Abstract
Background
Hemifacial spasm is a neuromuscular movement disorder characterized by brief or persistent involuntary contractions of the muscles innervated by the facial nerve. Its prevalence has been estimated at 11 cases per 100 000 individuals. Among the patients who were operated on by our team, the mean interval from diagnosis to surgery was 8.2 years, and more than half of them learned of the possibility of surgical treatment only through a personal search for information on the condition. These facts motivated us to write this article to raise the awareness of hemifacial spasm and its neurosurgical treatment among physicians who will encounter it.
Methods
This review article is based on a selective literature search and on our own clinical experience.
Results
Hemifacial spasm is usually caused by an artery compressing the facial nerve at the root exit zone of the brainstem. 85–95% of patients obtain moderate or marked relief from local injections of botulinum toxin (BTX), which must be repeated every 3 to 4 months. Alternatively, microvascular decompression has a success rate of about 85%.
Conclusion
Local botulinum-toxin injection is a safe and well-tolerated symptomatic treatment for hemifacial spasm. In the long term, however, lasting relief can only be achieved by microvascular decompression, a microsurgical intervention with a relatively low risk and a high success rate.
Hemifacial spasm is a movement disorder of the muscles innervated by the facial nerve (cranial nerve VII). This movement disorder triggers involuntary short or longer contractions of the facial muscles. Although the disorder does not have life-threatening consequences, affected persons often suffer immensely and tend to increasingly withdraw socially. As a severe psychosocial stressor, the condition requires timely diagnosis and therapy.
The lengthy medical history of about 8.2 years in our own patients, in addition to the fact that more than 50% of these patients found out about surgical treatment options owing to their own research initiative, prompted us to write this article. With this review article, we aim to increase awareness of and attention for this disorder.
In the article we will discuss clinical characteristics, differential diagnoses, diagnostic approaches, and therapeutic options for hemifacial spasm. In addition to established symptomatic treatment in the shape of local injections of botulinum toxin, we will focus in particular on the option of microvascular decompression, since this currently still represents the only long-term and causal therapeutic option.
Methods
This review article is based on a selective literature search and the scientific analysis of data from our own patients. The literature search focused primarily on studies from the past 20 years, which described larger case series including more than 50 patients.
Epidemiology
Few epidemiological data are available for the pathological entity that is hemifacial spasm. One study evaluated data from patients in Olmsted County, Minnesota, from 1960 through 1984 (1). The mean annual incidence was 0.81 per 100 000 in women and 0.74 per 100 000 in men. The mean prevalence was 11 per 100 000 in the total population. Women developed the disorder at a rate of 14.5 per 100 000 and men at 7.4 per 100 000. The distribution by sex is thus 2:1. In Germany, the estimated prevalence is 8000 to 9000 persons.
A study from Oslo, Norway, showed comparable results (2). Differences were, however, seen in the age distribution of the patients. In the US study the age peak with the highest prevalence was between 40 and 59 years, whereas the Norwegian study showed a continual increase in the prevalence to a maximum of 39.7 per 100 000 in those older than 70. The mean age at disease onset in the Norwegian study was 54. Only 1% to 6% of all patients with hemifacial spasm before age 30 (3). In our own group of patients, too, more women than men developed the disease (women 60%, men 40%) (Table).
Table. Data from our own patient collective (in total: 110 patients with 116 microvascular decompression procedures, 83 patients with follow-up of more than 6 months).
| Distribution by sex | ||||
| Women | 69 | (62.7%) | ||
| Men | 41 | (37.3%) | ||
| Average age (years) | 56.6 | |||
| Women | 55.9 | |||
| Men | 57.6 | |||
| Side affected by hemifacial spasm | ||||
| Left | 72 | |||
| Right | 38 | |||
| Mean duration of problems (years) | 8.1 | |||
| Follow-up | > 6 months (83 patients) | > 18 months (40 patients) | ||
| Completely free of problems | 69.9% | 77.5% | ||
| Reduction in spasms by >90% | 13.3% | 10.0% | ||
| Reduction in spasms >50% | 10.8% | 2.5% | ||
| No improvement | 6.0% | 10.0% | ||
| Complications (in a total of 116 procedures) | ||||
| Permanent anacusis | 2% | |||
| Persistent vertigo | 1% | |||
| Temporary, delayed facial nerve paralysis | 3% | |||
Familial clustering is rare for this condition (4). The prevalence of bilateral hemifacial spasm has been reported to be 2.6% of cases (5). None of our own patients had bilateral symptoms.
Clinical presentation, differential diagnoses
Hemifacial spasm is characterized by progressive, involuntary, irregular, clonic or tonic movements of the muscles innervated by the facial nerve (cranial nerve VII) (6). Typically, these symptoms are strictly unilateral. At disease onset, in many cases involuntary spasms occur in the region of the orbicularis oculi muscle, which then gradually extend into other parts of the affected half of the face. In pronounced cased, the platysma muscle is also affected (Figure 1). In most patients the symptoms persist during sleep (6). Five of our own patients also complained of a “clicking” in the ear, which can be explained with contractions of the stapedius muscle.
Figure 1.
Patient with pronounced right-sided hemifacial spasm. Involvement of the platysma muscle is clearly visible
Patients with hemifacial spasm suffer primarily from the associated psychosocial stress. Furthermore, pronounced spasm of the orbicularis oculi muscle can impair bilateral vision, which is required for reading or driving (7). The symptoms are often accentuated by psychological tension and during speaking. In many patients, low-grade facial nerve paralysis develops after many years of hemifacial spasm. In contrast to other movement disorders, psychopathological abnormalities—for example, anxiety disorders—are no more common in patients with hemifacial spasm than in the normal population (8).
A problem in diagnosing hemifacial spasm can be the differentiation from other movement disorders in the cranio-cervical region. The main differential diagnoses include:
Blepharospasm
Oromandibular dystonia
Facial nerve tic
Hemimasticatory spasm
Focal seizures
Synkinesias after facial nerve paralysis (6).
In contrast to the strictly unilateral symptoms of hemifacial spasm, blepharospasm presents with bilateral, involuntary, mostly synchronous, symmetrical contractions of the eyelids. Oromandibular dystonia presents with involuntary, repeated, sustained muscle contractions that affect primarily the lower part of the face, the mouth, the mandible and maxilla, the tongue, and the pharynx. Facial nerve tic presents with more complex, coordinated, multifocal movement patterns and switches between the right and the left of the face. In contrast to hemifacial spasm, the tics can typically be suppressed (6). Simple focal seizures can also be confused with hemifacial spasm if they affect the facial muscles. Hemimasticatory spasm describes painful contractions of the muscles of mastication. Synkinesias after facial nerve paralysis also lead to activation of several muscles innervated by the facial nerve. Typically this only occurs in the context of voluntary movement.
The diagnosis of hemifacial spasm ultimately needs to be made by a specialist.
Etiology and pathophysiology
The underlying cause of hemifacial spasm is in most cases an ectatic or atypically aberrant blood vessel, which compresses the facial nerve at the place where it exits the brainstem (9). Pathoanatomically this so-called root-exit zone has some particular features: The nerve is ensheathed by an arachnoidal membrane only, without the epineurium. Furthermore, no connective tissue septa traverse the individual fascicles. This region is also the transition zone between central (oligodendroglial cells) and peripheral (Schwann cells) myelination (10). All the particular features result in increased vulnerability and therefore susceptibility to stimuli, such as compression.
The pathogenesis of hemifacial spasm by compression of the facial nerve is explained by using several theories. According to the “peripheral” hypothesis, ephaptic and ectopic excitations occur in the root-exit zone. The ephaptic impulse conduction is characterized by a pathological transfer of impulses between neighboring nerve fibers (10– 12). Ectopic impulse conduction describes the spontaneous development of neural impulses in the compression area. By contrast, the “central” hypothesis assumes hyperexcitability of the facial motor nucleus in the brainstem (10, 11). The explanation for patients’ relatively old age at disease onset is that in the course of life, progressive ectatic changes and elongations may affect the vessels in the cerebellopontine angle. This is particularly common in patients with arterial hypertension. Over time, this results in contact between vessel and nerve, which causes the compression. It is assumed that this leads in turn to focal demyelination, which leads to the electrophysiological processes mentioned above.
Further, albeit much rarer, causes of hemifacial spasm include all kinds of space-occupying lesions in the cerebellopontine angle—for example, Schwannomas, meningiomas, and arachnoidal cysts (4, 6). Certain processes in the brainstem can also result in symptoms. These include gliomas, demyelinating neurological disorders such as multiple sclerosis, or brainstem infarction. It has been reported that symptoms can also be triggered by Bell’s palsy owing to injury to the facial nerve (4).
In our own series of 110 patients, we found that clear compression of the root-exit zone was the cause of hemifacial spasm in all but two patients.
Diagnostic evaluation
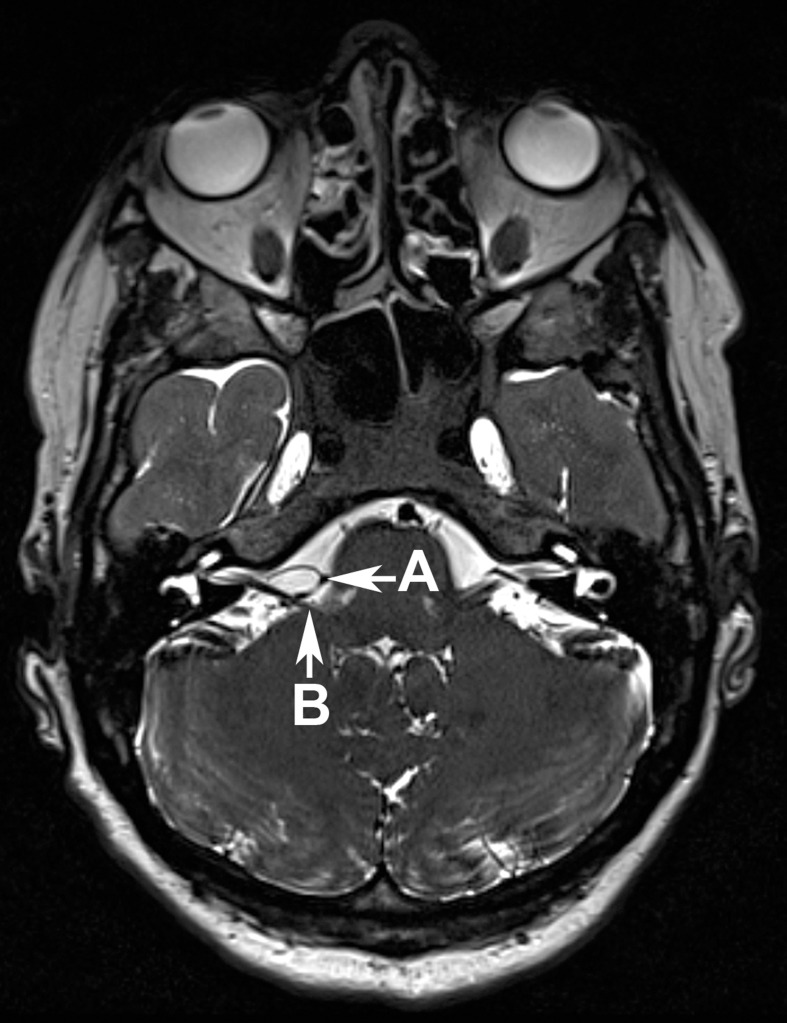
The clinical features are crucial to making a diagnosis of hemifacial spasm. Additional methods to find a diagnosis include electromyography (EMG) (13) and magnetic resonance imaging (MRI). MRI is also a useful modality to exclude pathological changes in the cerebellopontine angle, such as tumors or brainstem lesions. To display possible vascular compression, high-resolution, T2-weighted sequences are particularly useful—for example, an axial-plane CISS (constructive interference in steady state) sequence, since this yields images with a high contrast between cerebrospinal fluid, nerves, and vessels (Figure 2).
Figure 2.
The axial-plane CISS sequence shows a loop of the posterior inferior cerebellar artery (Arrow A) which compresses the facial nerve (Arrow B), where it exists the brainstem
In most cases, the compression is caused by the inferior posterior cerebellar artery (PICA) or the inferior anterior cerebellar artery (AICA). The compression is rarely caused by the vertebral artery (VA) or a combination of these arteries. In very rare cases, the compression can also be caused by a vein.
Treatment
Since hemifacial spasm has a low incidence and prevalence, large studies are lacking that compare the possible therapeutic options in a randomized and controlled fashion. Untreated symptoms will persist for life, and over time, the spasms are often progressive in terms of intensity and frequency, as well as area affected. An indication for treatment exists when a patient feels impaired in his/her quality of life by the disease or if functional impairments—for example, of the visual field—are present.
The therapeutic options for hemifacial spasm range from simple application of heat to medication treatment and botulinum injections to microvascular decompression surgery.
Medication treatment
The drugs used to treat hemifacial spasm include carbamazepine, clonazepam, and baclofen, as well as newer anticonvulsive drugs, such as gabapentin. Reportedly, success has been poor, sporadic, and not sustained (6). For this reason, medication therapy has been assessed as unsatisfactory in most studies (6, 14) and is used only for very mild forms of the disease. It should also be mentioned that some patients are notably affected by adverse effects, such as fatigue, exhaustion, and poor performance.
Botulinum toxin
Botulinum toxin (BTX) is a neurotoxin that paralyses the muscles by irreversibly blocking the cholinergic signal transmission at the presynaptic nerve endings (15). Since the early 1980s it has been used for local injection therapy for hemifacial spasm. Since then BTX treatment has become the standard symptomatic treatment for the condition. This treatment reportedly results in notable to moderate alleviation of symptoms in 85% to 95% of patients (6, 16, 17). Common adverse effects include temporary facial nerve paralysis (23%), the occurrence of double vision (17%), and ptosis (15%) (6, 14, 16). Nausea, allergic reactions, and antibodies to BTX have been observed less often.
The crucial disadvantage of this treatment modality is its limited effectiveness; the injections have to be repeated at intervals of 3–4 months (6, 14, 17). The treatment is merely symptomatic. Furthermore, patients treated for many years have reported a reduction in the effectiveness of BTX after a few years.
Altogether, however, botulinum injection is a minimally invasive option to alleviate the symptoms of hemifacial spasm that has few side effects and can be given on an outpatient basis.
For certain groups of patients, local BTX injection is the only effective symptomatic treatment option. This is mainly the case when surgery is out of the question—for example, in patients put at high risk by anesthesia or those patients whose symptoms are not caused by vascular compression.
Mikrovascular decompression
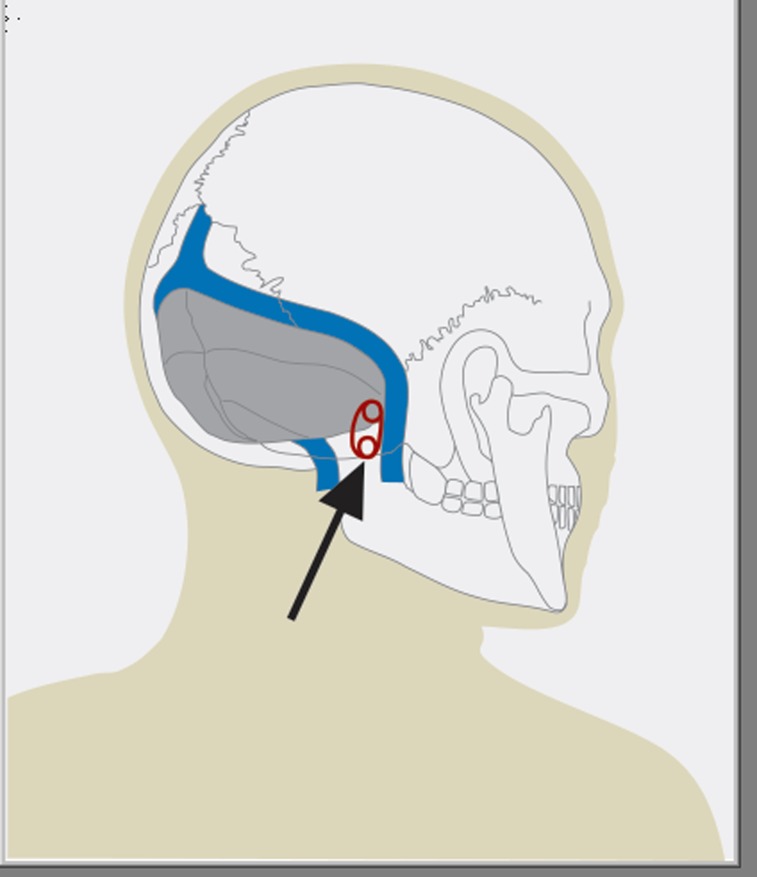
Microvascular decompression of the facial nerve is the only therapeutic option to treat the cause of hemifacial spasm. The surgery aims to remove the vascular compression in the root-exit zone of the nerve from the brainstem that is the cause of the illness. The surgery is done under general anesthesia. In order to detect alterations to the cochlear and facial nerves early on during the operation, the procedure is done under continual intraoperative neuromonitoring with facial nerve EMG and auditory evoked potentials. After retrosigmoidal craniotomy (Figure 3) the cerebellopontine angle is exposed. Subsequently the course, but especially the exit zone of the facial nerve from the brainstem are inspected (Figure 5). In order to identify the location of the vascular compression, using endoscopy is often helpful (Figures 4 and 6). In our institution, this procedure is therefore always endoscopy guided.
Figure 3.
The figure shows the location of the lower retrosigmoidal craniotomy (Arrow)
Figure 5.
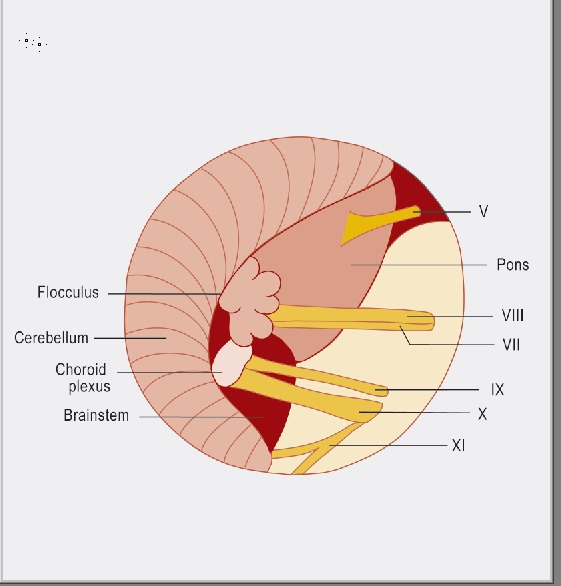
The figure shows schematically the anatomical conditions in the cerebellopontine angle: V = trigeminal nerve, VIII = cochlear nerve, VII = facial nerve, IX = glossopharyngeal nerve, X = vagus nerve, XI = spinal accessory nerve
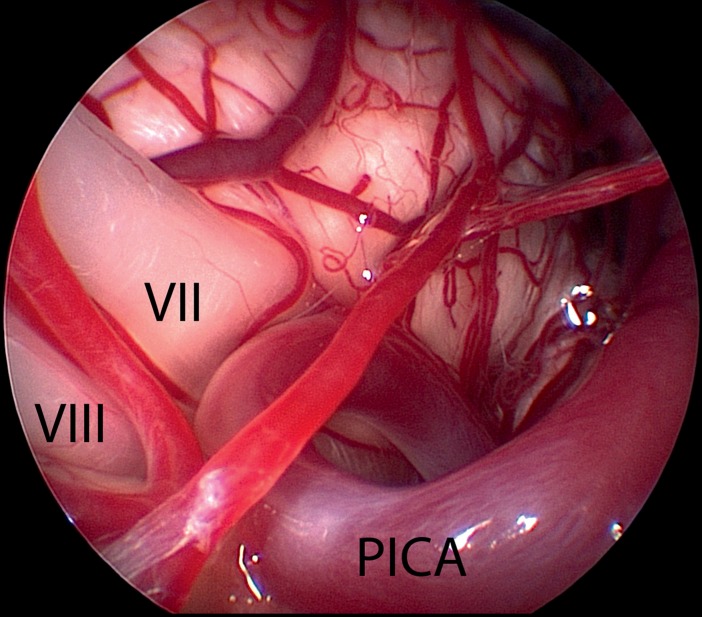
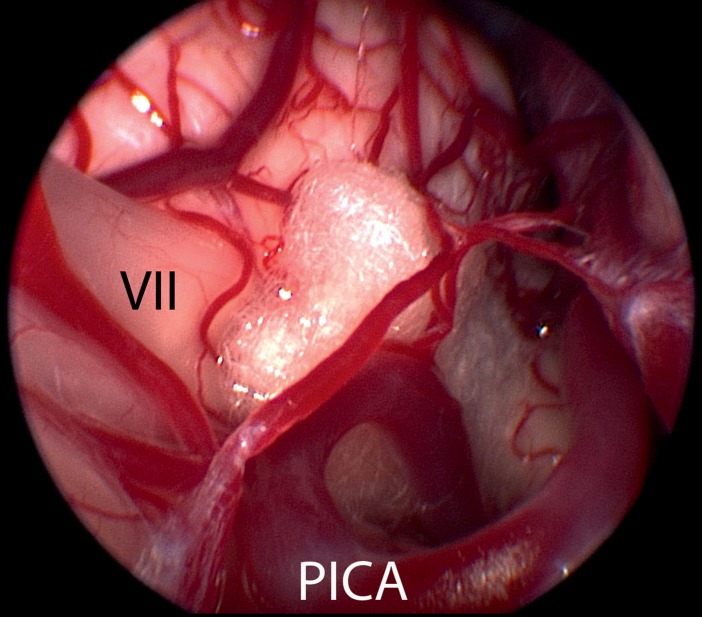
Figure 4.
Endoscopic image—taken using a 30°endoscope—shows the proximal part of the cochlear nerve (VIII) and the facial nerve with its root-exit zone (VII), which is compressed by a loop of the posterior inferior cerebellar artery (PICA) (same patient as in Figure 2)
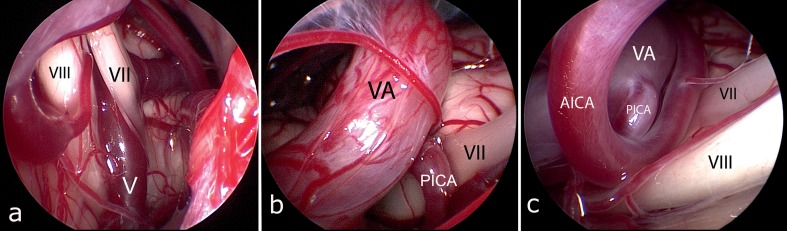
Figure 6.
Different forms of vascular compression
In this case the compression is caused by a vein (V) in close proximity to the brainstem (VII = facial nerve, VIII = cochlear nerve)
Here, the compression is caused by a combination of the vertebral artery (VA) and posterior inferior cerebellar artery (PICA). (VII = facial nerve)
The compression is caused by a combination of vertebral artery (VA), the descending posterior inferior cerebellar artery (PICA), and the anterior inferior cerebellar artery (AICA)
The actual decompression is usually done by placing a Teflon sponge between the vessel and the brainstem (Figure 7). In more complex cases—in patients with large-caliber and arteriosclerotic vessels—it may be necessary to stitch the compressing vessel to the dura mater with a teflon sling, in order to remove the compression completely.
Figure 7.
After microsurgical dissection of the vessel from the facial nerve, a teflon sponge is placed between brainstem and vessel, in order to permanently prevent vascular compression (VII = facial nerve, PICA = posterior inferior cerebellar artery)
The literature has reported an average success rate of the surgery of 80% to 88% within the first postoperative year (14, 18, 19). This is consistent with the results in our own series (Table). At follow-up, the success rate (that is, a reduction in spasms by more than 90%) was 83% at 6 months and 87% at 18 months. In patients with a small improvement in symptoms, or none at all, the reason for the lack of success was mostly individual anatomy, such as large-caliber veins or arteries running between the facial nerve and the vestibulocochlear nerve, or extremely elongated and ectatic vertebral arteries in a small perimedullary space. The recurrence rate in our series has been 4% to date; this, again, is consistent with other studies (18, 14, 19).
As for every surgical procedure, certain risks exist for microvascular decompression. The main risk is temporary or permanent hearing impairment, even to the extent of unilateral deafness. The risk reported in larger studies was 1.5% to 8% (14, 18– 21). The risk for permanent facial paralysis reported in the literature is 0.7% to 0.9%. Delayed facial paralysis is more common (in 3% to 8% of cases [19, 22]). The function of the facial nerve is initially completely intact after the procedure. After a mean of 12 days, however, acute high-grade paralysis of the facial muscles can develop on the operated side. One of the possible causes associated with this is the possible reactivation of Herpes zoster. Ultimately, however, the etiology of the delayed facial paralysis has not been identified to date (22). The symptoms recede completely over time in nearly all patients. The perioperative risk for life-threatening complications in the context of microvascular decompression—such as space-occupying hemorrhages or cerebellar or brainstem infarctions—is less than 1%, and no such complications have as yet occurred in our own case series. In conclusion, we can say that this risk profile seems acceptable in view of the high success rate and the fact that patients as a result are symptom-free and often remain so for the duration.
Conclusion
Local botulinum injections are a well tolerated, low-risk symptomatic treatment option for hemifacial spasm. Especially for patients with mild hemifacial spasm, who have a high anesthetic risk, and for patients who refuse surgery, this is the treatment of choice.
Freedom from symptoms in the longer term can, however, be achieved only by means of microvascular decompression surgery, a relatively low-risk intervention with a high likelihood of success. The option of surgical treatment should be explained to all patients with unequivocal clinical symptoms, since it represents the only causal form of treatment. Especially in young patients, the authors recommend early surgery, since our own case series showed that the results in patients with long-term symptoms were significantly poorer. Often by then the facial nerve already has developed structural damage, which becomes obvious during surgery.
Key Messages.
Hemifacial spasm is a movement disorder affecting the muscles innervated by the facial nerve. The result is involuntary tonic or clonic contractions of the facial muscles, which are almost always unilateral.
The cause in most cases is a compression of the facial nerve in its root-exit zone from the brainstem by a vessel with an aberrant or ectatic blood vessel.
The diagnosis is led by the clinical features. Important in deciding treatment is the differential diagnostic differentiation from other craniofacial movement disorders. Magnetic resonance imaging using CISS sequence is helpful for diagnosing a possible vascular compression or other intracranial cause.
The most important symptomatic treatment is local injection of botulinum toxin. In more than 85% of patients this leads to a notable alleviation of symptoms. The disadvantage associated with his procedure is the fact that repeated administration at intervals of several months is necessary.
Microvascular decompression surgery is the only causal treatment option with a success rate of about 85% in terms of permanent freedom of symptoms.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
Professor Schroeder has received honoraria and reimbursement of travel expenses for consulting and tutoring from Karl Storz GmbH und Co KG, Tuttlingen.
The other authors declare that no conflict of interest exists.
References
- 1.Auger RG, Whisnant JP. Hemifacial spasm in Rochester and Olmsted County, Minnesota, 1960 to 1984. Arch Neurol. 1990;47:1233–1234. doi: 10.1001/archneur.1990.00530110095023. [DOI] [PubMed] [Google Scholar]
- 2.Nilsen B, Le KD, Dietrichs E. Prevalence of hemifacial spasm in Oslo, Norway. Neurology. 2004;63:1532–1533. doi: 10.1212/01.wnl.0000142080.85228.e8. [DOI] [PubMed] [Google Scholar]
- 3.Tan EK, Chan LL. Young onset hemifacial spasm. Acta Neurol Scand. 2006;114:59–62. doi: 10.1111/j.1600-0404.2006.00650.x. [DOI] [PubMed] [Google Scholar]
- 4.Yaltho TC, Jankovic J. The many faces of hemifacial spasm: differential diagnosis of unilateral facial spasms. Mov Disord. 2011;26:1582–1592. doi: 10.1002/mds.23692. doi: 10.1002/mds.23692. [DOI] [PubMed] [Google Scholar]
- 5.Felício AC, Godeiro-Junior Cde O, Borges V, Silva SM, Ferraz HB. Bilateral hemifacial spasm: a series of 10 patients with literature review. Parkinsonism Relat Disord. 2008;14:154–156. doi: 10.1016/j.parkreldis.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Wang A, Jankovic J. Hemifacial spasm: clinical findings and treatment. Muscle Nerve. 1998;21:1740–1747. doi: 10.1002/(sici)1097-4598(199812)21:12<1740::aid-mus17>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Wilkins RH. Hemifacial spasm: a review. Surg Neurol. 1991;36:251–277. doi: 10.1016/0090-3019(91)90087-p. [DOI] [PubMed] [Google Scholar]
- 8.Scheidt CE, Schuller B, Rayki O, Kommerell G, Deuschl G. Relative absence of psychopathology in benign essential blepharospasm and hemifacial spasm. Neurology. 1996;47:43–45. doi: 10.1212/wnl.47.1.43. [DOI] [PubMed] [Google Scholar]
- 9.Girard N, Poncet M, Caces F, Tallon Y, Chays A, Martin-Bouyer P, Magnan J, Raybaud C. Three-dimensional MRI of hemifacial spasm with surgical correlation. Neuroradiology. 1997;39:46–51. doi: 10.1007/s002340050366. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen VK. Electrophysiology of the facial nerve in hemifacial spasm: ectopic/ephaptic excitation. Muscle Nerve. 1985;8:545–555. doi: 10.1002/mus.880080702. [DOI] [PubMed] [Google Scholar]
- 11.Møller AR. Vascular compression of cranial nerves: II: pathophysiology. Neurol Res. 1999;21:439–443. [PubMed] [Google Scholar]
- 12.Møller AR, Jannetta PJ. On the origin of synkinesis in hemifacial spasm: results of intracranial recordings. J Neurosurg. 1984;61:569–576. doi: 10.3171/jns.1984.61.3.0569. [DOI] [PubMed] [Google Scholar]
- 13.Wilkins RH. Hemifacial spasm: a review. Surg Neurol. 1991;36:251–277. doi: 10.1016/0090-3019(91)90087-p. [DOI] [PubMed] [Google Scholar]
- 14.Barker FG, 2nd, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg. 1995;82:201–210. doi: 10.3171/jns.1995.82.2.0201. [DOI] [PubMed] [Google Scholar]
- 15.Jitpimolmard S, Tiamkao S, Laopaiboon M. Long term results of botulinum toxin type A (Dysport) in the treatment of hemifacial spasm: a report of 175 cases. J Neurol Neurosurg Psychiatry. 1998;64:751–757. doi: 10.1136/jnnp.64.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimura DM, Aminoff MJ, Tami TA, Scott AB. Treatment of hemifacial spasm with botulinum toxin. Muscle Nerve. 1992;15:1045–1049. doi: 10.1002/mus.880150909. [DOI] [PubMed] [Google Scholar]
- 17.Simpson DM, Blitzer A, Brashear A, et al. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699–1706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan Y, Wang Y, Zhang SX, Zhang L, Li R, Guo J. Microvascular decompression in patients with hemifacial spasm: report of 1200 cases. Chin Med J (Engl) 2005;118:833–836. [PubMed] [Google Scholar]
- 19.Dannenbaum M, Lega BC, Suki D, Harper RL, Yoshor D. Microvascular decompression for hemifacial spasm: long-term results from 114 operations performed without neurophysiological monitoring. J Neurosurg. 2008;109:410–415. doi: 10.3171/JNS/2008/109/9/0410. [DOI] [PubMed] [Google Scholar]
- 20.Jo KW, Kim JW, Kong DS, Hong SH, Park K. The patterns and risk factors of hearing loss following microvascular decompression for hemifacial spasm. Acta Neurochir (Wien) 2011;153:1023–1030. doi: 10.1007/s00701-010-0935-8. Epub 2011 Jan 15. [DOI] [PubMed] [Google Scholar]
- 21.Samii M, Günther T, Iaconetta G, Muehling M, Vorkapic P, Samii A. Microvascular decompression to treat hemifacial spasm: long-term results for a consecutive series of 143 patients. Neurosurgery. 2002;50:712–718. doi: 10.1097/00006123-200204000-00005. discussion 718-9. [DOI] [PubMed] [Google Scholar]
- 22.Lovely TJ, Getch CC, Jannetta PJ. Delayed facial weakness after microvascular decompression of cranial nerve VII. Surg Neurol. 1998;50:449–452. doi: 10.1016/s0090-3019(97)00314-5. [DOI] [PubMed] [Google Scholar]