Abstract
Background/Aims
Exacerbation of innate immune responses can contribute to development of acute lung injury. Multiple cell populations, including the bronchiolar epithelium, coordinate these inflammatory responses. Clara cells, non-ciliated epithelial cells, are located in the distal airways in humans and conducting airways in mice. These cells actively participate in innate immune responses but their precise contributions remain poorly defined.
Methods
To test the hypothesis that E. coli lipopolysaccaride (LPS) treatment stimulates production of pro-inflammatory mediators in mouse transformed Clara cells (MTCC), MTCC were treated with E. coli lipopolysaccaride (LPS).
Results
LPS increased COX-2 expression and stimulated production of prostaglandins, including prostaglandin E2 (PGE2). Enhanced mitogen activated protein kinase (MAPK) activation, nuclear factor-κB (NFκB) activation, and chemokine production were observed in MTCC in response to LPS treatment.
Conclusions
While the role for Clara cells in the regulation of host defense and the progression of acute lung injury needs further characterization, our data suggests the importance of this unique cell population in the pathogenesis of LPS-induced acute lung injury.
Key Words: Clara cells, LPS, Inflammation, Chemotaxis, COX-2, Prostaglandins
Introduction
Acute lung injury (ALI) can result from persistent lung infection which exacerbation of pro-inflammatory responses is a contributing factor. Clinically, ALI can further progress into life-threatening acute respiratory distress syndrome (ARDS) [1]. ALI and ARDS are characterized by excess production of pro-inflammatory mediators, including eicosanoids [2]. Lipopolysaccaride (LPS), a component of gram negative bacteria, activate Toll-like receptor 4 (TLR4) to stimulate intracellular signaling cascades. Through the activation of mitogen activated protein kinase (MAPK) and nuclear factor-κB (NFκB) pathways, these signaling pathways lead to increased production of cytokines, chemokines, and the synthesis of a broad group of lipid inflammatory mediators [3, 4].
During the inflammatory process, activation of phospholipase A2 (PLA2) causes release of arachidonic acid from membrane phospholipids which can be enzymatically metabolized by cyclooxgenases (COX) to form products including prostaglandins (PG) and thromboxanes (TX). Prostanoid products of COX are produced by multiple cell types including leukocytes and lung epithelial cells [5]. COX-2 is highly expressed in the lung, can be further induced by pro-inflammatory stimuli [6], and is increased in animal models of acute lung injury [7]. Pro-inflammatory lipid products of cycloxygenases include PGD2', PGF2α', PGE2', and TXB2. PGD2 and PGF2α' stimulate constriction of pulmonary airways and the systemic vasculature [5]. TXB2 is involved in regulation of vascular permeability. PGE2 serves as a potent bronchodilator and regulator of macrophage activation [5, 8].
The airway epithelium contributes to innate immune defenses in the lung by serving as a physical barrier and participating in coordinated pro- and anti-inflammatory responses [9]. Clara cells are non-ciliated epithelial cells located in the distal airways of human lungs and modulate immune responses [10]. These immune properties are largely attributed to the properties of their signature protein product, Clara cell secretory protein (CCSP) [11]. CCSP is suggested to have anti-inflammatory properties based on associations with acute lung injury in humans [12] and multiple mouse models of lung injury [13, 14, 15, 16]. Recent investigations have supported a role for NFκB activation in Clara cells to initiate pulmonary inflammation [17, 18, 19]. Additionally, Clara cells respond to LPS administration by increasing secretion of pro-inflammatory chemokines [20, 21].
Mouse transformed Clara cells (MTCC), a cell line generated from transgenic mice expressing SV40 T antigen under control of the CCSP promoter, have physiologic properties similar to primary Clara cells [22, 23]. The studies described in this report utilized MTCC to elucidate the contribution of COX-derived lipid mediators in Clara cell responses to lipopolysaccharide (LPS) treatment. We hypothesized that LPS treatment would increase COX-2 protein expression and prostanoid production in MTCC. Our data indicate that COX-2 is expressed in small airways and LPS induces COX-2 expression in MTCC leading to increased production of inflammatory lipid products, suggesting a previously undefined role for Clara cells during innate immune responses.
Materials and Methods
E. Coli Treatment and Immunohistochemistry
All mouse studies were performed using protocols that are approved by the IACUC at The Research Institute at Nationwide Children's Hospital. Eleven to twelve week old female C57BL/6 mice were anesthetized with isofluorane, and then inoculated intratracheally with vehicle (PBS) or 1 × 107 CFU/mouse E. coli (strain #12014, American Type Culture Collection, Manassas, VA). Twenty four hours after infection, mice were sacrificed and lungs were fixed with formalin. Fixed lungs were paraffin embedded and microsections were mounted on microscope slides. Slides of lung sections were stained with antibodies specific for COX-2 (rabbit polyclonal, 1:150, Cayman, Ann Arbor, MI) and CCSP (rabbit polyclonal, 1:1000, Seven Hill Bioreagents, Cincinnati, OH). Photomicrographs were taken of conducting or bronchiolar airways with a light microscope at 400x magnification.
Cell Culture and Treatments
Mouse transformed Clara cells (MTCC) and RAW 264.7 cells (a mouse macrophage cell line) were grown to 70-80% confluence for all studies. MTCC or RAW 264.7 cells were cultured in 1X DMEM with 4.5 g/L glucose (Mediatech, Inc., Manassas, VA) with 10% Fetal Bovine Serum (Mediatech, Inc.), and 1% Penicillin-Streptomycin (Mediatech, Inc.). MTCC or RAW 264.7 macrophages were treated with Dulbecco's PBS (Mediatech, Inc.) or E. coli O111: B4 lipopolysaccaride (cat# 437627, Calbiochem, Darmstadt, Germany) in serum-free media for the length of time indicated in each experiment. Dose response studies were performed and 1 ng/mL LPS was determined to be the lowest dose that caused keratinocyte-derived chemokine (KC) levels to be higher than the vehicle control. In additional studies, MTCC were treated with DMSO, 20 µM SB203580 (Invivogen, San Diego, CA), 20 µM SP600125 (Invivogen), and 1 or 10 µM NS-398 (Cayman).
ELISA
MTCCs or RAW 264.7 macrophages were cultured on 24-well plates and allowed to adhere overnight. Cells were treated with DPBS, 1, 10, 100, or 1000 ng/mL LPS and media was collected after 24 h. Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and KC levels were measured in media using ELISA (Duoset ELISA kits, R&D Systems, Minneapolis, MN) according to the manufacturers’ protocols. Absorbance was determined spectrophometrically using a Spectramax M2 Plate Reader (Molecular Devices, Sunnyvale, CA).
Western blots
Protein concentrations of cell lysates were determined by Bradford assay. Samples (25 µg protein) were separated by SDS-PAGE, and transferred to nitrocellulose membranes. Following blocking for 1.5 h, blots were probed with primary antibodies for phospho-p38 (rabbit monoclonal, 1:1000, Cell Signaling, Danvers, MA), phospho-ERK (rabbit monoclonal, 1:1000, Cell Signaling), phospho-JNK (rabbit monoclonal, 1:1000, Cell Signaling), total p38 (rabbit monoclonal, 1:1000, Cell Signaling), total ERK (rabbit monoclonal, 1:1000, Cell Signaling), total JNK (rabbit monoclonal, 1:1000, Cell Signaling), IκB-α (rabbit monoclonal, 1:1000, Cell Signaling), CCSP (1:1000), COX-1 (rabbit polyclonal, Cayman), or COX-2 (rabbit monoclonal, 1:200, Abcam, Cambridge, MA). For loading controls, α-tubulin (rabbit polyclonal, 1:10000, Abcam) or β-actin (rabbit monoclonal, 1:10000, Abcam) primary antibodies were used. Horseradish peroxidase conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (1:12000, BioRad Laboratories, Hercules, CA) were applied for 1 h. Immunoblots were developed using enhanced chemiluminescence western blotting detection (GE Healthcare, Buckinghamshire, UK) and band densities were quantified using Image Quant TL software, version 5.0 (GE Healthcare). During band quantification, background was subtracted.
Quantitative real-time PCR
RNA was isolated from cells using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. cDNAs were synthesized using Oligo d(T) primers (Invitrogen), dNTP, Superscript III Reverse Transcriptase kit (Invitrogen). cDNAs were loaded onto 96-well plates containing specific primers for KC, TNF-α, IL-6, CCSP, and β-actin (Table 1; Integrated DNA Technologies, San Diego, CA), and SYBR green/ROX master mix (Qiagen, Valencia, CA). Quantitative real time PCR (qRT-PCR) was performed using 7500 Applied Biosystems Real time PCR System (Qiagen). CT values of specified proteins were normalized to the CT values of β-actin. Fold change was calculated by normalizing to vehicle (calculated using 2(−δδCT)). Melt curves were utilized to ensure formation of a single product. Statistical analyses were performed on β-actin normalized values to assess differences between treatment groups.
Table 1.
Primer Sequences.
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| KC | CTTGAAGGTGTTGCCCTCAG | AAGGGAGCTTCAGGGTCAAG |
| COX-2 | TGAGCAACTATTCCAAACCAGC | GCACGTAGTCTTCGATCACTATC |
| TNFα | AGAAGTTCCCAAATGGCCTC | TTGTCTTTGAGATCCATGCC |
| IL-6 | TTCCATCCAGTTGCCTTC | TTCTCATTTCCACGATTTCC |
| CCSP | TGCAATAAACTGCGAGCATC | TGGTTCCCTCACTCACTCC |
| β-actin | CCTGACAGACTACCTCATGAAGATC | TAGAGCAACATAGCACAGCTTCTC |
Eicosanoid measurements
Analyses were performed by LC-MS/MS techniques using MRM and stable isotope dilution for quantitation as previously described [24]. Individual calibration curves were generated for each class of lipids and sample concentrations were calculated using isotope dilution corrections.
Statistics
Values from ELISA, density values, and prostaglandin levels were analyzed using one-way ANOVA with Newman-Keuls post-hoc. δCT values were analyzed by two-tailed student's t test. Significant differences are indicated as p>0.05. Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
Results
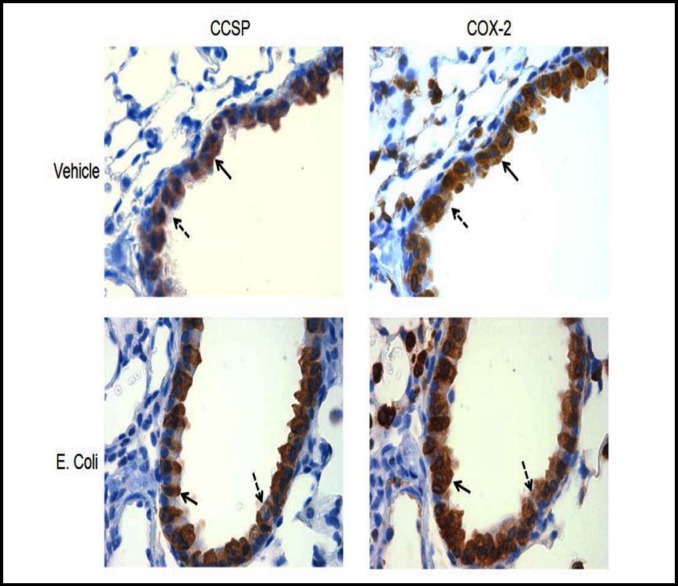
Although COX-2 is highly expressed in the lung, the cell type specificity in the airway epithelium has not been previously defined. COX-2 expression, in vivo, was assessed in lung sections from mice treated with vehicle or E. coli. Immunohistochemical staining revealed intense COX-2 protein expression in many cell types in the lung including conducting airway epithelial cells (Fig. 1).
Fig. 1.
COX-2 is expressed in CCSP expressing cells. Eleven to twelve week old mice were treated with vehicle or E. coli intratracheally and tissues were harvested 24 h post treatment. Lung serial sections were stained with CCSP or COX-2 antibodies. Photomicrographs were taken at 400x magnification. CCSP expressing cells are identified by solid arrow and non-CCSP expressing cells are identified by dashed arrow.
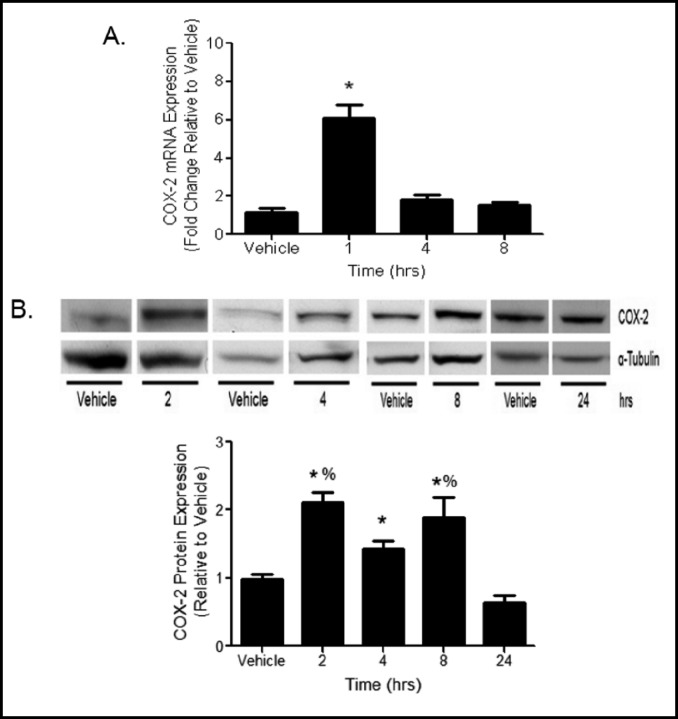
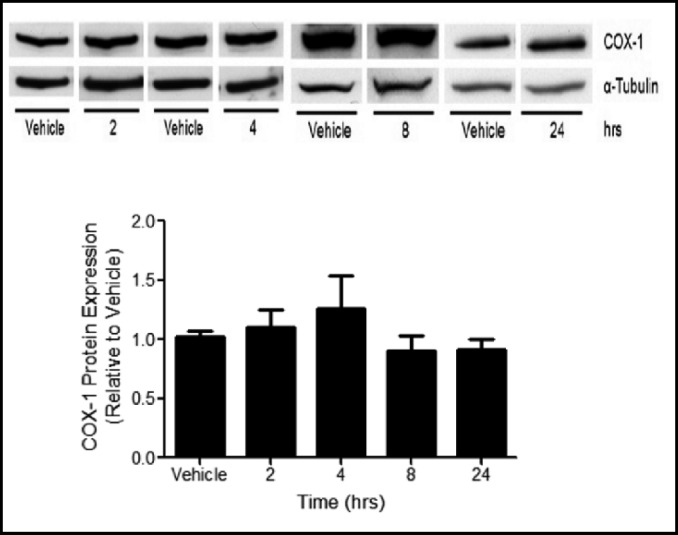
The mechanisms by which Clara cells contribute to pulmonary responses to E. coli infection were modeled using LPS treatment to MTCC in vitro. COX-2 mRNA levels, measured by qRT-PCR, were increased 6 fold 1 h following LPS treatment and returned to baseline levels at 4 and 8 h (Fig. 2A). COX-2 protein expression was increased in lysates from LPS treated MTCC when compared to vehicle controls at 2, 4, and 8 h, but was not different than vehicle treated cells at 24 h (Fig. 2B). Protein levels of COX-1 were not different between vehicle and LPS treated MTCC at any time point (Fig. 3).
Fig. 2.
LPS increases COX-2 mRNA and protein expression. (A) MTCC were treated with vehicle or 100 ng/mL LPS for 1, 4, or 8 h. COX-2 mRNA expression levels were determined by qRT-PCR. Ct values were normalized to ß-actin, and AACt were normalized to vehicle controls. Data are presented as fold change. Statistical analyses were performed on ACt values and analyzed using two tailed student's t test. * indicates a significant difference compared to vehicle control. Data represent means ± SEM from 2 independent experiments (n=6, p<0.05). (B) MTCC were treated with vehicle or 100 ng/mL LPS for 2, 4, 8, or 24 h. COX-2 protein levels were determined by western blot using enhance chemiluminescense and autoradiography COX-2 densities were normalized to oc-tubulin and quantified by densitometry. Data were analyzed using oneway ANOVA with Newman Keuls post-hoc. * indicates a significant difference compared to vehicle control and 24 h; % indicates a significant difference compared to 4 h. Data represents means ± SEM (normalized to vehicle controls) from at least 3 independent experiments (n=9-12, p<0.05).
Fig. 3.
COX-1 protein expression is not altered by LPS. MTCC were treated with vehicle or 100 ng/mL LPS for 2, 4, 8, or 24 h. COX-1 protein levels were determined by western blot using enhance chemiluminescense and autoradiography. COX-1 densities were normalized to α-tubulin and quantified by densitometry. Data were analyzed using one-way ANOVA with Newman Keuls post-hoc. No significant differences were observed. Data represents means ± SEM (normalized to vehicle controls) from 3–4 independent experiments (n=9–12, p≤0.05).
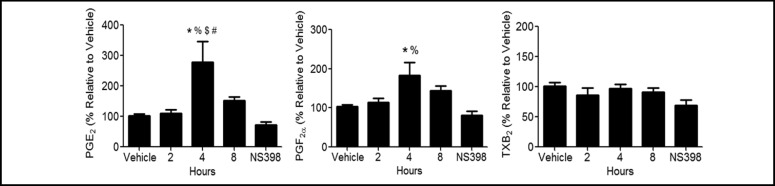
The contribution of LPS induced COX-2 expression on the formation of lipid metabolites was investigated. Prostaglandin and thromboxane levels were measured in media at 2, 4, and 8 h after treatment (Fig. 4). Significant increases of PGF2α and PGE2 levels were observed at 4 h, and returned to baseline levels by 8 h. TXB2 levels were not significantly altered by LPS treatment at the time points tested. Pretreatment with 1 µM (data not shown) or 10 µM NS-398, a COX-2 selective inhibitor, prevented the LPS-induced increases observed at 4 h (Fig. 4). These data demonstrate that LPS induced increases in specific prostaglandin products are mediated by COX-2.
Fig. 4.
Prostaglandin levels are increased by LPS. MTCC were treated with vehicle or 100 ng/mL LPS for 2, 4, or 8 h. Additionally, MTCC were pretreated with vehicle or 10 µM NS-398 for 30 min, then vehicle or 100 ng/mL LPS for 4 h. Lipid values were normalized to vehicle control for each experiment. Data was analyzed using one-way ANOVA with Newman Keuls post-hoc. * indicates a significant difference compared to vehicle control; % indicates a significant difference compared to NS-398; * indicates a significant difference compared to 2 h; # indicates a significant difference compared to 8 h. Data represents means ± SEM (normalized to vehicle controls) from at least 3 independent experiments (n=9–12, p≤0.05).
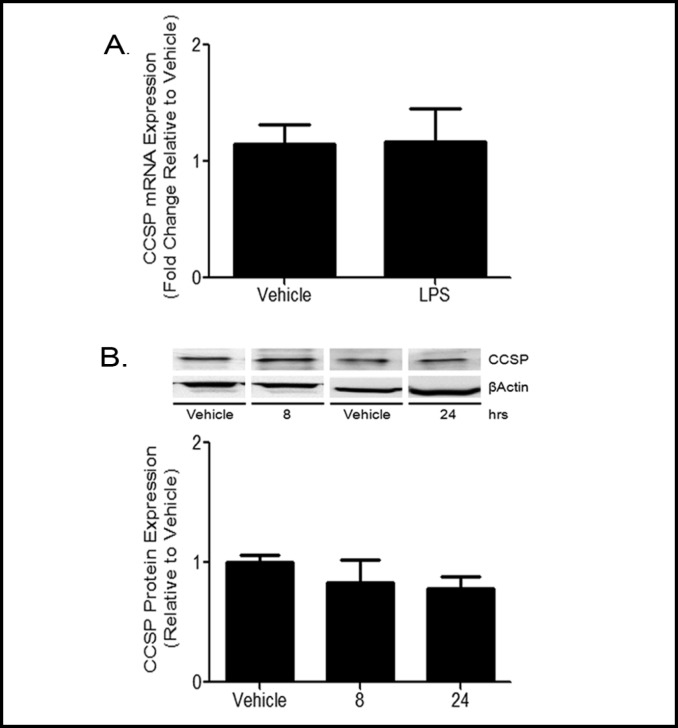
The immune function of Clara cells has been previously attributed to their production of CCSP protein [25]. To determine the effect of LPS treatment on CCSP expression, MTCC were treated with vehicle or 100 ng/ mL LPS for 8 or 24 h. CCSP mRNA levels, measured by qRT-PCR, were not increased by LPS treatment at 8 h. Furthermore, CCSP protein levels were not different between vehicle and LPS treatments at either 8 or 24 h (Fig. 5). Our data indicate that LPS treatment had no effect on CCSP mRNA or protein expression in MTCC at the time points tested.
Fig. 5.
CCSP expression is not altered by LPS treatment. MTCC were treated with vehicle or 100 ng/mL LPS for 8 or 24 h. (A) Cells were harvested at 8 h and CCSP mRNA expression levels were determined by qRT-PCR. Ct values were normalized to ß-actin, and AACt were normalized to vehicle controls. Data are presented as fold change. Statistical analyses were performed on ACT values and analyzed using two tailed student's t test. Data represent means ± SEM from at least 3 independent experiments (n=13, p<0.05). (B) CCSP protein levels at 8 and 24 h were determined by Western blot using enhance chemiluminescense and autoradiography and quantified by densitometry. CCSP band density was normalized to the band density of ß-actin. Data were analyzed using two tailed student's t test. Data represents means ± SEM (normalized to vehicle controls) from at least 3 independent experiments (n=13, p<0.05).
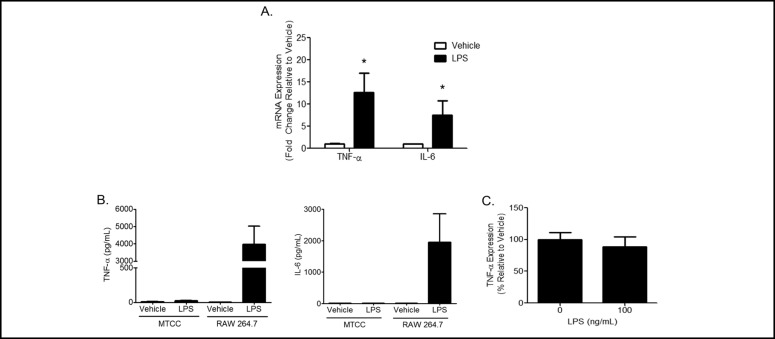
Classical cellular responses to LPS include increased expression of cytokines and chemokines. Cytokine and chemokine mRNA expression levels were measured by qRT-PCR and protein levels in media were measured by ELISA. Significant increases in TNF-α and IL-6 mRNA expression were observed in response to LPS when compared to vehicle treatment (Fig. 6A). However, significant increases in protein levels of TNF-α and IL-6 were not observed in media from either LPS or vehicle treated cells (Fig. 6B). As a positive control, RAW 264.7 macrophages were treated with LPS and significant induction of TNF-α protein secretion was observed (Fig. 6B). In addition, TNF-α and IL-6 protein levels were measured in whole cell lysates following LPS treatment.
Fig. 6.
LPS increases cytokine transcription. (A) MTCC were treated with vehicle or 100 ng/mL LPS for 8 h. Transcription of TNF-oc and IL-6 was evaluated by qRT-PCR. Ct values were normalized to ß-actin, and ddCt were normalized to vehicle controls. Data are presented as fold change. Statistical analyses were performed on ACT values and analyzed using two tailed student's t test. * indicates a significant difference compared to vehicle control. Data represent means ± SEM from 3 independent experiments (n=10, p<0.05). (B) MTCC and RAW 264.7 macrophages were treated with vehicle or 100 ng/mL LPS for 24 h. Media was collected, TNF-oc and IL-6 protein levels were measured in media by ELISA (n=6). (C) MTCC were treated with vehicle or 100 ng/mL LPS for 24 h. TNF-oc protein levels were measured in cell lysates by ELISA. Data represent means ± SEM from 2 independent experiments (n=6).
TNF-α protein levels were detected in lysates of vehicle and LPS treated MTCC (Fig. 6C) but were not different between treatments. IL-6 levels in cell lysates from vehicle and LPS treated MTCC were below the limit of detection (data not shown). These data suggest that upon stimulation with LPS, MTCC increase TNF-α and IL-6 mRNA levels however this does not result in elevated protein levels in cell lysates or media.
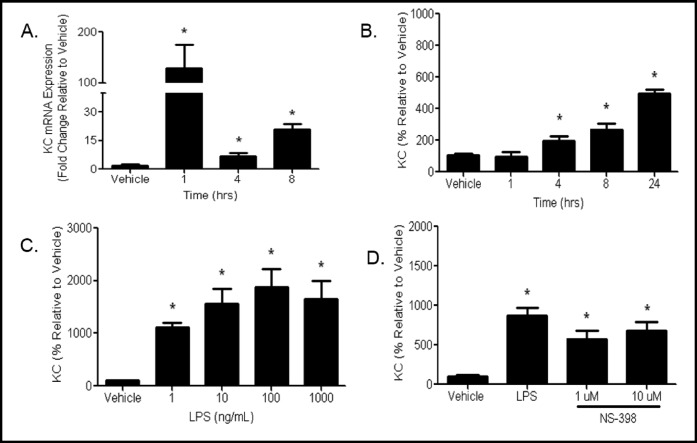
LPS treatment significantly increased KC mRNA expression 1 h post treatment and mRNA levels remained elevated through 8 h (Fig. 7A). Similar increases in macrophage inflammatory protein (MIP-2) and monocyte chemoattractant protein-1 (MCP-1) mRNA were observed at 8 h (data not shown). The increase in mRNA levels in LPS treated cells translated to increases in KC protein levels at 4 h and protein levels continued to increase through 24 h (Fig. 7B). Dose response studies indicate that at 24 h KC protein levels are increased by as little as 1 ng/mL with no further significant increases in doses up to 1000 ng/mL (Fig. 7C).
Fig. 7.
Chemokine mRNA and protein expression is increased by LPS treatment. (A) MTCC were treated with vehicle or 100 ng/mL LPS for 1, 4, or 8 h. mRNA expression levels were determined by qRT-PCR. Ct values were normalized to ß-actin, and AACt were normalized to vehicle controls. Data are presented as fold change. Statistical analyses were performed on ACt values and analyzed using two tailed student's t test (n=6-9, p<0.05). (B) MTCC were treated with vehicle or 1 ng/mL LPS for 1, 4, 8, or 24 h (n=9). (C) MTCC were treated with vehicle 1 10 100 1000 ng/mL LPS for 24 h (n=12). (D), mTCC we, re pre-treated with vehicle 1, or 10 µM NS-398 for 30 min then treated with vehicle or 1 ng/mL LPS (n=9). Media were collected for (B) (C) and (D) KC protein levels were measured by ELISA. Data were analyzed for (B) (C) and (D) using one-way ANOVA with Newman Keuls post-hoc (p<0 05) * indicates a significant difference compared to compared to vehicle. Data represents means ± SEM (normalized to vehicle controls) from 3-4 independent experiments.
We assessed the autocrine effect of LPS induced prostaglandin formation on chemokine expression by inhibition of COX-2 expression and activity on KC production by MTCC. Cells were pretreated with vehicle, 1, or 10 µM NS-398 and subsequently treated with vehicle or LPS. KC expression was not significantly altered in MTCC pretreated with NS-398 prior to LPS treatment (Fig. 7D) at the doses tested, suggesting that KC expression is not influenced by COX-2 activity or the concentration of inhibitor is insufficient to overcome the responses.
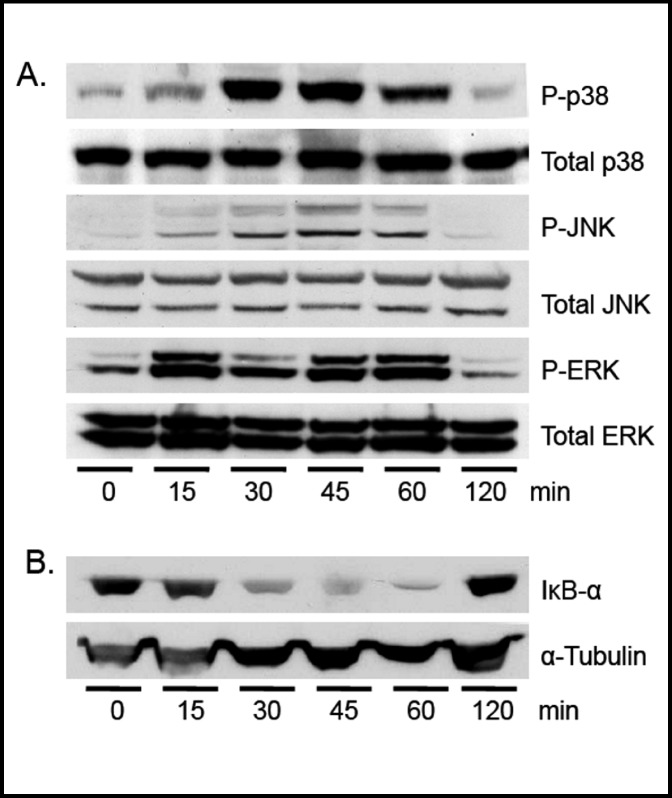
In vitro studies in macrophages and mouse studies have shown that LPS stimulation leads to phosphorylation of the MAPK proteins, JNK, p38, and/or ERK and activation of NFκB [3, 26]. Phosphorylation of JNK, p38, and ERK were measured in whole cell lysates from MTCC treated with vehicle or 100 ng/mL LPS for 0, 15, 30, 45, 60, and 120 min. The phosphorylation of JNK, p38, and ERK was significantly increased 15 min after LPS treatment, was sustained for at least 60 min, and returned to basal levels by 120 min (Fig. 8A). IκB-α directly binds NFκB subunits, thereby sequestering them in the cytoplasm and preventing NFκB activation. Inflammatory stimuli causes phosphorylation, ubiquitination, and degradation of IκB-α releasing NFκB subunits [27]. IκB-α protein levels were measured by western blot in MTCC treated with vehicle or 100 ng/mL LPS. Our data indicate a reduction in IκB-α protein levels at 30 min in MTCC treated with LPS compared to controls (Fig. 8B). As with the MAPK proteins, IκB-α protein expression levels returned to basal levels 120 min after LPS treatment.
Fig. 8.
LPS induces MAP kinase phosphory-lation and IκB-α degradation. MTCC were treated with vehicle or 100 ng/mL LPS for 0, 15, 30, 45, 60, or 120 min. (A) p38, JNK, and ERK phospho-rylation and total levels were assessed by Western blot using enhance chemiluminescense and autoradiography. (B) IκB-α and α-tubulin protein levels were evaluated by western blot. Blots are representative of at least 3 independent experiments.
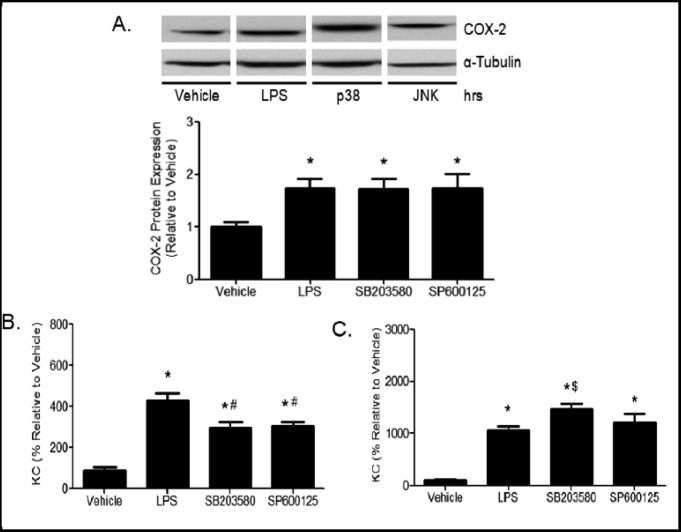
MAPK pathways are known to regulate expression of pro-inflammatory mediators. Consequently, we hypothesized that p38 and JNK may regulate the rapid induction of COX-2 (Fig. 2) and KC (Fig. 7) protein levels. To test this hypothesis, MTCC were pretreated with vehicle, SB203580 (p38 inhibitor), or SP600125 (JNK inhibitor), and subsequently treated with vehicle or LPS. COX-2 protein levels in cell lysates were not affected by inhibition of p38 or JNK after 2 h LPS stimulation (Fig. 9A). However, p38 and JNK inhibition suppressed LPS-induced KC protein levels after 4 h (Fig. 9B). KC protein levels were no longer suppressed at 24 h (Fig. 9C). Surprisingly, p38 inhibition slightly increased KC expression at 24 h. Together, these data suggest that p38 and JNK may regulate KC and play a role in the increase in protein expression at 4 h, but are either not involved or the inhibition is no longer effective in modulating the increases in KC translation after 24 h.
Fig. 9.
Regulation of COX-2 and chemokines by SB203580 and SP600125. MTCC were pretreated with vehicle, 20 µM SB203580 (p38 inhibitor), or 20 µM SP600125 (JNK inhibitor) for 1 h. (A) COX-2 expression was assessed after treatment with 100 ng/mL LPS for 2 h by western blot using enhance chemiluminescense and autoradiography. Band densities were quantified. Media was collected and KC secretion was analyzed by ELISA after treatment with 1 ng/mL LPS for (B) 4 h or (C) 24 h. Data were analyzed using one-way ANOVA with Newman Keuls post-hoc. * indicates a significant difference compared to vehicle, # and * indicates a significant difference compared to LPS treatment. Data represents means ± SEM (normalized to vehicle controls) from 2–3 independent experiments (n=6–9 p≤0.05).
Discussion
Clara cells are emerging as an important cell population within the airway epithelium during lung injury [28]. However, the specific mediators that are produced by Clara cells during inflammation are not well characterized. The role of COX-2 during inflammation is dynamic and recent studies demonstrate that COX-2 has a central role during the initiation as well as resolution of inflammation [29]. A seminal report by Gilroy et al. [30] indicated bi-phasic responses associated with COX-2 activation during a model of acute inflammation in the pleural cavity. The first phase was a classical pro-inflammatory response early in the course of injury, followed by a second anti-inflammatory phase involving the production of lipids with anti-inflammatory properties [30]. We observed changes in COX-2 expression in MTCC treated with LPS suggesting a potential role for Clara cells in response to LPS.
Immunohistochemical analyses demonstrated that COX-2 expression in small airways is present in non-ciliated airway epithelial cells in addition to other cell types (Fig. 1). Positive staining in vehicle treated mice suggests that COX-2 may have a homeostatic role in the small airways. While elevations in COX-2 and subsequent prostaglandin expression during inflammation are likely transient and tightly regulated, we speculate that Clara cells exhibit increased COX-2 mRNA and protein, and production of prostaglandins during initial responses to E. coli infection.
The expression of COX-2 in CCSP expressing cells led us to investigate COX-2 expression and activity in an immortalized Clara cell line, using LPS treatment as a model of gram negative infection. Our studies in MTCC indicated a rapid and substantial increase in COX-2 mRNA and protein expression in response to LPS (Fig. 2). Increases in COX-2 expression preceded the elevation in PGF2α and PGE2 levels in response to LPS (Fig. 4). The specific effects of elevated PGF2α remain less characterized than other prostaglandins, however PGF2α is associated with inflammatory responses [5, 31]. The primary physiological functions of PGF2α include vaso-and broncho-constriction by stimulation of smooth muscle cells that is observed early in the course of pulmonary infection.
The known roles of PGE2 during inflammatory responses are complex and depend on the cell type, expression of receptors, and the microenvironment. During the initiation of inflammation in the lung, PGE2 regulates vascular and airway constriction increasing vascular and airway permeability [5, 32]. After the initial response, PGE2 is implicated in “class switching” to initiate pathways involved in reprogramming of inflammatory cells to activate inflammatory resolution pathways [2, 7, 33]. Studies using COX-2 deficient mice have demonstrated an essential role for PGE2 expression in prevention of pulmonary fibrosis induced by vanadium pentoxide or bleomycin [34, 35] and bronchoconstriction in response to LPS [36]. Increases in PGE2 production by MTCC suggest an active role for Clara cells in both stages of the inflammatory response. The lack of changes in the levels of TXB2 was surprising. One possible explanation is that the subsequent enzyme activity required for TXB2 formation, thromboxane A2 synthase, is not activated by LPS in Clara cells.
Previous studies in Clara cells have focused on the expression of CCSP and have demonstrated that intratracheal LPS administration decreased CCSP levels in bronchoalveolar lavage fluids compared to vehicle controls [37]. In the present study, CCSP mRNA or protein levels were not affected by LPS administration in MTCC (Fig. 5). Differences between our results and previous studies could be attributable to cell-cell interactions present in the whole lung, differences in timing, or dose of LPS in our in vitro model.
Cytokine and chemokine responses to LPS by MTCC were similar to previous reports investigating inflammatory responses of Clara cells. Elizur et al. reported increased chemokine mRNA and protein secretion in both immortalized and primary Clara cells treated with LPS [21]. Similarly, we observed increases in KC transcription and secretion in MTCC treated with LPS (Fig. 7). Similar increases in mRNA and protein levels were also observed with other chemokines, MIP-2 and MCP-1 (data not shown), however the most robust responses were observed and further characterized with KC. The prostanoids PGF2α and PGE2 can influence inflammatory responses and alter chemokine expression [38, 39]. In our studies, inhibition of COX-2 and thus prostaglandin production, did not alter LPS-induced KC secretion in MTCC (Fig. 7D). These data suggest that COX-2 metabolic products do not influence KC expression in response to LPS in MTCC.
Interestingly, transcription of TNF-α and IL-6 mRNA were increased in MTCC treated with LPS compared to vehicle-treated controls however protein levels in media were below the limit of detection (Fig. 6). TNF-α, but not IL-6, protein was detected in cell lysates from MTCC (Fig. 6C) but was not different between LPS and vehicle treated cells. We observed a similar finding upon treatment with Pam2CSK, a TLR2 agonist, which stimulated KC secretion but did not stimulate TNF-α and IL-6 secretion in MTCC (data not shown), suggesting these mechanisms are not specific to LPS treatment. Similar observations have been previously reported in primary Clara cells treated with LPS [21]. Furthermore, CCSP-positive primary airway epithelial cells isolated from mice expressing constitutively active NFκB produce multiple chemokines but do not produce TNF-α or interleukin-1β (IL-1β) protein [18]. The physiological significance or mechanisms responsible for the absence of significant cytokine production upon LPS stimulation are not clear. However, we speculate that Clara cells may contribute to chemotactic gradients for leukocyte recruitment and facilitate production of cytokines by other cell types such as macrophages.
Our data indicate that LPS treatment induces phosphorylation of p38, JNK, and ERK and reduces IκB-α protein levels in MTCC (Fig. 8) within 30 min. We demonstrated rapid decreases in IκB-α protein levels which is a marker for NFκB nuclear translocation. Previous studies have suggested that rapid induction of COX-2 expression may be regulated by p38 [40]. We found that COX-2 protein expression was not directly related to activation of p38 or JNK in MTCC (Fig. 9A), but may be dependent on NFκB-mediated mechanisms. Additional studies using the IκB-α inhibitor, BAY-117082, resulted in cytotoxicity of MTCC; consequently, we were unable to test this hypothesis. We speculate that other cell signaling pathways may be involved in the regulation of LPS induced COX-2 expression in Clara cells.
Although the KC mRNA levels are maximal at 1 h, KC protein expression continued to increase for 24 h after LPS stimulation (Fig. 7). Inhibition of p38 and JNK activation significantly reduced protein expression of KC after 4 h LPS treatment but had little effect on KC protein levels at 24 h (Figs. 9B, C). These data suggest that p38 and JNK may regulate early induction of KC expression, but their effects were not sustained, likely due to continued enhanced transcription of KC mRNA.
Further investigation is needed to understand the transcriptional regulation of COX-2 and KC expression in Clara cells.
In addition to the previously described roles for Clara cells in the pathogenesis of acute lung injury, our studies indicate that LPS modulates COX-2 expression in MTCC and the subsequent production of prostaglandins. Furthermore, these novel findings suggest a previously unidentified mechanism by which Clara cells may modulate pro-inflammatory responses within the small airways. Enhanced understanding of the role of COX-2 in response to LPS in Clara cells could lead to directed therapies that would be clinically useful in the treatment of acute lung injury.
Acknowledgements
The authors would like to thank Dr. Francesco DeMayo for providing the MTCC, Dr. Lyn Wancket for assisting in immunohistochemical assessments of COX-2, and funding support from the F31HL097619 (NIH/ NHLBI), 1K08HL093365-01A2 (NIH/NHLBI), and American Thoracic Society.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Bonnans C, Levy BD. Lipid mediators as agonists for the resolution of acute lung inflammation and injury. Am J Respir Cell Mol Biol. 2007;36:201–205. doi: 10.1165/rcmb.2006-0269TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals R, Hiemstra PS. Innate immunity in the lung: How epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 5.Park GY, Christman JW. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol. 2006;290:L797–805. doi: 10.1152/ajplung.00513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu KK. Control of cyclooxygenase-2 transcriptional activation by pro-inflammatory mediators. Prostaglandins Leukot Essent Fatty Acids. 2005;72:89–93. doi: 10.1016/j.plefa.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174:5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 8.Nataraj C, Thomas DW, Tilley SL, Nguyen MT, Mannon R, Koller BH, Coffman TM. Receptors for prostaglandin e(2) that regulate cellular immune responses in the mouse. J Clin Invest. 2001;108:1229–1235. doi: 10.1172/JCI13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato A, Schleimer RP. Beyond inflammation: Airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder JC, Reynolds SD, Hollingsworth JW, Li Z, Kaminski N, Stripp BR. Clara cells attenuate the inflammatory response through regulation of macrophage behavior. Am J Respir Cell Mol Biol. 2010;42:161–171. doi: 10.1165/rcmb.2008-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh G, Katyal SL. Clara cells and clara cell 10 kd protein (cc10). Am J Respir Cell Mol Biol. 1997;17:141–143. doi: 10.1165/ajrcmb.17.2.f138. [DOI] [PubMed] [Google Scholar]
- 12.Kropski JA, Fremont RD, Calfee CS, Ware LB. Clara cell protein (cc16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest. 2009;135:1440–1447. doi: 10.1378/chest.08-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol. 1998;275:L924–930. doi: 10.1152/ajplung.1998.275.5.L924. [DOI] [PubMed] [Google Scholar]
- 14.Johnston CJ, Mango GW, Finkelstein JN, Stripp BR. Altered pulmonary response to hyperoxia in clara cell secretory protein deficient mice. Am J Respir Cell Mol Biol. 1997;17:147–155. doi: 10.1165/ajrcmb.17.2.2676. [DOI] [PubMed] [Google Scholar]
- 15.Hayashida S, Harrod KS, Whitsett JA. Regulation and function of ccsp during pulmonary pseudomonas aeruginosa infection in vivo. Am J Physiol Lung Cell Mol Physiol. 2000;279:L452–459. doi: 10.1152/ajplung.2000.279.3.L452. [DOI] [PubMed] [Google Scholar]
- 16.Wang SZ, Rosenberger CL, Espindola TM, Barrett EG, Tesfaigzi Y, Bice DE, Harrod KS. Ccsp modulates airway dysfunction and host responses in an ova-challenged mouse model. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1303–1311. doi: 10.1152/ajplung.2001.281.5.L1303. [DOI] [PubMed] [Google Scholar]
- 17.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial nf-kappa b activation in lipopolysaccharide-induced airway inflammation. J Immunol. 2003;170:6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]
- 18.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, Blackwell TS. Airway epithelium controls lung inflammation and injury through the nf-kappa b pathway. J Immunol. 2007;178:6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 19.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287:L143–152. doi: 10.1152/ajplung.00030.2004. [DOI] [PubMed] [Google Scholar]
- 20.Elizur A, Adair-Kirk TL, Kelley D G, Griffin GL, Demello DE, Senior RM. Tumor necrosis factor-alpha from macrophages enhances lps-induced clara cell expression of keratinocyte-derived chemokine. Am J Respir Cell Mol Biol. 2008;38:8–15. doi: 10.1165/rcmb.2007-0203OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elizur A, Adair-Kirk TL, Kelley D G, Griffin GL, deMello DE, Senior RM. Clara cells impact the pulmonary innate immune response to lps. Am J Physiol Lung Cell Mol Physiol. 2007;293:L383–392. doi: 10.1152/ajplung.00024.2007. [DOI] [PubMed] [Google Scholar]
- 22.DeMayo FJ, Finegold MJ, Hansen TN, Stanley LA, Smith B, Bullock DW. Expression of sv40 t antigen under control of rabbit uteroglobin promoter in transgenic mice. Am J Physiol. 1991;261:L70–76. doi: 10.1152/ajplung.1991.261.2.L70. [DOI] [PubMed] [Google Scholar]
- 23.Ramsay PL, Luo Z, Major A, Park MS, Finegold M, Welty SE, Kwak I, Darlington G, Demayo FJ. Multiple mechanisms for oxygen-induced regulation of the clara cell secretory protein gene. Faseb J. 2003;17:2142–2144. doi: 10.1096/fj.03-0048fje. [DOI] [PubMed] [Google Scholar]
- 24.Rogers LK, Tipple TE, Nelin LD, Welty SE. Differential responses in the lungs of newborn mouse pups exposed to 85% or >95% oxygen. Pediatr Res. 2009;65:33–38. doi: 10.1203/PDR.0b013e31818a1d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson TM, Reynolds SD, Mango GW, Boe IM, Lund J, Stripp BR. Altered lung gene expression in ccsp-null mice suggests immunoregulatory roles for clara cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1523–1530. doi: 10.1152/ajplung.2001.281.6.L1523. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: Attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem. 2005;280:8101–8108. doi: 10.1074/jbc.M411760200. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh S, Hayden MS. New regulators of nf-kappab in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 28.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc. 2008;5:328–333. doi: 10.1513/pats.200711-167DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: State of the art, definitions and terms. Faseb J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 31.Basu S. Novel cyclooxygenase-catalyzed bioactive prostaglandin f2alpha from physiology to new principles in inflammation. Med Res Rev. 2007;27:435–468. doi: 10.1002/med.20098. [DOI] [PubMed] [Google Scholar]
- 32.Goggel R, Hoffman S, Nusing R, Narumiya S, Uhlig S. Platelet-activating factor-induced pulmonary edema is partly mediated by prostaglandin e(2), e-prostanoid 3-receptors, and potassium channels. Am J Respir Crit Care Med. 2002;166:657–662. doi: 10.1164/rccm.200111-071OC. [DOI] [PubMed] [Google Scholar]
- 33.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 34.Hodges RJ, Jenkins RG, Wheeler-Jones CP, Copeman DM, Bottoms SE, Bellingan GJ, Nanthakumar CB, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin e(2) production. Am J Pathol. 2004;165:1663–1676. doi: 10.1016/S0002-9440(10)63423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonner JC, Rice AB, Ingram JL, Moomaw CR, Nyska A, Bradbury A, Sessoms AR, Chulada PC, Morgan DL, Zeldin DC, Langenbach R. Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. Am J Pathol. 2002;161:459–470. doi: 10.1016/S0002-9440(10)64202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeldin DC, Wohlford-Lenane C, Chulada P, Bradbury JA, Scarborough PE, Roggli V, Langenbach R, Schwartz DA. Airway inflammation and responsiveness in prostaglandin h synthase-deficient mice exposed to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 2001;25:457–465. doi: 10.1165/ajrcmb.25.4.4505. [DOI] [PubMed] [Google Scholar]
- 37.Arsalane K, Broeckaert F, Knoops B, Wiedig M, Toubeau G, Bernard A. Clara cell specific protein (cc16) expression after acute lung inflammation induced by intratracheal lipopolysaccharide administration. Am J Respir Crit Care Med. 2000;161:1624–1630. doi: 10.1164/ajrccm.161.5.9812157. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Wang H, Brown J, Daikoku T, Ning W, Shi Q, Richmond A, Strieter R, Dey SK, DuBois RN. Cxcl1 induced by prostaglandin e2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006;203:941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sales KJ, Maldonado-Perez D, Grant V, Catalano RD, Wilson MR, Brown P, Williams AR, Anderson RA, Thompson EA, Jabbour HN. Prostaglandin f(2alpha)-f-prostanoid receptor regulates cxcl8 expression in endometrial adenocarcinoma cells via the calcium-calcineurin-nfat pathway. Biochim Biophys Acta. 2009;1793:1917–1928. doi: 10.1016/j.bbamcr.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lasa M, Mahtani KR, Finch A, Brewer G, Saklatvala J, Clark AR. Regulation of cyclooxygenase 2 mrna stability by the mitogen-activated protein kinase p38 signaling cascade. Mol Cell Biol. 2000;20:4265–4274. doi: 10.1128/mcb.20.12.4265-4274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]