012, and 0.023), respectively.
The solid/trabecular-like architecture appeared in one of three different types of groupings as (I) relatively small, clonal, multicellular clusters distributed throughout the cancer; (II) larger groupings (each composed of a few multicellular clusters) distributed throughout the cancer, or as (III) a few large groupings that occupied a major portion (>50%) of the cancer volume. Interestingly, 6 of 10 PTC cases with rearranged ALK had solid/trabecular-like architecture corresponding to types (I) and (II) above (numbering 3 and 1, respectively) in several areas within the cancers, and two cases had such architecture in more than 50% of the cancer (type III and shown in Supplementary Fig. 1A, B). However, only 2 of 15 PTC cases with no rearranged ALK had such architecture; they were small and found in several areas scattered throughout the cancer (type I). The 25 PTC cases with nondetected gene alterations showed no necrosis or mitotic figures, but did show PTC-specific nuclear features such as nuclear groves, intranuclear cytoplasmic inclusions, and psammoma bodies. Taking into account the diagnostic criteria proposed by Volante et al. (26), it is reasonable to conclude that two PTC cases harboring solid/trabecular-like architecture in more than 50% of cancer regions are a mixed type of PTC and solid variant, or a solid variant of PTC rather than poorly differentiated thyroid cancer. This is true even though the size of one solid/trabecular nest was small and uniform compared with that of post-Chernobyl childhood PTC, and that the other six PTC cases (four and two with and without rearranged ALK, respectively) harboring such architecture in several cancer regions are typical PTC.
PTC cases harboring both rearranged ALK and solid/trabecular-like architecture were significantly associated not only with a greater radiation dose but also with younger age at the time of bombing and at diagnosis, compared with the remaining PTC cases. One plausible interpretation of this finding is that in the process of radiation-induced papillary thyroid carcinogenesis involving ALK rearrangements, unknown gene alterations may additionally occur and generate solid/trabecular-like architecture. Considering this, further analysis will be required to identify such gene alterations and to assess the pathogenic role of ALK rearrangements and its potential link to solid/trabecular-like architecture.
Supplementary Material
Acknowledgments
The RERF, Hiroshima and Nagasaki, Japan, is a private, nonprofit foundation funded by Japan's Ministry of Health, Labour, and Welfare, and the U.S. Department of Energy (DOE), the latter in part through DOE Award DE-HS0000031 to the National Academy of Sciences. This publication was supported by RERF Research Protocols RP 5-02, and in part by a Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science, and Technology, and a Grant-in Aid for Cancer Research from Japan's Ministry of Health, Labour, and Welfare, as well as research grant for the Princess Takamatsu Cancer Research Fund (08-24015). We thank Dr. J. Cologne for his helpful statistical advice, Mr. K. Koyama for his excellent technical assistance, and Ms. M. Yonezawa for her good help with preparation of article. The views of the authors do not necessarily reflect those of the two governments.
Disclosure Statement
The authors declare that they have no commercial associations that might create a conflict of interest in connection with this article.
References
- 1.Imaizumi M. Usa T. Tominaga T. Neriishi K. Akahoshi M. Nakashima E. Ashizawa K. Hida A. Soda M. Fujiwara S. Yamada M. Ejima E. Yokoyama N. Okubo M. Sugino K. Suzuki G. Maeda R. Nagataki S. Eguchi K. Radiation dose-relationships for thyroid nodules and autoimmune thyroid diseases in Hiroshima and Nagasaki atomic bomb survivors 55–58 years after radiation exposure. JAMA. 2006;295:1011–1022. doi: 10.1001/jama.295.9.1011. [DOI] [PubMed] [Google Scholar]
- 2.Kazakov VS. Demidchik EP. Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992;359:21. doi: 10.1038/359021a0. [DOI] [PubMed] [Google Scholar]
- 3.Astakhova LN. Anspaugh LR. Beebe G.W. Bouville A. Drozdovitch VV. Garber V. Gavrilin YI. Khrouch VT. Kuvshinnikov AV. Kuzmenkov YN. Minenko VP. Moschik KV. Nalivko AS. Robbins J. Shemiakina EV. Shinkarev S. Tochitskaya SI. Waclawiw MA. Chernobyl-related thyroid cancer in children of Belarus: A case-control study. Radiat Res. 1998;150:349–356. [PubMed] [Google Scholar]
- 4.Thompson DE. Mabuchi K. Ron E. Soda M. Tokunaga M. Ochikubo S. Sugimoto S. Ikeda T. Terasaki M. Izumi S. Preston DL. Cancer incidence in atomic bomb survivors. Part II: Solid tumors, 1958–1987. Radiat Res. 1994;137:S17–S67. [PubMed] [Google Scholar]
- 5.Preston DL. Ron E. Tokuoka S. Funamoto S. Nishi N. Soda M. Mabuchi K. Kodama K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi M. Evdokimova V. Nikiforov YE. Mechanisms of chromosomal rearrangements in solid tumors: the model of papillary thyroid carcinoma. Mol Cell Endcrinol. 2010;321:36–43. doi: 10.1016/j.mce.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greco A. Miranda C. Pierotti MA. Rearrangements of NTRK1 gene in papillary thyroid carcinoma. Mol Cell Endocrinol. 2010;321:44–49. doi: 10.1016/j.mce.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321:86–93. doi: 10.1016/j.mce.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi K. Eguchi H. Arihiro K. Ito R. Koyama K. Soda M. Cologne J. Hayashi Y. Nakata Y. Nakachi K. Hamatani K. The presence of BRAF point mutaion in adult papillary thyroid carcinomas from atomic bomb survivors correlates with radiation dose. Mol Carcinog. 2007;46:242–248. doi: 10.1002/mc.20277. [DOI] [PubMed] [Google Scholar]
- 10.Hamatani K. Eguchi E. Ito R. Mukai M. Takahashi K. Taga M. Imai K. Cologne J. Soda M. Arihiro K. Fujihara M. Abe K. Hayashi T. Nakashima M. Sekine I. Yasui W. Hayashi Y. Nakachi K. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 2008;68:7176–7182. doi: 10.1158/0008-5472.CAN-08-0293. [DOI] [PubMed] [Google Scholar]
- 11.Morris SW. Kirstein MN. Valentine MB. Dittmer KG. Shapiro DN. Saltman DL. Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 12.Shiota M. Fujimoto J. Tanegawa M. Satoh H. Ichinohasama R. Abe M. Nakano M. Yamamoto T. Mori S. Diagnosis of t(2;5)(p23;q35)-associated Ki-1 lymphoma with immunohistochemistry. Blood. 1994;84:3648–3652. [PubMed] [Google Scholar]
- 13.Pulford K. Morris SW. Turturro F. Anaplastic lymphoma kinase proteins in growth control and cancer. J Cell Physiol. 2004;199:330–358. doi: 10.1002/jcp.10472. [DOI] [PubMed] [Google Scholar]
- 14.Soda M. Choi YL. Enomoto M. Takada S. Yamashita Y. Ishikawa S. Fujiwara S. Watanabe H. Kurashina K. Hatanaka H. Bando M. Ohno S. Ishikawa Y. Aburatani H. Niki T. Sohara Y. Sugiyama Y. Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 15.Mano H. Non-solid oncogenes in solid tumors: EML4-ALK fusion genes in lung cancer. Cancer Sci. 2008;99:2349–2355. doi: 10.1111/j.1349-7006.2008.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi YL. Takeuchi K. Soda M. Inamura K. Togashi Y. Hatano S. Enomoto M. Hamada T. Haruta H. Watanabe H. Kuruhashi K. Hatanaka H. Ueno T. Takda S. Yamashita Y. Sugiyama Y. Ishikawa Y. Mano H. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res. 2008;68:4971–4976. doi: 10.1158/0008-5472.CAN-07-6158. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi K. Choi YL. Soda M. Inamura K. Togashi Y. Hatano S. Enomoto M. Takada S. Yamashita Y. Satoh Y. Okumura S. Nakagawa K. Ishikawa Y. Mano H. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14:6618–6624. doi: 10.1158/1078-0432.CCR-08-1018. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi K. Choi YL. Togashi Y. Soda M. Hanata S. Inamura K. Takada S. Ueno T. Yamashitaa Y. Satoh Y. Okumura S. Nakagawa K. Ishikawa Y. Mano H. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15:3143–3149. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 19.Lin E. Li L. Guan Y. Soriano R. Rivers CS. Mohan S. Pandita A. Tang J. Modrusan Z. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol Cancer Res. 2009;7:1466–1476. doi: 10.1158/1541-7786.MCR-08-0522. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Classification of Tumours., Pathology and Genetics of Tumours of Endocrine Organs. In: DeLellis RA, editor; Lloyd RV, editor; Heitz PU, editor; Eng C, editor. IARC Press; Lyon, France: 2004. [Google Scholar]
- 21.Hamatani K. Eguchi H. Takahashi K. Koyama K. Mukai M. Ito R. Taga M. Yasui W. Nakachi K. Improved RT-PCR amplification for molecular analyses with long-term preserved formalin-fixed, paraffin-embedded tissue specimens. J Histochem Cytochem. 2006;54:773–780. doi: 10.1369/jhc.5A6859.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hamatani K. Eguchi H. Mukai M. Koyama K. Taga M. Ito R. Hayashi Y. Nakachi K. Improved methods for analysis of RNA present in long-term preserved thyroid cancer tissue of atomic bomb survivors. Thyroid. 2010;20:43–49. doi: 10.1089/thy.2009.0098. [DOI] [PubMed] [Google Scholar]
- 23.Reassessment of the Atomic Bomb Radiation Dosimetry for Hiroshima and Nagasaki Dosimetry System 2002. In: Young RW, editor; Kerr GD, editor. Radiation Effects Research Foundation; Hiroshima, Japan: 2005. [Google Scholar]
- 24.Chiarle R. Voena C. Ambrogio C. Piva R. Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 25.Shaw ST. Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res. 2011;17:2081–2086. doi: 10.1158/1078-0432.CCR-10-1591. [DOI] [PubMed] [Google Scholar]
- 26.Volante M. Collini P. Nikiforov YE. Sakamoto A. Kakudo K. Katoh R. Lloyd RV. LiVolsi VA. Papotti M. Sobrinho-Simoes M. Bussolati G. Rosai J. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Sug Pathol. 2007;31:1256–1264. doi: 10.1097/PAS.0b013e3180309e6a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.