Abstract
Increased risk of vasospasm, a spontaneous hyperconstriction, is associated with atherosclerosis, cigarette smoking, and hypertension—all conditions involving oxidative stress, lipid peroxidation, and inflammation. To test the role of the lipid peroxidation- and inflammation-derived aldehyde, acrolein, in human vasospasm, we developed an ex vivo model using human coronary artery bypass graft (CABG) blood vessels and a demonstrated acrolein precursor, allylamine. Allylamine induces hypercontraction in isolated rat coronary artery in a semicarbazide-sensitive amine oxidase activity (SSAO) dependent manner. Isolated human CABG blood vessels (internal mammary artery, radial artery, saphenous vein) were used to determine: (1) vessel responses and sensitivity to acrolein, allylamine, and H2O2 exposure (1 μM–1 mM), (2) SSAO dependence of allylamine-induced effects using SSAO inhibitors (semicarbazide, 1 mM; MDL 72274-E, active isomer; MDL 72274-Z, inactive isomer; 100 μM), (3) the vasoactive effects of two other SSAO amine substrates, benzylamine and methylamine, and (4) the contribution of extracellular Ca2+ to hypercontraction. Acrolein or allylamine but not H2O2, benzylamine, or methylamine stimulated spontaneous and pharmacologically intractable hypercontraction in CABG blood vessels that was similar to clinical vasospasm. Allylamine-induced hypercontraction and blood vessel SSAO activity were abolished by pretreatment with semicarbazide or MDL 72274-E but not by MDL 72274-Z. Allylamine-induced hypercontraction also was significantly attenuated in Ca2+-free buffer. In isolated aorta of spontaneously hypertensive rat, allylamine-induced an SSAO-dependent contraction and enhanced norepinephrine sensitivity but not in Sprague–Dawley rat aorta. We conclude that acrolein generation in the blood vessel wall increases human susceptibility to vasospasm, an event that is enhanced in hypertension.
Keywords: Aldehydes, Allylamine, Amine oxidase, EC 1.4.3.6, H2O2, Hypertension, Vasospasm
Introduction
Lipid peroxidation and inflammation can generate acrolein, a highly reactive α,β-unsaturated aldehyde. Oxidation of unsaturated fatty acids of lipoproteins and lipids forms alkoxyl and peroxyl radicals that decompose into several metastable carbonyl end products including acrolein (Grosch, 1987; Porter et al., 1995). Several disease states are associated with enhanced lipid peroxidation that lead to the accumulation of acrolein and acrolein–protein and acrolein–DNA adducts. Increased levels of acrolein have been measured in plasma of patients with renal failure (Sakata et al., 2003) and in Alzheimer's disease brain tissues (Lovell et al., 2001). Moreover, increased acrolein–protein adducts have been detected in vascular tissues of patients with diabetes (Daimon et al., 2003; Uesugi et al., 2004), Alzheimer's disease (Calingasan et al., 1999; Lovell et al., 2001), and atherosclerosis (Uchida, 1999; Uchida et al., 1998; Shao et al., 2005). Acrolein is also formed by myeloperoxidase-mediated oxidation of l-threonine, and thus activated neutrophils may contribute to vascular damage at sites of inflammation (Anderson et al., 1997). Other sources of tissue acrolein include glucose autoxidation-mediated oxidation of fatty acids (Medina-Navarro et al., 2004) and the oxidation of polyamines by amine oxidase (Gahl and Pitot, 1982), which is linked to polyamine-induced phase 2 enzymes (Kwak et al., 2003) and cytotoxicity (Sharmin et al., 2001).
Acrolein is also generated during metabolism of drugs and toxins. Metabolism of cyclophosphamide produces acrolein, which is implicated in the toxic action of the drug (i.e., hemorrhagic cystitis) (Kehrer and Biswal, 2000; Ren et al., 1999). Acrolein is formed in metabolism of allylamine (AA; an aliphatic amine used in the dye industry) by semicarba-zide-sensitive amine oxidase (SSAO; EC 1.4.3.6; Boor and Nelson, 1982). Acute exposure of rats to AA in vivo causes SSAO-dependent subendocardial necrosis (Boor et al., 1979; Boor and Hysmith, 1987), and limited in vitro data indicate that this may result from coronary artery vasospasm (Conklin et al., 2001; Conklin and Boor, 1998). Thus, increased acrolein generation in the vascular wall from AA or other sources may well lead to vascular instability, altered vasoreactivity, and vasospasm.
Several diseases are associated with enhanced vascular inflammation, oxidative stress, and increased formation of acrolein and acrolein adducts in the blood vessel wall (Uchida et al., 1998). However, the effects of acrolein generation and accrual on vascular reactivity in human blood vessels are unknown. In a study of isolated rat coronary artery, we showed that direct acrolein exposure induces vasospasm-like effects and we validated the use of AA as an “acrolein generator” by showing that AA evokes SSAO-dependent effects similar to those of acrolein alone (Conklin et al., 2001; Conklin and Boor, 1998). Moreover, we confirmed the presence of a relatively high level of SSAO activity in human coronary arteries and coronary artery bypass graft (CABG) blood vessels that mediate methylamine-induced relaxation in CABG blood vessels (Conklin et al., 2001, 2004). Thus, to simulate increased vascular production of acrolein associated with inflammation and lipid peroxidation and determine if acrolein generation promotes vasospasm in human blood vessels, we tested the direct effects of the acrolein generator AA. Herein we show that acrolein formation initiates hypercontraction via a calcium overload mechanism in human CABG blood vessels.
Methods
Human subjects
Adults (age years, mean±SE, all, 62.8±1.8; males, 59.9± 2.4, ≈77% of total; females, 71.9±1.9; Table 1) undergoing CABG surgery at Luther Hospital/Midelfort Clinic (Eau Claire, WI) were the source of blood vessels. Unused sections of internal mammary artery (IMA), radial artery (RA), and saphenous vein (SV) were placed in lactated Ringers and refrigerated (4 °C) at the hospital. Vessels were retrieved between 4 and 16 h after surgery, cleaned of blood, staples, thread, and extraneous tissue, and placed in fresh physiological saline solution with glucose (PSS; pH 7.4; 4 °C). All isolated vessel experiments were conducted within 24 h of surgical removal. This research adhered to the principles of the Declaration of Helsinki as well as to Title 45, U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects and patient consent was obtained with Luther Hospital/Midelfort Clinic IRB approval (#T-4028).
Table 1. Age, sex, and risk factor characteristics of coronary artery bypass graft (CABG) surgery patients from whom CABG blood vessel segments were obtained for use in this study.
| Total (n) | Female (n) | Male (n) | |
|---|---|---|---|
| Age (years) | 63.0±11.8 (40) | 71.4±10.9 (10) | 60.2± 10.8 (30) |
| Range (years) | 43–86 | 46–86 | 43–84 |
| Sex | 100 (40) | 25 (10) | 75 (30) |
| Risk factor | |||
| Hypertension | 80 (32) | 100 (10) | 73 (22) |
| Familial coronary artery disease | 75 (30) | 80 (8) | 73 (22) |
| Hypercholesterolemia | 75 (30) | 80 (8) | 73 (22) |
| Diabetes | 25 (10) | 30 (3) | 23 (7) |
| Morbid obesity | 15 (6) | 30 (3) | 10 (3) |
| Smoking | 12.5 (5) | 20 (2) | 10 (3) |
| Cerebrovascular accident | 7.5 (3) | 10 (1) | 7 (2) |
| Renal failure | 2.5 (1) | 0 (0) | 3 (1) |
(n)=number of patients. Values=means±SD (Age only) or percentage.
Experimental animals
Hypertension was the most prevalent cardiovascular risk factor in these patients (Table 1). To address the potential of hypertension to enhance AA-induced effects, we compared aortic responses of spontaneously hypertensive rats (SHR) and age-matched Sprague–Dawley (SD) rats to AA exposure (Harlan Sprague–Dawley, Indianapolis, IN). Aortas of SHR are more contractile, have less EDRF capacity, and exhibit enhanced aldehyde content, increased advanced glycation end products, and more superoxide formation than control rat aortas (Wang et al., 2005; Yang et al., 2004; Rodriguez-Martinez et al., 1998) although no differences in aortic SSAO activity are detected (Guffroy and Strolin, 1984). After verifying that 18-week-old male SHR were hypertensive by non-invasive tail cuff methodology (NIBP Model 229; IITC, Woodland Hills, CA; systolic blood pressure, mm Hg: SD, 159±7; SHR, 200±4; n=4, 4; p<0.05) (Conklin and Boor, 1998), thoracic aortas were isolated from CO2-euthanized rats, placed in cold PBS, and treated as indicated below (Conklin et al., 2001). Animal research adhered to Guiding Principles in the Use of Animals in Toxicology and protocols were approved by local IACUC.
Vascular ring physiology
All vascular rings were subjected to an identical, initial 4-step sequence: (1) rings were equilibrated to a loading tension for 30 min (≈1 g for SV and rat aorta or ≈3 g for IMA and RA) (Conklin et al., 2004; Cracowski et al., 1999), (2) rings were stimulated with 100 mM potassium-PBS (HI K+) as a test of viability, (3) rings were washed 3 times with PBS over 30 min and re-equilibrated to resting tension, and (4) rings were precontracted with norepineprine (NE, 1 or 10 μM) and then stimulated with acetylcholine (ACh, 1 μM) to test for the presence of EDRF response (Conklin et al., 2004).
Human CABG blood vessels
After the initial protocol, each human CABG blood vessel ring was assigned to one of the following experimental protocols:
To compare the direct effects of acrolein exposure to that of the acrolein generator, AA, uncontracted or NE-precontracted rings were exposed to cumulative concentrations of AA or acrolein (1, 10, 100, or 1000 μM) followed by a contraction/relaxation cycle (due to limited availability of RA, acrolein curves were performed with IMA and SV).
To show that AA metabolism was necessary for AA-induced effects, IMA rings were pretreated with SSAO inhibitors, MDL 72274-E-isomer (active, 0.1 mM; 20 min), MDL 72274-Z-isomer (inactive, 0.1 mM; 20 min), or semicarbazide (SEMI, 1 mM; 20 min), then precontracted with NE followed by exposure of up to 1 mM of AA then ACh addition (IMA only).
To validate that AA-induced hypercontraction was dependent on acrolein generation, NE-precontracted rings were exposed up to 1 mM of AA, benzylamine, methylamine, or H2O2 before addition of ACh (IMA only).
To test the role of calcium in AA-induced hypercontraction, IMA and SV rings were washed 3 times in Ca2+-free PBS, precontracted with NE (10 μM), and exposed to AA (1 mM; 10 min). Vessels were then washed in normal PBS (3×) and exposed to HI K+, ACh, and SNP.
AA-induced hypercontraction was preceded by robust relaxation, so to test for involvement of nitric oxide synthase (NOS) and/or cyclooxygenase (COX) activity in AA-induced relaxation, 1 of 4 rings from each patient (n=4) was assigned either (1) control (no pretreatment), (2) l-nitroarginine methyl ester (l-NAME, 200 μM/L, 20 min), (3) indomethacin (INDO, 100 μM, 20 min), or (4) l-NAME+INDO pretreatment. Pretreated rings were NE-precontracted, relaxed with ACh, followed immediately by exposure to 1 mM AA (IMA only).
AA-induced hypercontraction was preceded by robust relaxation, so the effect of relaxation on hypercontraction was tested in NE-precontracted IMA treated with a potent vasorelaxant: diltiazem (calcium channel blocker; 100 μM), HA-1077 (rho-kinase inhibitor; 10 μM), papaverine (phospho-diesterase inhibitor, 100 μM), or sodium nitroprusside (NO donor, SNP; 100 μM) followed by exposure to 1 mM AA (IMA only).
Rat aortic experiments
Aortic rings were assigned one of four pretreatments: (1) control, (2) AA at 1, 10, 100 or 1000 μM for 10 min, (3) semicarbazide pretreatment (1 mM; 20 min), or (4) semicarbazide pretreatment plus 1 mM AA. Following pretreatment, rings were exposed to cumulative concentrations of NE (10−9 M–10−5 M) followed by cumulative concentrations of ACh (10−9 M–10−6 M). After treatment and 3 bath changes (30 min), rings were precontracted with 100 mM HI K+ followed by exposure to ACh (1 μM) and then SNP (100 μM). Each experiment lasted 5–6 h.
Vascular reactivity calculations
Blood vessel contractile tension (e.g., residual tension, HI K+-induced tension) was normalized as a percentage of the initial NE-induced contractile tension. Maximum vessel relaxation to agonists (e.g., ACh, SNP, amines, acrolein, H2O2, etc) was calculated as a percentage reduction of the preceding agonist-induced tension. Because relaxation preceded hypercontraction in some vessels but not all, hypercontraction was calculated two ways as a percentage of: (1) the initial NE-induced tension, and (2) the remaining NE-induced tension following relaxation.
Semicarbazide-sensitive amine oxidase (SSAO) assay
Standard assay protocols for measuring SSAO activity radiometrically using 14C-benzylamine·HCl as substrate were followed (59 μCi/mmol; Amersham Inc., Rockford, IL) (Conklin et al., 2004; Lewinsohn et al., 1978). SSAO activity was measured in homogenized human IMA (mean±SD: patient age years, 65±10, n=40), RA (58±9, n=19; mean patient age was significantly less than in IMA and SV patient age), and SV (65±9, n=15) without and with MDL 72274-E or MDL 72274-Z isomers (10−9–10−4 M in log molar steps) at 37 °C. SSAO activity was calculated as the nmol benzylamine substrate metabolized per 30 min per mg protein. SSAO inhibition was calculated as a percentage of the control SSAO activity (i.e., without inhibitor=100%). The concentration producing 50% inhibition of control activity (IC50) was determined by interpolation from individual cumulative concentration response curves.
Chemicals and solutions
Solutions were composed of the following in mM: PBS, NaCl, 130; KCl, 4.7; MgSO4·7H2O, 1.17; KH2PO4, 1.18; NaHCO3, 14.9; CaCl2, 2.0; glucose, 5.0; pH 7.4, HI K+ PBS (as PBS except), NaCl, 34.7; KCl, 100, and Ca2+-free PBS (as PBS except), CaCl2, 0; EGTA, 2.0. The MDL 72274-E and-Z isomers were synthesized as previously described (McDonald et al., 1985; Kim et al., 2006). Additional chemicals were purchased commercially (rho-kinase inhibitor, HA-1077, Calbiochem, Darmstadt, FGR; allylamine·HCl, Pfaltz and Bauer, Inc., Stamford, CT; others from Sigma Chemical Co., St. Louis, MO) and dissolved in dH2O except indomethacin, which was dissolved in 0.1 N NaOH in dH2O.
Statistics
Values are means±standard error of the mean (SE). Statistical comparisons were performed with Student's paired or unpaired t-tests and between more than two groups using one-way ANOVA and post-test comparison using Student–Newman–Keuls test (SigmaStat, SPSS, Inc., Chicago, IL). Statistical significance was set at p<0.05.
Results
Acrolein generator AA induces hypercontraction in human blood vessels
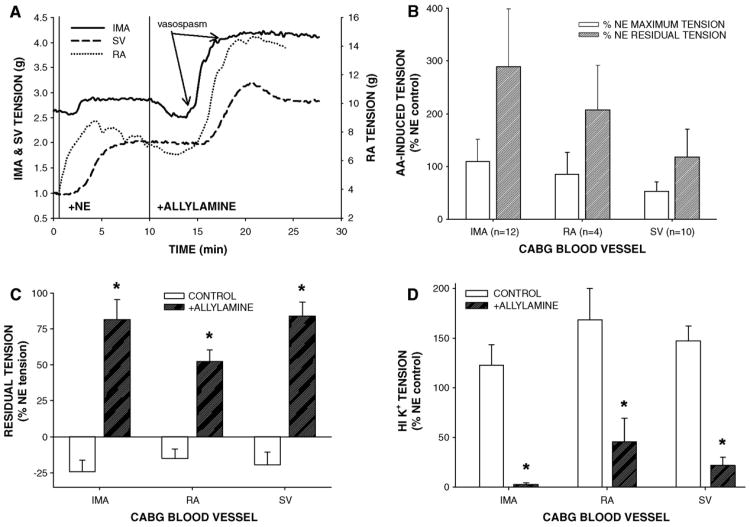
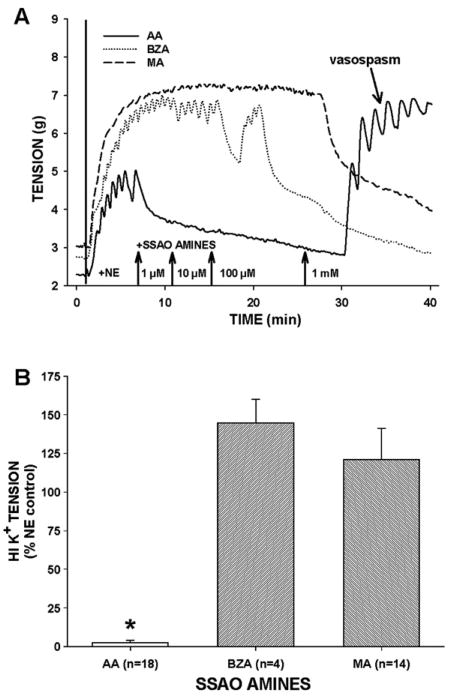
AA pretreatment suppressed NE-induced tension in uncontracted vessels (data not shown), but in NE-precontracted CABG rings, 1 mM AA generally induced: (1) a triphasic response consisting of rapid and small contraction, sustained and robust relaxation, and spontaneous hypercontraction, (2) rapid inhibition of ACh- or SNP-induced relaxation, (3) sustained and elevated residual tension, and (4) a reduction in tension of HI K+-induced contraction (Fig. 1). Typically the onset of 1 mM AA-induced hypercontraction was spontaneous but usually occurred about 5 min after AA addition following relaxation in tension, although an AA-induced relaxation was occasionally absent in SV (Fig. 1A). Since AA-induced robust relaxation in NE-precontracted IMA, AA-induced hypercontraction percentage was more dramatic in IMA compared to RA or SV (Fig. 1B). After 10 min of AA (1 mM) exposure and 3 bath changes, residual tension was significantly greater in AA-treated rings than in control rings indicating intractable contraction (Fig. 1C). Moreover, AA-induced residual tension was unaltered by addition of ACh, in fact, in 2 of 10 IMA, ACh addition provoked hypercontraction in the presence of 1 mM AA (data not shown). The NO donor, SNP, was unable to relax 1 mM AA-induced residual tension. In an additional experiment in IMA, 1 mM AA exposure (2–10 min) reduced SNP-induced relaxation in a temporally dependent manner indicating rapid inhibition of smooth muscle responsiveness to NO (data not shown). Elevated residual tension was inversely predictive of tension production in response to depolarizing 100 mM HI K+ buffer in AA-treated vessels compared to controls (Fig. 1D), indicating an irreversible AA-induced effect. The levels of residual tension and strength of the HI K+-induced contractions after AA treatment also were vessel-specific. Residual tension was greatest in IMA and SV and least in RA while HI K+-induced tension after AA exposure was greatest in RA and least in the IMA whereas the SV produced an intermediate level of tension. Thus, CABG vessel resistance to 1 mM AA-induced effects was inversely proportional to level of residual tension obtained following exposure to AA.
Fig. 1.
Acrolein generator allylamine (AA) stimulated hypercontraction in NE-precontracted isolated human coronary artery bypass graft (CABG) blood vessels. (A) Representative tracings of IMA, RA, and SV responses to AA (1 mM) addition. AA-induced initial relaxation in NE-precontracted IMA and RA but not in SV that was followed by a spontaneous and relatively rapid increase in tension of all three vessels after about 5 min of AA exposure that resulted in sustained hypercontraction (B). Following agonist washouts, significant residual tension remained in AA-exposed vessels compared to controls indicating intractable hypercontraction (C). Additionally, AA-exposed blood vessels contracted significantly less to depolarizing HI K+ stimulus (100 mM) compared to untreated controls indicating irreversible toxicity (D). Abbreviations: IMA, internal mammary artery; RA, radial artery; SV, saphenous vein. Values=means±SE. n=number of vessels. *=significantly different from control.
Validation of allylamine-induced hypercontraction—SSAO-dependence
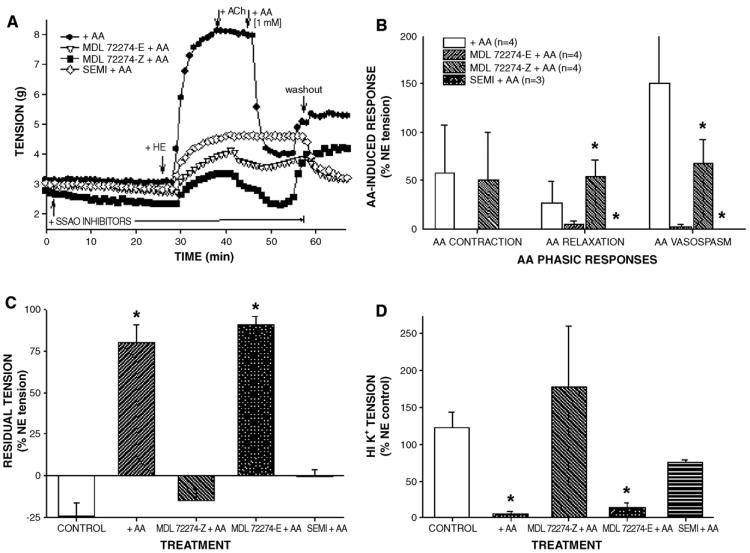
Our previous study showed that SSAO activity was necessary for methylamine-induced relaxation in NE-precontracted CABG vessels (Conklin et al., 2004), therefore, we confirmed that AA-induced hypercontraction was also SSAO-dependent. Pretreatment of IMA with SSAO inhibitors, semicarbazide (1 mM) or MDL 72274-E (active isomer; 0.1 mM) but not with MDL 72274-Z (0.1 mM; inactive isomer; 20 min), effectively prevented AA-induced responses including hypercontraction (Figs. 2A and B). After 3 bath changes, AA and MDL 72274-Z+ AA-treated vessels had significantly elevated residual tension hile semicarbazide+AA or MDL 72274-E+AA-treated vessels developed tension indistinguishable from controls (no AA, no inhibitor; Fig. 2C). Similarly, MDL 72274-Z pretreatment failed to prevent AA-induced attenuation of HI K+-stimulated contraction in IMA (Fig. 2D). Collectively, these data support a direct role of SSAO activity in AA-induced hypercontraction, presumably due to formation of acrolein. Moreover, SSAO inhibitors appeared specific since there was no effect of SSAO inhibitor pretreatment on NE-, ACh-, or HI K+-induced responses in IMA (data not shown) as previously shown for semicarbazide in CABG vessels (Conklin et al., 2004).
Fig. 2.
Semicarbazide-sensitive amine oxidase (SSAO) inhibitors prevented acrolein generator-induced hypercontraction and toxicity in isolated IMA. Representative tracings of IMA responses to allylamine (AA, 1 mM, 10 min) in the presence of active SSAO inhibitors (MDL 72274-E or semicarbazide) and inactive inhibitor MDL 72274-Z (A). Quantification of AA effects in NE-precontracted IMA shows that active SSAO inhibitors blocked AA action (B). SSAO inhibitor pretreatment blocked subsequent AA-induced toxicity as enhanced residual tension (C) and suppression of HI K+-stimulated contraction (D) indicating that AA-induced effects were SSAO-dependent. Values=means±SE. n=number of vessels. *=significant difference from control. Note: In SEMI+AA treatment of B, no hypercontraction was detected. In +AA treatment of B, AA VASOSPASM=151±150%.
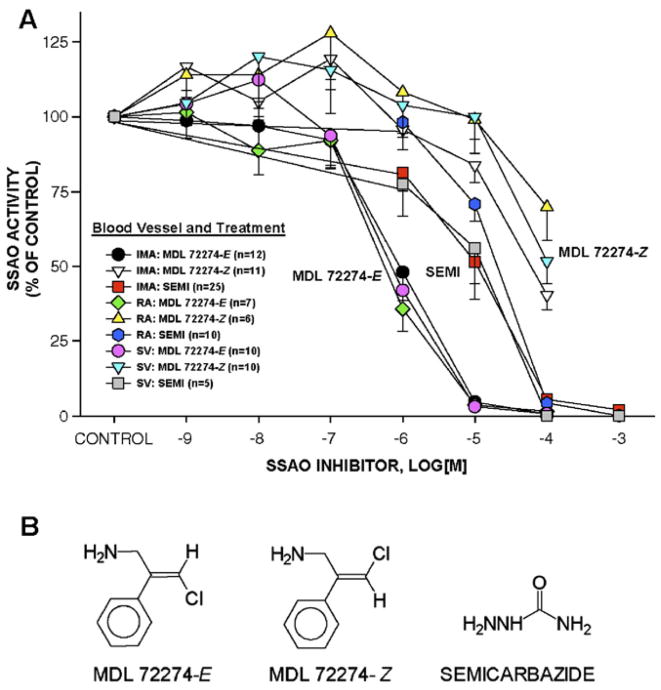
We confirmed that MDL 72274-E and semicarbazide were effective inhibitors of CABG blood vessel SSAO activity while MDL 72274-Z was significantly less effective (Fig. 3A; Table 2). Both MDL 72274-E and semicarbazide inhibited 100% of SSAO activity in CABG vessel homogenates at the concentrations used in these experiments, 0.1 and 1 mM, respectively, confirming that inhibition of AA-induced hyper-contraction was SSAO activity-dependent (compare Figs. 2A and 3A). The MDL 72274-Z compound at 0.1 mM inhibited <50% of SSAO activity in CABG homogenates, its IC50 values were 87–600 times greater than that of MDL 72274-E, and, importantly, MDL 72274-Z did not attenuate AA-induced hypercontraction indicating little inhibition, if any, of SSAO activity in intact IMA rings. The pA2 values of inhibitors were significantly different between compounds across all blood vessels (i.e., MDL 72274-E>semicarbazide>MDL 72274-Z), but no differences were observed between pA2 values for a single compound between blood vessels (Table 2). Moreover, SSAO activity level in all 3 types of CABG blood vessels was similar (Fig. 3B), and there was no correlation between patient age and blood vessel SSAO activity (R2 =0.00002; data not shown).
Fig. 3.
SSAO inhibitors produced concentration-dependent inhibition of SSAO activity in human CABG blood vessel homogenates. Summary inhibition curves for MDL 72274-E, semicarbazide, and MDL 72274-Z revealed relative effectiveness of MDL 72274-E and semicarbazide compared to MDL 72274-Z (A). Structures of SSAO inhibitors used in this study (B). Values=means±SE and are presented as a percentage of the control SSAO activity. n=number of vessels. *=significant difference between concentrations of SSAO inhibitors producing 50% inhibition of SSAO activity (IC50).
Table 2. The semicarbazide-sensitive amine oxidase (SSAO) inhibitor concentration producing 50% inhibition (IC50) of SSAO activity was determined in homogenates of human blood vessels (internal mammary artery, IMA; radial artery, RA; saphenous vein, SV) in the presence of cumulative concentrations of MDL 72274-E (active isomer), MDL 72274-Z (weakly active isomer), and semicarbazide.
| MDL 72274-E | MDL 72274-Z | Semicarbazide | ||
|---|---|---|---|---|
| IMA | IC50 | 1.04±0.76 | 90.5±87.7* | 16.7± 19.5 |
| pA2 | 6.11 ±0.39 (12) | 4.18±0.34 (11) | 5.10±0.60 (25) | |
| RA | IC50 | 1.42±2.04 | 853±936* | 21.2± 11.2 |
| pA2 | 6.12±0.50 (7) | 3.44±0.68 (6) | 4.74±0.25 (10) | |
| SV | IC50 | 0.74 ±0.40 | 135±156* | 14.7± 13.3 |
| pA2 | 6.24±0.39 (10) | 4.06±0.42 (10) | 5.14±0.69 (5) |
Values are means±SD. IC50 in μM was interpolated from individual cumulative response curves. pA2=−log[IC50]. Number of vessels shown in parentheses.
=significant difference between values with asterisks for same vessel (p<0.05).
Acrolein, not H2O2, stimulates hypercontraction
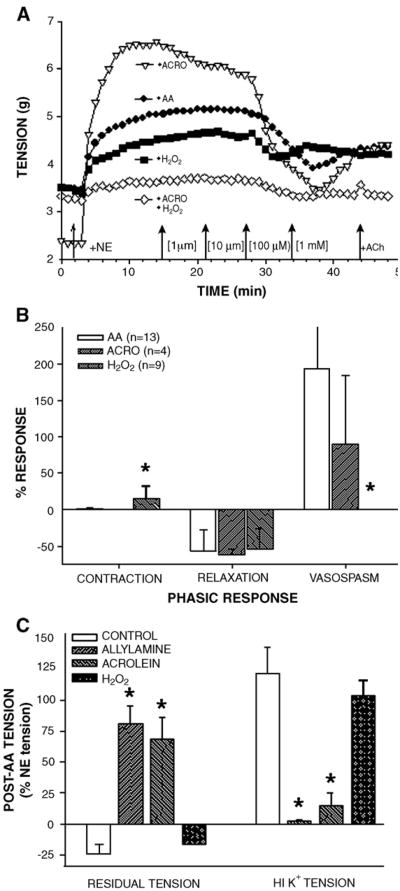
Since 2 structurally different SSAO inhibitors prevented AA-induced hypercontraction, these data indicated that AA metabolism by SSAO activity to equal molar amounts of acrolein, H2O2, and NH3 was responsible for AA action. Therefore, to confirm that acrolein was the metabolite responsible for hypercontraction, NE-precontracted IMA and SV were exposed to cumulative concentrations of AA, acrolein, or H2O2. Both IMA (Fig. 4A) and SV responded in vessel pecific and concentration-dependent manner to AA, acrolein, or H2O2 (data not shown). In IMA, AA at <1 mM induced biphasic responses (a small contraction followed by a robust relaxation) with a typical threshold around 10 μM, an EC50 of 171 μM for contraction, and EC50 of 88 μM for relaxation (Table 3). In SV, AA had about a 10 μM threshold, an EC50 of 97 μM for contraction, and an EC50 of 143 μM for relaxation (Table 3). There was no apparent difference in IMA sensitivity to AA between male and female patients or any correlation between AA sensitivity and patient age (data not shown). However, the biphasic response to AA was vessel specific with SV having the strongest AA-induced contraction and IMA having the greatest AA-induced relaxation (data not shown). Since AA-induced hypercontraction was usually preceded by a robust relaxation in IMA, we examined potential mechanisms of AA-induced relaxation. Although either l-NAME or INDO treatment significantly inhibited ACh-induced relaxation, these inhibitors alone or in combination had no effect on AA-induced relaxation in IMA (Table 4). Moreover, l-NAME or INDO treatments neither inhibited nor enhanced AA-induced hyper-contraction and toxicity in IMA (data not shown; n=4).
Fig. 4.
Acrolein-induced effects in IMA were similar to the effects stimulated by acrolein generator allylamine (AA). Representative tracings of cumulative concentration response curves in NE-precontracted IMA to AA, acrolein, H2O2, or acrolein+H2O2 demonstrated similarity between IMA responses to AA or acrolein alone (A). Quantification of agonist-induced responses in IMA revealed more similarity between AA- and acrolein-induced responses than between AA-and H2O2-induced effects (B). Moreover, acrolein and AA induced similar elevation of residual tension and suppression of HI K+-induced contraction whereas H2O2 exposure was reversible (C). Values=means±SE. n=number of vessels. *=significantly different from other treatments. Note: In AA treatment of B, VASOSPASM=193±76%. In H2O2 treatment of B, no hypercontraction was detected.
Table 3. The effective concentration of allylamine, acrolein, or H2O2 producing 50% response (EC50; contraction or relaxation) in isolated NE-precontracted human CABG blood vessels (internal mammary artery, IMA; saphenous vein, SV) exposed to cumulative concentrations of agonists.
| Allylamine | Acrolein | H2O2 | |
|---|---|---|---|
| IMA contraction | |||
| EC50 | 171 ±60 | ND | 175±58 |
| pD2 | 4.00±0.49 (2) | ND (4) | 4.02±0.33 (5) |
| IMA relaxation | |||
| EC50 | 88±26* | 28±18* | 162±41* |
| pD2 | 4.26±0.12 (13) | 4.62±0.14 (4) | 4.02±0.20 (9) |
| SV contraction | |||
| EC50 | 97±38* | ND | 220±38* |
| pD2 | 4.20±0.18 (5) | ND (2) | 3.75±0.14 (8) |
| SV relaxation | |||
| EC50 | 143±65* | 190±60 | 320±72* |
| pD2 | 4.26±0.38 (5) | 3.74±0.14 (2) | 3.49±0.0 (4) |
Values are means±SD. EC50 in μM was interpolated from individual cumulative response curves. pD2=(−log[EC50]). ND=response not detected. Number of vessels shown in parentheses.
=significant difference between values with asterisks for same vessel (p<0.05).
Table 4. Inhibitors of nitric oxide synthase (l-NAME) and cyclooxygenase (indomethacin, INDO) diminished ACh-induced relaxation but not AA-induced relaxation.
| Initial % | 2nd relaxation | AA relaxation | |
|---|---|---|---|
| Control (n=4) | 50.1 ±17.6 | 158±46.3 | 91.6±5.6 |
| l-NAME (n=4) | 41.7±16.3 | 20.6±20.6* | 77.6±3.8 |
| INDO (n=4) | 40.7±12.2 | 35.1± 14.3* | 76.7±7.8 |
| l-NAME+INDO (n=4) | 68.5 ±18.1 | 57.1± 19.6† | 90.3±3.8 |
NE-precontracted IMA were exposed to ACh (Initial %) and then washed ×3 over 30 min. Before a second NE precontraction, vessels were exposed to nothing (control), l-NAME, INDO, or l-NAME+INDO for 20 min, and then rings were exposed to ACh (2nd relaxation) followed by addition of AA (AA relaxation; 1 mM). Values are means±SE. 2nd relaxation=% retention of Initial ACh relaxation. AA relaxation=maximum relaxation of NE-induced tension.
=significant difference between values and control value (p<0.05);
=trend toward significance (p=0.071).
Acrolein stimulated biphasic responses in NE-precontracted IMA, and the EC50 for relaxation was much lower than the EC50 for contraction and was similar to the EC50 of AA-induced relaxation (Table 3). Acrolein stimulated relaxation without preceding contraction or subsequent hypercontraction in the 2 SV examined (Table 3). H2O2 produced biphasic responses with a prominent relaxation response in IMA and RA and a concentration-dependent and sustained contractile response in SV (data not shown). Thus, the EC50 of relaxation in IMA and SV stimulated by AA or acrolein was significantly lower than for H2O2-induced relaxation, indicating that acrolein acts as the primary AA metabolite inducing relaxation in IMA and SV. However, AA-induced small contractions in IMA and SV appeared more dependent on H2O2, especially in SV. Although some AA-induced effects could be attributed to either acrolein or H2O2, only acrolein at 1 mM stimulated hypercontraction in IMA and had irreversible effects (i.e., elevated residual tension and reduced HI K+ contraction tension) that mimicked AA-induced responses (Figs. 4B and C). Moreover, H2O2 effects were completely reversible and hypercontraction was not observed at any concentration in any vessel (Figs. 4A and B). In addition, there was no evidence of synergy during combined acrolein and H2O2 exposure in IMA since acrolein-induced effects predominated and were not enhanced by H2O2 (Fig. 4A).
Benzylamine or methylamine stimulated relaxation but not hypercontraction
In NE-precontracted IMA, addition of each of the SSAO amine substrates (AA, benzylamine or methylamine) produced concentration-dependent relaxations. IMA vessels were strikingly more sensitive to AA than to methylamine and showed intermediate sensitivity to benzylamine (Fig. 5A; EC50 in μM: AA, 88±26; benzylamine, 122±51; methylamine, 230±30; n=13, 4, 8, respectively). However, neither benzylamine nor methylamine invoked other responses characteristic of AA-induced effects in IMA, i.e., hypercontraction, elevated residual tension, weak HI K+ contractions, and decreased ACh and SNP relaxations. For example, after exposure to 1 mM benzylamine or methylamine, IMA rings had fully preserved HI K+-stimulated contractile responses (Fig. 5B). Moreover, it is well documented that methylamine stimulates a concentration- and SSAO-dependent, reversible, and robust relaxation without hypercontraction in isolated IMA and rat aorta (Conklin et al., 2004). These data further support the role of acrolein in AA-induced hypercontraction.
Fig. 5.
Allylamine (AA) but not two other SSAO amine substrates, benzylamine or methylamine, stimulated hypercontraction in isolated IMA. Representative tracings of IMA responses to increasing concentrations of amine substrates show all three amines induced relaxation in NE-precontracted IMA with different potency, however, only AA-induced spontaneous hypercontraction (A). Similarly, IMA exposed to AA but not to benzylamine or to methylamine had reduced responsiveness to HI K+ indicating that only AA metabolism resulted in intractable hypercontraction (B). Values=means±SE. n=number of vessels. *=significantly different from other treatments.
AA-induced hypercontraction was dependent on extracellular Ca2+
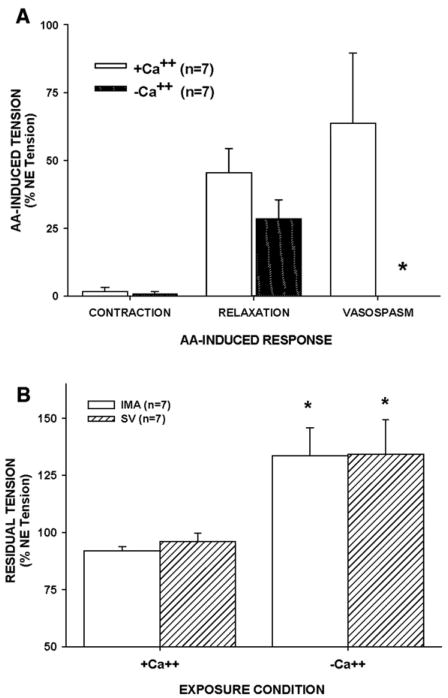
Because AA-induced hypercontraction was spontaneous, rapid, and resulted in a pharmacologically intractable rise in tension, we tested if AA-induced hypercontraction was due to Ca2+ overload by performing experiments in Ca2+-free buffer. NE-induced tension was significantly reduced in IMA (by 70%) and in SV (by 90%) in Ca2+-free buffer. However, AA-induced contraction or relaxation of NE-induced tone in Ca2+-free buffer was not different from responses in Ca2+-containing buffer. Surprisingly though, AA-induced hypercontraction was absent in Ca2+-free buffer (Fig. 6A), indicating hypercontraction was dependent on extracellular calcium in IMA. Moreover, tension increased rapidly in IMA exposed to AA in Ca2+-free buffer upon repletion of Ca+ and was significantly elevated above tension in matched rings exposed to AA in normal Ca2+-containing buffer (Fig. 6B). Subsequent HI K+-induced contractions and SNP relaxations were not different between matched IMA exposed to AA in calcium containing or Ca2+-free buffer (data not shown). These data indicate that AA-induced hypercontraction was dependent on extracellular calcium entry resulting in calcium overload.
Fig. 6.
Extracellular calcium was necessary for AA-induced hypercontraction in NE-precontracted IMA. Calcium-free buffer significantly depressed AA-induced hypercontraction but did not inhibit the preceding AA-induced relaxation in NE-precontracted IMA (A). Restoration of extracellular calcium significantly increased residual tension in AA-exposed IMA and SV to significantly higher levels than achieved in AA-exposed IMA and SV in normal calcium containing buffer, indicating an important role of extracellular calcium influx in sustained and intractable hypercontraction (B). Values=means±SE. n=number of vessels. *=significantly different from other treatments.
Vasorelaxants decreased tension rapidly in NE-precontracted IMA rings from 2 different patients and nearly completely: papaverine (102±2%), diltiazem (72±9%), SNP (106±6%), and HA-1077 (68±30%). Addition of AA caused relaxation in diltiazem- and HA-1077-treated rings and stimulated spontaneous hypercontraction in SNP- and HA-1077-treated rings but not in diltiazem- nor in papaverine-treated rings (data not shown). Similarly, residual tension was greater in SNP- or HA-1077-treated vessels than in diltiazem- or papaverine-treated rings (data not shown). However, subsequent HI K+-induced contraction was depressed similarly in all treatments and was not different from IMA response to AA alone (data not shown). Thus, these data indicate that extracellular calcium influx contributed to AA-induced hypercontraction and the elevated residual tension of post-AA exposure.
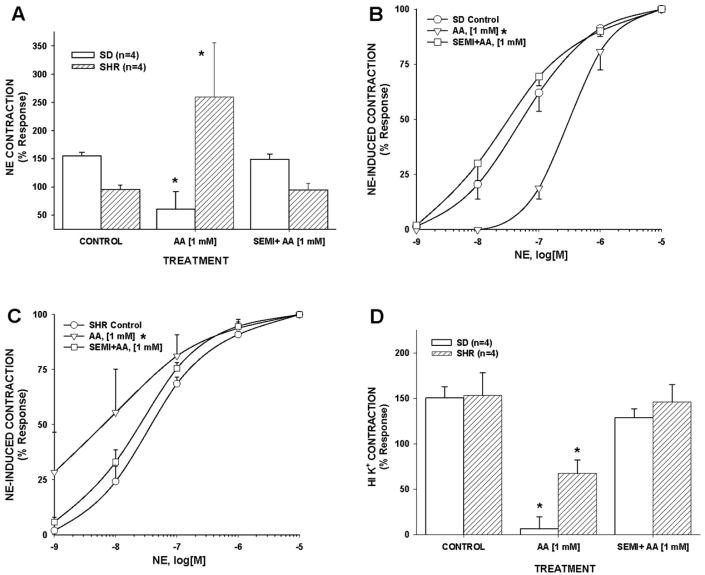
Hypertension enhances acrolein generator- and NE-induced contraction in rat aorta
Since hypertension was the most prevalent cardiovascular risk factor in these CABG patients (80%; Table 1), we tested whether hypertension altered rat aorta responses to acrolein generation (AA) and if these effects were SSAO-dependent. In aortic rings, AA stimulated concentration-dependent responses that were distinctly different in age-matched SHR and SD rats. For example, AA alone (1–1000 μM) failed to elicit contraction in uncontracted SD aorta but AA at 10, 100, and 1000 μM significantly increased tension by 95±3 mg, 158±61 mg, and 350±142 mg, respectively (means±SE; n=4), in uncontracted SHR aorta. Moreover, AA exposure (1 mM) significantly suppressed sensitivity and efficacy of NE-induced contraction in SD aorta while it significantly enhanced sensitivity and efficacy of NE contractile response in SHR aorta (Figs. 7A and B). Thus, the SHR aortic response to AA exposure mimicked the AA-induced hypercontraction observed in isolated human IMA (see Fig. 1), whereas aorta of normotensive SD exhibited only AA-induced toxicity. AA-induced effects in SD and SHR aortas were SSAO-dependent since semicarbazide pretreatment completely prevented AA effects (Figs. 7A, B, and C). These data indicate that hypertension enhances vascular contractility to acrolein (and/or H2O2) and that intravascular generation of acrolein may further exacerbate the risk of vasospasm in hypertensive humans.
Fig. 7.
Allylamine (AA) stimulated hypercontraction-like responses in SHR aorta but not in SD aorta in an SSAO-dependent manner. AA exposure either suppressed or enhanced NE-induced contractions in SD or SHR aorta, respectively, in an SSAO-dependent manner (A). Similarly, AA exposure decreased or increased aortic sensitivity to NE in SD or SHR, respectively, in an SSAO-dependent manner (B and C). Following AA exposure, HI K+-induced contractions were significantly more suppressed in SD aorta compared to SHR aorta and this effect also was SSAO-dependent (D). Values=means±SE. n=number of vessels. *=significantly different from other treatments.
Discussion
For many years, it has been proposed that reactive aldehydes as products of lipid peroxidation and inflammation contribute to progressive and deleterious changes in the hypertensive and atherosclerotic blood vessel wall (Shao et al., 2005; Uchida, 1999; Uchida et al., 1998; Anderson et al., 1997). In this study, we support this hypothesis in two specific ways: (1) real-time generation of acrolein in the blood vessel wall stimulates hypercontraction and (2) hypertension increases susceptibility to acrolein-induced hypercontraction. Moreover, our data are consistent with the proposed role of the acrolein generator, allylamine, as a toxin that stimulates subendocardial necrosis by initiating acute coronary artery vasospasm (Conklin et al., 2001; Boor et al., 1979).
Acrolein generator-induced hypercontraction is SSAO-dependent
We used the acrolein generator AA to simulate enhanced local generation of acrolein in the vascular wall as occurs in inflammation and lipid peroxidation (Anderson et al., 1997; Calingasan et al., 1999; Shao et al., 2005). For example, myeloperoxidase activity generates acrolein from 200 μM l-threonine at a rate of 0.1 nmol/min/106 activated neutrophils at sites of inflammation (Anderson et al., 1997). Thus, 106 activated neutrophils would produce 67 nM acrolein in 10 min in a 15 ml volume (i.e., bath volume in present study). Similarly, SSAO activity in CABG blood vessel homogenates (i.e., 1.5 nmol/30 min/mg protein) generates 1 nmol acrolein from 1 mM AA in 10 min resulting in an acrolein concentration of 67 nM in 15 ml—a level equal to that of activated neutrophils. However, using the Vmax reported by Yu (1990) for rat aortic SSAO activity using AA as substrate (i.e., 1.44 nmol/min/mg protein), we calculate an acrolein concentration of 1.92 μM—a level nearly 30 times greater than that generated by 106 activated neutrophils and nearer our observed threshold of AA effects.
We validated our acrolein generator model by demonstrating a necessary role of SSAO activity, which is a hallmark of AA cardiovascular toxicity in vivo, in isolated blood vessels, and in cultured cells (Conklin et al., 2001; Awasthi and Boor, 1994; Boor and Nelson, 1980; Ramos et al., 1988). For example, isolated IMA and SV pretreated with either of 2 structurally different SSAO inhibitors, semicarbazide or MDL 72274-E, abolishes AA-induced effects, including hypercontraction. Semicarbazide or MDL 72274-E also inhibits 100% of SSAO activity in homogenized vessels while the inactive isomer MDL 72274-Z is ineffective in preventing AA-induced hypercontraction and at the concentrations used in ex vivo vessel rings blocks <50% of SSAO activity. Moreover, the SSAO inhibitors appear specific as they did not alter physiological vascular responses to agonists, i.e., NE- or HI K+-induced contractions or ACh-stimulated relaxations, as shown previously (Conklin et al., 2001, 2004). Thus, these data are consistent with an SSAO-dependent mechanism.
Although we observed no differences between SSAO activity or the effectiveness of SSAO inhibitors in the different CABG blood vessels, each blood vessel had a distinctive resistance to allylamine toxicity (i.e., RA > SV > IMA). While blood vessel ring size may account for this effect, these data imply that blood vessels possess varying levels of protection against aldehyde exposure and, thus, the role of aldehyde metabolism in different blood vessels and vascular tunica deserves greater scrutiny (Srivastava et al., 2001; Wang et al., 2005). Additionally, these findings support the use of an acrolein generator as an alternative to direct acrolein exposure, where acrolein addition easily exceeds detoxification system capacity. For example, estimated maximal acrolein generation from AA at 28.8 nmol/10 min/ring exceeds by 5 to 6 times the estimated total GSH content of blood vessel ring of 5 nmol, while addition of acrolein at 1000 μM in a 15 ml bath equals 15,000 nmol acrolein, an excess of >3000 times the molar equivalents of GSH in blood vessel ring. Thus, the interplay between acrolein generation, GSH depletion, and acrolein metabolism likely is an important determinant of the onset, amplitude, and reversibility of vasospasm in human blood vessels.
Acrolein not H2O2 mediates AA-induced hypercontraction in human CABG blood vessels
We found that only acrolein induced hypercontraction similar to AA in IMA, RA, and SV. Additional evidence supports the role of acrolein as the sole mediator of AA-induced hypercon-traction. Acrolein exposure produced intractable residual tension and suppressed HI K+ contractility similar to that of AA, while the effects of 1 mM H2O2 are reversible (Conklin et al., 2004; Rodriguez-Martinez et al., 1998). Two other SSAO amine substrates, benzylamine and methylamine, did not stimulate hypercontraction but produce vasorelaxation and equal molar amounts of aldehyde (i.e., benzaldehyde or formaldehyde, respectively) and H2O2 when metabolized by SSAO activity. Although rates of metabolism vary by amine substrate, longer duration exposure with 1 mM methylamine (up to 40 min) did not produce hypercontraction in human IMA (Conklin et al., 2004). Additionally, our previous work in isolated rat coronary artery and rat aorta shows that acrolein induces vasospasm-like effects similar to that of AA, further indicating mechanistic overlap of acrolein and acrolein generator AA (Conklin and Boor, 1998; Conklin et al., 2001). Although H2O2 likely contributes to AA-induced relaxation in CABG blood vessels (vide infra), it neither induces hypercontraction alone nor does it appear to synergize with acrolein during combined exposure. These data indicate that AA-induced hypercontraction is an acrolein-dependent phenomenon.
Acrolein generator induces calcium overload—a working hypothesis
Several mechanisms are possible for AA- and acrolein-induced hypercontraction. For example, each may induce hypercontraction by: (1) Ca2+ overload (Hyvelin et al., 2000), (2) fixation of myofibrillar proteins (acrolein is an excellent fixative) (Murata et al., 1985), (3) rapid depletion of ATP levels and ATP production (Biagini et al., 1990), and (4) rapid depletion of reduced GSH level (Awasthi and Boor, 1994) (vide supra). Although these mechanisms are not mutually exclusive, our data implicate calcium overload in AA-induced (or acrolein-induced) hypercontraction. Calcium overload is an important mechanism of ischemic cardiomyocyte injury but has received less attention with regard to vasospasm (Orrenius et al., 1996). In our experiments, extracellular calcium contributes to AA-induced hypercontraction in IMA and SV. Calcium overload is indicated because AA-induced hypercontraction (but not AA-induced relaxation) is prevented in Ca2+-free buffer and by diltiazem pretreatment. Calcium channel blocker treatment is widely used to effectively lower and control arterial blood pressure in hypertensive patients, and verapamil is combined with nitroglycerin to prevent peri-operative vasospasm in CABG blood vessels (He, 1998). In our study, calcium repletion rapidly increased tension in AA-exposed blood vessels and thus extracellular calcium entry appears necessary for both hyper-contraction and sustained tension in IMA. Similarly, acrolein exposure stimulates airway smooth muscle hypercontractility and calcium entry ex vivo (Ben Jebria et al., 1995; Hyvelin et al., 2000). Collectively, these studies implicate a role for calcium overload in acrolein-induced hypercontraction.
Clinical significance of acrolein exposure and vasospasm
Vasospasm is a spontaneous and sustained blood vessel hyperconstriction that occurs in both physiological (e.g., hemorrhage) and pathophysiological settings (e.g., exertional angina, coronary spastic angina/variant angina, stroke, etc). Moreover, coronary artery vasospasm occurs in humans with (≈85%) and without (≈15%) underlying coronary artery disease (Ganz et al., 1991; Nakayama et al., 2000). In our study, all patients obviously had CAD but the CABG blood vessel segments used were free of overt atherosclerotic lesions and were susceptible to vasospasm. This agrees with clinical observations of peri- and post-operative vasospasm in CABG blood vessels (He, 2001; Sarabu et al., 1987). However, due to the relatively high rate of vasospasm in RA (≈5–10% spasm rate), the IMA (thoracic artery) and SVare used clinically as the CABG vessels of first and second choice, respectively (He, 2001). In our model, these CABG blood vessels are equally susceptible to acrolein-induced hypercontraction (see Fig. 1B). Moreover, susceptibility to hypercontraction appears independent of endothelial function since IMA vessels typically had the best preserved EDRF response and the strongest relative hypercontraction. Thus, other factors contribute to hypercon-traction susceptibility in our model.
Our findings suggest that hypertension increases susceptibility to acrolein-induced hypercontraction. Clearly the acrolein generator AA enhances NE sensitivity and hypercontractility in aorta of SHR rats – 2 characteristics favoring vasospasm – yet diminishes NE sensitivity and contractility in aorta of normotensive, age-matched SD rats although Wistar–Kyoto is the genetic background strain for SHR. Increased formation of endogenous aldehydes could contribute to the SHR phenotype (Wang et al., 2005) and subchronic exposure of rats to acrolein induces hypertension (Yousefipour et al., 2005). Moreover, hypertension is the most prevalent risk factor in the CABG patients in this study (100% in females and 80% overall), and AA-induced hypercontraction occurred in nearly all the IMA studied, indicating hypertension could enhance vascular response to acrolein (and/or H2O2) (Karasu, 1999). Intriguingly, hypertension in humans and SHR is associated with elevated myocyte intracellular Ca2+(Gasser, 1988), i.e., the mechanism proposed for AA-induced hypercontractility (Conklin and Boor, 1998).
There is considerable epidemiological evidence linking environmental exposure to aldehydes, specifically acrolein, and an enhanced risk of vasospasm via adrenergic stimulation and/or endothelial dysfunction. From measurements of acrolein present in foods, drinks, and air pollution (e.g., environmental tobacco smoke, vehicle, and industrial emissions), we estimate that human exposure is 5 mg/day. This value could increase dramatically with occupational exposures (e.g., fire fighters, bus drivers, bartenders, etc.). For a review of bioavailable sources of acrolein, see Bhatnagar (2004). Importantly, acrolein is considered a co-pollutant of particulate matter, and particulate matter exposure is associated with increased cardiovascular morbidity and mortality of which some may be vasospasm-related (Peters, 2005). For example, cigarette smokers are at increased risk of coronary artery vasospasm (Quillen et al., 1993; Winniford et al., 1986) and a T−786 →C eNOS mutation increases risk of coronary artery vasospasm among Japanese men, especially smokers (Nakayama et al., 2003). Moreover, there is an increased rate of mortality in CABG patients who continue to smoke after surgery (Ashraf et al., 2004). Although nicotine-induced catecholamine release likely contributes to coronary vasospasm in smokers (Winniford et al., 1986), passive smoking also induces endothelial dysfunction, and thus aldehydes in cigarette smoke could contribute to vasospasm (Hausberg et al., 1997; Celermajer et al., 1996). Our model suggests that both exogenous and endogenous sources of acrolein could contribute to the risk of vasospasm.
Acrolein generator-induced relaxation: a unique mechanism
In our current study, we find that acrolein and the acrolein generator AA produce sensitive and robust relaxation that precedes hypercontraction in CABG blood vessels. The AA-induced relaxation in IMA is independent of NO and prostaglandin formation as it was unaffected by l-NAME and indomethacin pretreatments. Similarly, we found that MA-induced relaxation is also NO-, prostaglandin-, and likely endothelium-independent in human IMA (Conklin et al., 2004). Endothelium independence is plausible with these amines – AA, benzylamine, and methylamine – since the majority of SSAO activity is localized in smooth muscle cells and not endothelial cells (Hysmith and Boor, 1988). However, we recently observed acrolein-induced vasodilation in perfused rat mesenteric bed that was endothelium- and EDHF-dependent (Awe et al., in press), so a role of the endothelium is not ruled out. Moreover, H2O2 is considered an EDHF in human arteries (Hamilton et al., 2001; Matoba et al., 2002) and H2O2 production likely contributes to AA-induced relaxation. Thus, oxidative deamination of certain amines in human blood vessels could result in efficacious relaxation although the specific mechanism(s) of amine-induced relaxation remains to be determined.
Acknowledgments
This work was supported by NIH Grant HL65416 (PJB), NIEHS AREA Grant 1R15 ES011141-01 (DJC), NIEHS 1P01 ES11860 (AB) NIEHS ES12062 (AB), NIH Grant GM48812 (LMS), and the University of Wisconsin-Eau Claire (UWEC) Office of Research and Sponsored Programs Student/Faculty Collaboration Grants, Summer Research Experience, University Research Creative Activity award (DJC), and the Ronald McNair Scholars Program at UWEC. We thank L. Eveland, M. Garney, D. Kranig, M. Phillips, E. Sackett, D. Schilling, and N. Xiong (UWEC) and S. Khan (UTMB) for technical assistance. We also thank staffs of the Departments of Cardiothoracic Surgery and Pathology at Luther Hospital/Midelfort Clinic (Eau Claire, WI).
References
- Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf MN, Mortasawi A, Grayson AD, Oo AY. Effect of smoking status on mortality and morbidity following coronary artery bypass surgery. Thorac Cardiovasc Surg. 2004;52:268–273. doi: 10.1055/s-2004-821103. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Boor PJ. Lipid peroxidation and oxidative stress during acute allylamine-induced cardiovascular toxicity. J Vasc Res. 1994;31:33–41. doi: 10.1159/000159029. [DOI] [PubMed] [Google Scholar]
- Awe SO, et al. Acrolein induces vasodilatation of rodent mesenteric bed via an EDHF-dependent mechanism. Toxicol Appl Pharmacol. doi: 10.1016/j.taap.2006.08.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Jebria A, Crozet Y, Eskew ML, Rudeen BL, Ultman JS. Acrolein-induced smooth muscle hyperresponsiveness and eicosanoid release in excised ferret tracheae. Toxicol Appl Pharmacol. 1995;135:35–44. doi: 10.1006/taap.1995.1206. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A. Cardiovascular pathophysiology of environmental pollutants. Am J Physiol: Heart Circ Physiol. 2004;286:H479–485. doi: 10.1152/ajpheart.00817.2003. [DOI] [PubMed] [Google Scholar]
- Biagini RE, Toraason MA, Lynch DW, Winston GW. Inhibition of rat heart mitochondrial electron transport in vitro: implications for the cardiotoxic action of allylamine or its primary metabolite, acrolein. Toxicology. 1990;62:95–106. doi: 10.1016/0300-483x(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Boor PJ, Hysmith RM. Allylamine cardiovascular toxicity. Toxicology. 1987;44:129–145. doi: 10.1016/0300-483x(87)90144-2. [DOI] [PubMed] [Google Scholar]
- Boor PJ, Moslen MT, Reynolds ES. Allylamine cardiotoxicity: I. Sequence of pathologic events. Toxicol Appl Pharmacol. 1979;50:581–592. doi: 10.1016/0041-008x(79)90413-7. [DOI] [PubMed] [Google Scholar]
- Boor PJ, Nelson TJ. Allylamine cardiotoxicity: III. Protection by semicarbazide and in vivo derangements of monoamine oxidase. Toxicology. 1980;18:87–102. doi: 10.1016/0300-483x(80)90072-4. [DOI] [PubMed] [Google Scholar]
- Boor PJ, Nelson TJ. Biotransformation of the cardiovascular toxin, allylamine, by rat and human cardiovascular tissue. J Mol Cell Cardiol. 1982;14:679–682. doi: 10.1016/0022-2828(82)90165-1. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer's disease. J Neurochem. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- Conklin DJ, Boor PJ. Allylamine cardiovascular toxicity: evidence for aberrant vasoreactivity in rats. Toxicol Appl Pharmacol. 1998;148:245–251. doi: 10.1006/taap.1997.8331. [DOI] [PubMed] [Google Scholar]
- Conklin DJ, Boyce CL, Trent MB, Boor PJ. Amine metabolism: a novel path to coronary artery vasospasm. Toxicol Appl Pharmacol. 2001;175:149–159. doi: 10.1006/taap.2001.9238. [DOI] [PubMed] [Google Scholar]
- Conklin DJ, Cowley HR, Wiechmann RJ, Johnson GH, Trent MB, Boor PJ. Vasoactive effects of methylamine in isolated human blood vessels: role of semicarbazide-sensitive amine oxidase, formaldehyde, and hydrogen peroxide. Am J Physiol: Heart Circ Physiol. 2004;286:H667–676. doi: 10.1152/ajpheart.00690.2003. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Stanke-Labesque F, Sessa C, Hunt M, Chavanon O, Devillier P, Bessard G. Functional comparison of the human isolated femoral artery, internal mammary artery, gastroepiploic artery, and saphenous vein. Can J Physiol Pharmacol. 1999;77:770–776. [PubMed] [Google Scholar]
- Daimon M, Sugiyama K, Kameda W, Saitoh T, Oizumi T, Hirata A, Yamaguchi H, Ohnuma H, Igarashi M, Kato T. Increased urinary levels of pentosidine, pyrraline and acrolein adduct in type 2 diabetes. Endocr J. 2003;50:61–67. doi: 10.1507/endocrj.50.61. [DOI] [PubMed] [Google Scholar]
- Gahl WA, Pitot HC. Polyamine degradation in foetal and adult bovine serum. Biochem J. 1982;202:603–611. doi: 10.1042/bj2020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz P, Weidinger FF, Yeung AC, Vekshtein VI, Vita JA, Ryan TJ, Jr, McLenachan JM, Selwyn AP. Coronary vasospasm in humans: the role of atherosclerosis and of impaired endothelial vasodilator function. Basic Res Cardiol. 1991;86(Suppl. 2):215–222. doi: 10.1007/978-3-642-72461-9_21. [DOI] [PubMed] [Google Scholar]
- Gasser RN. The interdependence of hypertension, calcium overload, and coronary spasm in the development of myocardial infarction. Angiology. 1988;39:761–772. doi: 10.1177/000331978803900809. [DOI] [PubMed] [Google Scholar]
- Grosch W. Reactions of hydroperoxides—Products of low molecular weights. In: Chan HWS, editor. Autoxidation of Unsaturated Lipids. Academic Press; London: 1987. pp. 95–139. [Google Scholar]
- Guffroy C, Strolin BM. Monoamine oxidase and semicarbazide-sensitive amine oxidase in spontaneously hypertensive and in normotensive control rats. Life Sci. 1984;34:535–545. doi: 10.1016/0024-3205(84)90486-7. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, McPhaden AR, Berg G, Pathi V, Dominiczak AF. Is hydrogen peroxide an EDHF in human radial arteries? Am J Physiol: Heart Circ Physiol. 2001;280:H2451–2455. doi: 10.1152/ajpheart.2001.280.6.H2451. [DOI] [PubMed] [Google Scholar]
- Hausberg M, Mark AL, Winniford MD, Brown RE, Somers VK. Sympathetic and vascular effects of short-term passive smoke exposure in healthy nonsmokers. Circulation. 1997;96:282–287. [PubMed] [Google Scholar]
- He GW. Verapamil plus nitroglycerin solution maximally preserves endothelial function of the radial artery: comparison with papaverine solution. J Thorac Cardiovasc Surg. 1998;115:1321–1327. doi: 10.1016/S0022-5223(98)70215-6. [DOI] [PubMed] [Google Scholar]
- He GW. Arterial grafts for coronary surgery: vasospasm and patency rate. J Thorac Cardiovasc Surg. 2001;121:431–433. doi: 10.1067/mtc.2001.113593. [DOI] [PubMed] [Google Scholar]
- Hysmith RM, Boor PJ. Purification of benzylamine oxidase from cultured porcine aortic smooth muscle cells. Biochem Cell Biol. 1988;66:821–829. doi: 10.1139/o88-094. [DOI] [PubMed] [Google Scholar]
- Hyvelin JM, Roux E, Prevost MC, Savineau JP, Marthan R. Cellular mechanisms of acrolein-induced alteration in calcium signaling in airway smooth muscle. Toxicol Appl Pharmacol. 2000;164:176–183. doi: 10.1006/taap.1999.8879. [DOI] [PubMed] [Google Scholar]
- Karasu C. Increased activity of H2O2 in aorta isolated from chronically streptozotocin-diabetic rats: effects of antioxidant enzymes and enzymes inhibitors. Free Radical Biol Med. 1999;27:16–27. doi: 10.1016/s0891-5849(99)00028-3. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- Kim J, Zhang Y, Ran C, Sayre LM. Inactivation of bovine plasma amine oxidase by haloallylamines. Bioorg Med Chem. 2006;14:1444–1453. doi: 10.1016/j.bmc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kwak MK, Kensler TW, Casero RA., Jr Induction of phase 2 enzymes by serum oxidized polyamines through activation of Nrf2: effect of the polyamine metabolite acrolein. Biochem Biophys Res Commun. 2003;305:662–670. doi: 10.1016/s0006-291x(03)00834-9. [DOI] [PubMed] [Google Scholar]
- Lewinsohn R, Bohm K, Glover V, Sandler M. A benzylamine oxidase distinct from monoamine oxidase B-widespread distribution in man and rat. Biochem Pharmacol. 1978;27:1857–1863. doi: 10.1016/0006-2952(78)90033-3. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun. 2002;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- McDonald IA, Lacoste JM, Bey P, Palfreyman MG, Zreika M. Enzyme-activated irreversible inhibitors of monoamine oxidase: phenylal-lylamine structure–activity relationships. J Med Chem. 1985;28:186–193. doi: 10.1021/jm00380a007. [DOI] [PubMed] [Google Scholar]
- Medina-Navarro R, Duran-Reyes G, Diaz-Flores M, Hicks JJ, Kumate J. Glucose-stimulated acrolein production from unsaturated fatty acids. Hum Exp Toxicol. 2004;23:101–105. doi: 10.1191/0960327104ht416oa. [DOI] [PubMed] [Google Scholar]
- Murata F, Suzuki S, Tsuyama S, Suganuma T, Imada M, Furihata C. Application of rapid freezing followed by freeze-substitution acrolein fixation for cytochemical studies of the rat stomach. Histochem J. 1985;17:967–980. doi: 10.1007/BF01417946. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Yasue H, Yoshimura M, Shimasaki Y, Ogawa H, Kugiyama K, Mizuno Y, Harada E, Nakamura S, Ito T, Saito Y, Miyamoto Y, Ogawa Y, Nakao K. T(−786)→C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with myocardial infarction, especially without coronary organic stenosis. Am J Cardiol. 2000;86:628–634. doi: 10.1016/s0002-9149(00)01041-9. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Yoshimura M, Sakamoto T, Shimasaki Y, Nakamura S, Ito T, Abe K, Yamamuro M, Miyamoto Y, Saito Y, Nakao K, Yasue H, Ogawa H. Synergistic interaction of T-786→C polymorphism in the endothelial nitric oxide synthase gene and smoking for an enhanced risk for coronary spasm. Pharmacogenetics. 2003;13:683–688. doi: 10.1097/00008571-200311000-00004. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Ankarcrona M, Nicotera P. Mechanisms of calcium-related cell death. Adv Neurol. 1996;71:137–149. [PubMed] [Google Scholar]
- Peters A. Particulate matter and heart disease: evidence from epidemiological studies. Toxicol Appl Pharmacol. 2005;207:477–482. doi: 10.1016/j.taap.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- Quillen JE, Rossen JD, Oskarsson HJ, Minor RL, Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22:642–647. doi: 10.1016/0735-1097(93)90170-6. [DOI] [PubMed] [Google Scholar]
- Ramos K, Grossman SL, Cox LR. Allylamine-induced vascular toxicity in vitro: prevention by semicarbazide-sensitive amine oxidase inhibitors. Toxicol Appl Pharmacol. 1988;95:61–71. doi: 10.1016/s0041-008x(88)80008-5. [DOI] [PubMed] [Google Scholar]
- Ren S, Kalhorn TF, Slattery JT. Inhibition of human aldehyde dehydrogenase 1 by the 4-hydroxycyclophosphamide degradation product acrolein. Drug Metab Dispos. 1999;27:133–137. [PubMed] [Google Scholar]
- Rodriguez-Martinez MA, Garcia-Cohen EC, Baena AB, Gonzalez R, Salaices M, Marin J. Contractile responses elicited by hydrogen peroxide in aorta from normotensive and hypertensive rats. Endothelial modulation and mechanism involved. Br J Pharmacol. 1998;125:1329–1335. doi: 10.1038/sj.bjp.0702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata K, Kashiwagi K, Sharmin S, Ueda S, Irie Y, Murotani N, Igarashi K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem Biophys Res Commun. 2003;305:143–149. doi: 10.1016/s0006-291x(03)00716-2. [DOI] [PubMed] [Google Scholar]
- Sarabu MR, McClung JA, Fass A, Reed GE. Early postoperative spasm in left internal mammary artery bypass grafts. Ann Thorac Surg. 1987;44:199–200. doi: 10.1016/s0003-4975(10)62041-3. [DOI] [PubMed] [Google Scholar]
- Shao B, O'brien KD, McDonald TO, Fu X, Oram JF, Uchida K, Heinecke JW. Acrolein modifies apolipoprotein A-I in the human artery wall. Ann N Y Acad Sci. 2005;1043:396–403. doi: 10.1196/annals.1333.046. [DOI] [PubMed] [Google Scholar]
- Sharmin S, Sakata K, Kashiwagi K, Ueda S, Iwasaki S, Shirahata A, Igarashi K. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem Biophys Res Commun. 2001;282:228–235. doi: 10.1006/bbrc.2001.4569. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Liu SQ, Conklin DJ, Zacarias A, Srivastava SK, Bhatnagar A. Involvement of aldose reductase in the metabolism of atherogenic aldehydes. Chem-Biol Interact. 2001:130–132. 563–571. doi: 10.1016/s0009-2797(00)00299-4. [DOI] [PubMed] [Google Scholar]
- Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci U S A. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi N, Sakata N, Nangaku M, Abe M, Horiuchi S, Hisano S, Iwasaki H. Possible mechanism for medial smooth muscle cell injury in diabetic nephropathy: glycoxidation-mediated local complement activation. Am J Kidney Dis. 2004;44:224–238. doi: 10.1053/j.ajkd.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Wang X, Desai K, Chang T, Wu L. Vascular methylglyoxal metabolism and the development of hypertension. J Hypertens. 2005;23:1565–1573. doi: 10.1097/01.hjh.0000173778.85233.1b. [DOI] [PubMed] [Google Scholar]
- Winniford MD, Wheelan KR, Kremers MS, Ugolini V, van den Berg E, Jr, Niggemann EH, Jansen DE, Hillis LD. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73:662–667. doi: 10.1161/01.cir.73.4.662. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Endothelium-dependent contractions to acetylcholine, ATP and the calcium ionophore A 23187 in aortas from spontaneously hypertensive and normotensive rats. Fundam Clin Pharmacol. 2004;18:321–326. doi: 10.1111/j.1472-8206.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Yousefipour Z, Ranganna K, Newaz MA, Milton SG. Mechanism of acrolein-induced vascular toxicity. J Physiol Pharmacol. 2005;56:337–353. [PubMed] [Google Scholar]
- Yu P. Oxidative deamination of aliphatic amines by rat aorta semicarbazide-sensitive amine oxidase. J Pharm Pharmacol. 1990;42:882–884. doi: 10.1111/j.2042-7158.1990.tb07048.x. [DOI] [PubMed] [Google Scholar]