Abstract
von Hippel-Lindau disease (VHL) is an autosomal dominant neoplasia syndrome that is the result of a germline mutation of the VHL tumor suppressor gene on the short arm of chromosome 3. VHL patients are predisposed to develop lesions of the central nervous system (CNS) and viscera. CNS lesions include hemangioblastomas (the most common tumor in VHL) and endolymphatic sac tumors (ELSTs). Visceral manifestations include renal carcinomas and cysts, pancreatic neuroendocrine tumors and cysts, pheochromocytomas and cystadenomas of the reproductive adnexal organs. Despite their benign pathology, hemangioblastomas and ELSTs are a frequent cause of morbidity and mortality in VHL patients. Recent molecular biologic investigations into these VHL-associated CNS lesions provide new insight into their origin and development. Emerging data from serial imaging and clinical surveillance protocols provide insight into the natural history of these lesions. Because of the dissimilar pathobiology and clinical course between hemangioblastomas and ELSTs, the optimal management strategies for these neurologic manifestations of VHL are very different.
Case Presentation
This 39-year-old female was first evaluated at the National Institutes of Health (NIH) in August 2003. Three years before assessment she experienced an acute episode of vertigo and left tinnitus. Magnetic resonance (MR)-imaging of the brain and temporal bones at that time was unremarkable and an audiogram revealed normal hearing (Figure 1). Her symptoms were attributed to Meniere’s disease and she was placed on a low sodium diet without relief. One-year later the patient experienced an episode of sudden left hearing loss that coincided with an acute exacerbation of vertigo and left tinnitus. An audiogram confirmed a mild to severe left sensorineural hearing loss. MR-imaging demonstrated hemorrhage in the left labyrinth but no evidence of endolymphatic sac tumor (ELST) (Figure 1). The patient was continued on a low sodium diet with no relief in symptoms and her hearing loss remained unchanged.
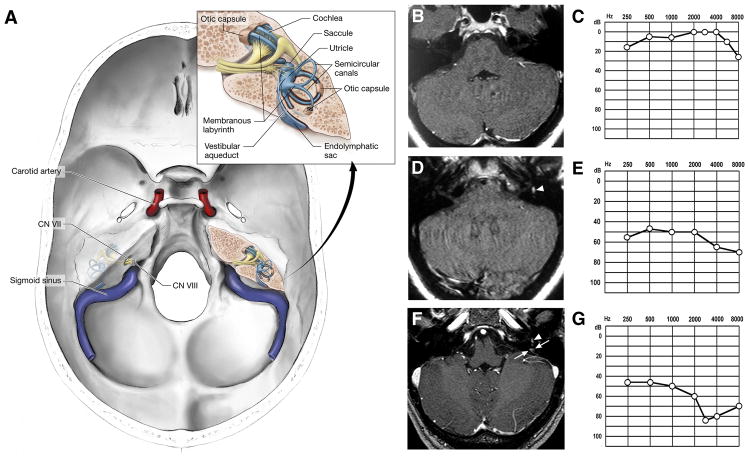
Figure 1.
(A) Illustration of the base of skull demonstrating the relationship between the carotid artery, sigmoid sinus, seventh (CN VII) and eighth (CN VIII) cranial nerves, and the inner ear. Inset, detailed view of the inner ear showing the bony otic capsule (filled with perilymph) that houses the membranous labyrinth (filled with endolymph). The endolymphatic duct lies within the bony vestibular aqueduct and connects the endolymphatic sac to the remainder of the membranous labyrinth. Serial temporal bone magnetic resonance (MR)-imaging and audiograms (B-G). Serial MR-imaging of the temporal bones and corresponding audiograms (performed with imaging) from the patient demonstrating the clinical and imaging features associated with development of a left endolymphatic sac tumor. Three years before evaluation at the National Institutes of Health experienced an acute onset of vertigo and left tinnitus. (B) Axial, T1-weighted, enhanced MR-imaging at that time demonstrated no evidence of endolymphatic sac tumor and (C) audiogram was normal. One year later, the patient developed worsening tinnitus associated with acute onset of vertigo and left hearing loss. (D) Axial, T1-weighted, non-enhanced MR-imaging revealed left intralabyrinthine hemorrhage (arrowhead), but no evidence of enhancing tumor. (E) Audiogram at that time revealed mild to severe left sensorineural hearing loss. One week before evaluation, the patient developed worsening left hearing loss, while continuing to have vertigo and left tinnitus. (F) Axial, T1-wieghted, enhanced MR-imaging again revealed evidence of intralabyrinthine hemorrhage (arrowhead), as well as an enhancing lesion in the region of the left endolymphatic duct (arrows). (G) Audiogram revealed progressive moderate to severe left sensorineural hearing loss.
Nine months after the patient’s acute left hearing loss, her mother was diagnosed with von Hippel-Lindau disease (VHL). Subsequently, the patient was confirmed to have VHL both by clinical criteria (Figure 2) and by VHL gene germline mutation testing. Based on her diagnosis of VHL and audiovestibular symptoms, the patient was referred to the NIH. During the week before evaluation, she experienced a sudden onset of acute vertigo and left tinnitus with concomitant sudden progression of left hearing loss that was confirmed by audiometry. Computed tomography (CT)- and MR-imaging of the temporal bones again demonstrated left intralabyrinthine hemorrhage, as well as a small ELST in the left vestibular aqueduct (Figure 1). MR-imaging of the craniospinal axis revealed hemangioblastomas of the medullary obex and thoracic spinal cord. CT-imaging of the abdomen revealed bilateral renal cell carcinomas (RCCs), as well as renal and pancreatic cysts (Figure 2). Four weeks after evaluation, the ELST was resected resulting in resolution of her vertigo and tinnitus. Postoperatively, her hearing loss remained unchanged compared to preoperative hearing levels. Bilateral partial nephrectomies were performed to resect multiple RCCs 1 month before and 1 month after ELST resection.
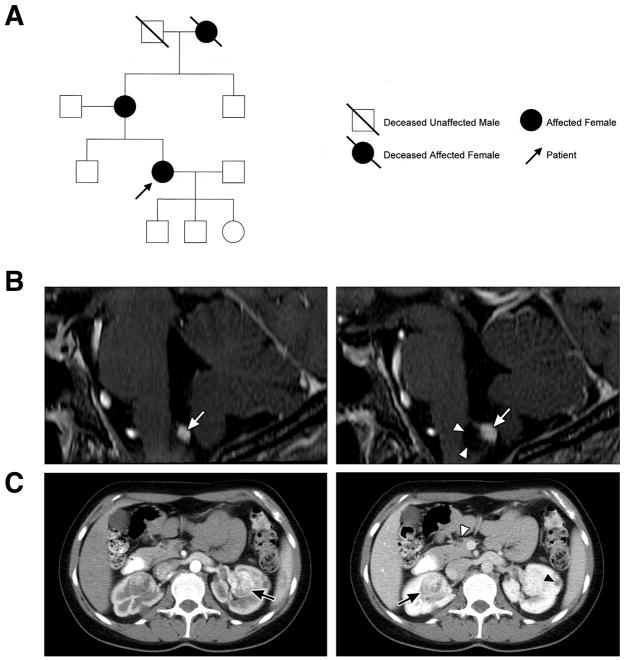
Figure 2. Pedigree of the case, serial magnetic resonance (MR)-imaging of brainstem hemangioblastoma and computed tomography (CT) of the abdomen.
(A) Pedigree of patient. The grandmother of the patient met the clinical criteria for the diagnosis of von Hippel-Lindau disease (VHL). Review of her medical history revealed that she had retina and cerebellar hemangioblastomas, as well as renal cell carcinoma. The children of the patient do not have clinical evidence of VHL but have not been genetically tested. (B) Sagittal, post-contrast, T1-weighted, MR-images of the brainstem of the patient demonstrating a hemangioblastoma of the obex and development of an associated peritumoral cyst. (Left) Twelve months before evaluation (2002) at the National Institutes of Health, contrast enhanced, T1-weighted, MR-imaging revealed an enhancing hemangioblastoma (arrow) of the obex in the medulla and edema (hypointensity in brainstem adjacent to tumor). The patient was asymptomatic the time. (Right) Thirty months later, the patient presented with headaches, frequent hiccups and swallowing difficulties. Contrast enhanced, T1-weighted, MR-imaging revealed development of a peritumoral cyst (arrowheads) associated with the obex hemangioblastoma (arrow). (C) Axial, post-contrast CT revealed renal cell carcinomas bilaterally (arrows), renal cysts (black arrowhead) and pancreatic cysts (white arrowhead).
Nineteen months after ELST resection, the patient returned with new complaints of headaches, frequent hiccups and swallowing difficulties. Craniospinal MR-imaging at that time revealed development of a peritumoral cyst associated with the obex hemangioblastoma (Figure 2). To alleviate the symptoms associated with mass effect from the peritumoral cyst and tumor, the patient underwent resection of the obex hemangioblastoma. Postoperative MR-imaging revealed complete tumor removal and secondary cyst resolution. The patient’s signs and symptoms resolved and she remains symptom free at last follow-up (21 months after brainstem hemangioblastoma resection). Serial abdominal CT-imaging revealed stable renal cyst, RCC and pancreatic cysts.
Discussion
VHL
VHL is an autosomal dominant neoplasia syndrome. It has a prevalence of 1 in 39,000 live births.1 VHL is the result of a germline mutation of the VHL gene.2,3 While this disorder has variable expression, it has greater than 90% penetrance by 65 years of age.4 Patients with VHL are predisposed to develop specific central nervous system (CNS) and visceral lesions (Table 1).5,6 In the CNS, tumors including hemangioblastomas and ELSTs can develop. In the viscera, renal cysts, RCCs, pancreatic cysts, pancreatic neuroendocrine tumors, pheochromocytomas and cystadenomas of the adnexal organs (broad ligament in females and epididymis in males) can develop. Before the advent of routine surveillance and better-defined treatment recommendations for VHL-associated lesions (Table 2), median survival of VHL patients was 50 years of age.6,7 The primary cause of death in VHL patients is from complications linked to CNS hemangioblastomas or RCCs.
Table 1.
Approximate distribution, mean age of onset, range of onset age, and frequency of lesions associated with von Hippel-Lindau disease (Adapted from Lonser et al.5)
| Location | Mean age of onset (years) | Age range (years) | Frequency of patients (%) |
|---|---|---|---|
| Central nervous system | |||
| Retinal hemangioblastomas | 25 | 1 to 67 | 25 to 60% |
| Craniospinal hemangioblastomas | 33 | 9 to 78 | 60 to 80% |
| Cerebellum | 33 | 9 to 78 | 44 to 72% |
| Brainstem | 32 | 12 to 46 | 10 to 25% |
| Spinal cord | 33 | 12 to 66 | 13 to 50% |
| Supratentorial | 34 | unknown | 3 to 6% |
| Lumbosacral nerve roots | unknown | unknown | 1 to 3% |
| Endolymphatic sac tumors | 22 | 12 to 50 | 10 to 15% |
| Visceral | |||
| Renal cell carcinoma/cysts | 39 | 16 to 67 | 25 to 60% |
| Pheochromocytomas | 30 | 5 to 58 | 10 to 20% |
| Pancreatic neuroendocrine tumor | 38 | 16 to 68 | 8 to 17% |
| Pancreatic cyst | 36 | 5 to 70 | 17 to 56% |
| Epididymal cystadenoma | unknown | unknown | 25 to 60% |
| Broad ligament cystadenoma | unknown | 16 to 46 | unknown |
Table 2.
Suggested surveillance modalities and frequency for neurologic manifestations of von Hippel-Lindau disease (Adapted from Choyke et al.54)
| Test | Lesions Evaluated | Start age (frequency) |
|---|---|---|
| Opthalmoscopy | Retinal hemangioblastoma | Infancy (yearly) |
| Magnetic resonance imaging of craniospinal axis* | Craniospinal hemangioblastoma | 11 years of age (yearly) |
| Computed tomography and magnetic resonance imaging of internal auditory canals* | Endolymphatic sac tumor | Onset of symptoms (hearing loss, tinnitus, vertigo, or unexplained balance difficulties) |
| Audiologic function tests | Endolymphatic sac tumor | When clinically indicated |
| Ultrasound of abdomen | Renal carcinoma/cyst, pancreatic neuroendocrine tumor/cyst | 8 years of age (yearly; magnetic resonance imaging as clinically indicated) |
| Computed tomography of abdomen* | Renal carcinoma/cyst, pancreatic neuroendocrine tumors/cysts | 18 years of age or earlier if clinically indicated (yearly) |
| Plasma or 24-hour urinary catecholamines and metanephrines | Pheochromocytoma | 2 years of age (yearly and when blood pressure is elevated) |
Imaging typically performed with and without intravenous contrast
Diagnosis
Diagnosis of VHL can be established by clinical criteria or by genetic testing. Patients with a family history of VHL (Figure 2) and a CNS hemangioblastoma (including retinal hemangioblastomas), RCC, pheochromocytoma or ELST meet the clinical criteria for diagnosis of VHL. Approximately 20% of patients will not have a family history but fulfill the clinical diagnostic criteria for VHL if they have 2 or more CNS hemangioblastomas or 1 CNS hemangioblastoma and a VHL-associated visceral tumor.7,8 Frequently, at risk patients (including patients with an isolated CNS hemangioblastoma)9 undergo testing for a germline VHL mutation. The detection rate of VHL mutations in patients with a family history of VHL is nearly 100%.10 However, de novo mutations in patients without a family history may result in a disease mosaicism where some but not all tissues carry the mutation.11 These patients may test negative if their peripheral blood leukocytes do not carry the VHL gene mutation.
Molecular Pathogenesis
The VHL tumor suppressor gene was mapped to the short arm of chromosome 3 by Seizinger and colleagues12 in 1988 and isolated by Latif and colleagues2 in 1993. Similar to our patient, most VHL patients inherit a VHL gene (allele) with a germline mutation from their affected parent and a normal (wild-type) VHL gene from the unaffected parent. While all cells have a VHL germline mutation in patients that inherit the trait, tumors only form in cells that have lost function of the wild-type allele13 and that are located within specific VHL susceptible target organs.
The VHL gene is widely expressed in tissues, including those not affected by VHL.2 VHL mRNA encodes for VHL protein (pVHL). Post-translation, pVHL complexes elongin B, elongin C, Rbx 1 and Cullin 2(1;2) to form an ubiquitin ligase that proteolyzes the alpha-subunit of hypoxia-inducible factor (HIF).5,14,15 Under normal circumstances, HIF coordinates cellular response to hypoxia through transcriptional regulation. HIF enhances glucose uptake and increases the expression of angiogenic, growth and mitogenic factors including, vascular endothelial growth factor (VEGF), platelet derived growth factor-beta chain (PDGF-B), erythropoietin and transforming growth factor (TGF).5,14
With absent or abnormal pVHL function, HIF may constitutively stimulate angiogenesis.15 HIF-mediated angiogenesis may result from increased levels of VEGF and/or PDGF-B and could explain the vascular nature of VHL-associated tumors.16 Moreover, VEGF-mediated increased tumor vascular permeability may underlie the frequent formation of peritumoral edema and cysts in VHL. Another potential mechanism of HIF-mediated carcinogenesis is the overproduction of TGF-alpha. Besides being a potent mitogenic factor, TGF-alpha stimulates cellular over-expression of the epidermal growth factor receptors (the receptors for TGF-alpha) creating a potential autocrine loop.17
Possible mechanisms of tumorigenesis independent of HIF regulation caused by absent or abnormal pVHL include, disruption of normal cell cycling, increased angiogenesis and abnormalities in the extracellular matrix. The incapacity to leave the cell cycle occurs in cells lacking pVHL and could be an early event in VHL tumorigenesis.18 Moreover, absence of pVHL may increase VEGF expression through the release of both transcriptional and post-translational regulation and can augment the angiogenic effects mediated through HIF and increase tumor vessel permeability.19 Finally, cells lacking pVHL are unable to properly assemble a fibronectin extracellular matrix further contributing to carcinogenesis.20
VHL-Associated Lesions (Excluding Craniospinal Hemangioblastomas and ELSTs)
RCC and renal cysts
RCC is the most common malignant neoplasm in VHL (Table 1). A RCC and/or cyst can be found in 60% of patients. Renal cysts are typically asymptomatic and seldom need treatment. However, complex cysts need serial monitoring because they often harbor solid components of RCC. While small RCCs tend to be low-grade and minimally invasive, the growth rate of these lesions is highly variable.21 Because RCCs can remain asymptomatic for long periods of time, serial imaging is useful for early diagnosis (Table 2). Rarely, in more advanced cases, patients present with hematuria, flank pain or a flank mass. Some clinicians recommend nephron-sparing resection of RCCs when the largest tumor reaches a diameter of 3 cm. Nephron or renal-sparing surgery is based on the ability to preserve renal function while reducing the risk of metastasis. Walter and colleagues21 reported that renal sparing for RCC less than 3 cm was not associated with metastasis or need for renal transplantation or dialysis. Tumor enucleation or partial nephrectomy may be needed for resection of larger tumors (greater than 3 cm). Less invasive percutaneous treatments of RCC are being explored.
Pancreatic neuroendocrine tumors and cysts
Thirty-five to 70% of VHL patients will develop a pancreatic neuroendocrine tumor and/or cyst (Table 1). Pancreatic cysts are generally asymptomatic and do not require treatment. Pancreatic neuroendocrine tumors are often non-functional and asymptomatic but are malignant and can behave malignantly in up to 8% of cases.(leave reference 22 in) These tumors are identified on post-contrast, abdominal CT or MR-imaging (Table 2). Recent data indicate that optimal timing of surgical resection of pancreatic neuroendocrine tumors may be based on tumor size, exon 3 VHL mutation status and tumor doubling time.22
Pheochromocytoma
Pheochromocytomas can be multiple and bilateral in nature in VHL (Table 1). They can also be found as extra-adrenal paragangliomas in the carotid body, glomus jugulare and peri-aortic tissues. Five percent of pheochromocytomas are malignant.23 While frequent clinical findings associated with pheochromocytomas include intermittent or sustained hypertension, palpitations, tachycardia, headaches, episodic sweating, pallor or nausea, 30% of patients with pheochromocytomas will be asymptomatic.23 The diagnosis of pheochromocytoma is made by laboratory and imaging studies (Table 2). Pre-operative evaluation is critical in VHL patients to prevent a perioperative hypertensive crisis. Early intervention with adrenal cortical-sparing surgery results in low recurrence and long-term corticosteroid independence.
Cystadenomas of reproductive adnexal organs
Cystadenomas of the epididymis and broad ligament are benign lesions that can be found frequently in VHL patients (Table 1). They are often bilateral and multiple in nature. They can be identified by ultrasound (epididymal) or by abdominal CT (broad ligament). Because these lesions are benign and most often symptomless, they are managed conservatively.
Retinal hemangioblastomas
Retinal hemangioblastomas are often bilateral and multifocal in VHL (Table 1). They can arise in the periphery or near/on the optic disk. Despite frequently being asymptomatic, they can cause visual symptoms by progressive growth, edema or development of hard exudates. Opthalmoscopy with iris dilation allows identification of most retinal hemangioblastomas. Early diagnosis and treatment (photocoagulation and cryotherapy) of peripheral tumors can prevent visual loss.
Hemangioblastomas
Epidemiology
Craniospinal hemangioblastomas are the most common tumor (located viscerally or in the CNS) associated with VHL (Table 1). Sixty to 80% of VHL patients will develop a CNS hemangioblastoma of the cerebellum, brainstem or spinal cord during their lifetime.5 Nearly all (90%) of these patients will develop multiple hemangioblastomas. Despite their benign histology, hemangioblastomas can cause significant morbidity and mortality in VHL. CNS hemangioblastomas (outside the retina) are found nearly exclusively (95% of tumors) in the brainstem, cerebellum or spinal cord.5,24,25
Imaging findings
Contrast-enhanced craniospinal MR-imaging is the most sensitive and accurate imaging modality for detecting and monitoring changes in hemangioblastomas. Hemangioblastomas are highly vascular tumors that vividly and discretely enhance on T1-weighted MR-imaging sequences (Figure 2). Even small tumors (2 mm in diameter) are reliably detected and can be precisely characterized over serial studies. Because hemangioblastomas are frequently associated with peritumoral edema and cysts, imaging to detect the development or changes in edema and cysts using FLAIR and T2-weighted MR-imaging is critical.
Presenting signs and symptoms
Signs and symptoms related to CNS hemangioblastomas are attributable to the anatomic region where they arise. Cerebellar hemangioblastomas cause headache (75% of patients), gait ataxia (55%), dysmetria (29%), hydrocephalus (28%) or nausea/vomiting (28%).26 Brainstem hemangioblastomas cause hypesthesia (55%), gait ataxia (22%), dysphagia (22%), hypereflexia (22%) or headache (11%).5,27 Spinal cord hemangioblastomas cause hypesthesia (83%), weakness (65%), gait ataxia (65%), hypereflexia (52%) or pain (17%).5
Natural history
To define the natural history of hemangioblastomas in VHL patients and to correlate features of hemangioblastomas that were associated with symptom development and need for treatment, Wanebo and colleagues27 reviewed the imaging and clinical features of 160 consecutive VHL patients harboring 655 CNS hemangioblastomas followed at the NIH (mean follow-up, 21±27 months). The clinical circumstance was dynamic and tumors were found to have variable growth patterns. Although symptom formation appeared to be associated with tumor size, tumor growth rate and the presence of a peritumoral cyst, no reliable threshold for tumor size or growth could be identified that would predict symptom formation and need for treatment.
Recently, Ammerman and colleagues28 studied the pattern of growth of CNS hemangioblastomas in 19 VHL patients serially imaged for at least 10 years. Hemangioblastomas grew in a saltatory growth pattern consisting of periods of rapid growth followed by periods of quiescence in nearly every case (97% of 143 tumors). Hemangioblastomas had an average of 1.85 quiescent periods between growth periods before becoming symptomatic requiring resection. Periods of growth averaged 13±15 months, while the periods of growth quiescence averaged 25±19 months. Nearly all (97%) the hemangioblastomas demonstrated radiographic progression but only 50% required treatment for symptom formation. Moreover, nearly half (45%) of tumors that required resection (symptomatic) were not apparent on initial MR- imaging.
Peritumoral cyst formation
While the development of signs and symptoms can occasionally be directly attributed to the mass effect of the hemangioblastoma itself, most frequently neurologic dysfunction is caused by the combined mass effect of the tumor and an associated peritumoral cyst (Figure 2). Most (70%) symptomatic cerebellar and brainstem hemangioblastomas will be associated with a peritumoral cyst and over 90% of symptomatic spinal hemangioblastomas will be associated with a peritumoral cyst (syringomyelia).27,28 In such cases, the cyst accounts for the bulk of the mass burden. Specifically, Wanebo and colleagues25 found in the cerebellum the average cyst volume was 4 times larger than the associated tumor and in the brainstem it was 12 times larger than the associated tumor of symptomatic patients. Conversely, a small fraction (5 to 10%) of asymptomatic cerebellar, brainstem or spinal cord hemangioblastomas are associated with peritumoral cysts.
Recent studies have shown that peritumoral cyst formation follows a defined sequence initiated by increased hemangioblastoma vascular permeability.29,30 This results in plasma ultrafiltrate extravasation into the interstitial spaces of the tumor and high interstitial tumor pressure drives this fluid into the surrounding parenchyma. This results in edema formation as the resorptive capacity of the peritumoral tissue is exceeded. As the mismatch between fluid production and tissue resorbtion increases, the surrounding tissues swell due to solid stresses (stretching) that favor cyst formation. Formation of the cyst alters interstitial flow patterns so that the majority of excess interstitial fluid flows into the peritumoral cyst (path of least resistance) resulting in cyst expansion.
Understanding the mechanism underlying peritumoral cyst development and propagation with VHL-associated hemangioblastomas has led to several critical clinical insights. First, because the tumor is the source of peritumoral edema/cyst formation, edema/cyst resolution after tumor removal will occur reliably and treatment does not require cyst wall resection or fenestration. Second, reducing tumor vascular permeability could be beneficial and clinical improvements using anti-VEGF tumor therapies have been associated with edema reduction despite having no effect on tumor size.31,32 Third, increasing vascular permeability can be deleterious and studies have shown that radiation induces increases in vascular permeability that can lead to peritumoral edema and cyst formation suggesting judicious irradiation of hemangioblastomas with significant peritumoral edema/cysts.33 Finally, peritumoral cysts can stop growing when the surface area of the cyst wall is sufficiently large to absorb the excess fluid and become quiescent and remain asymptomatic. Imaging evidence of edema and/or cyst formation in asymptomatic patients is not an absolute indication for surgery.
Tumor features
Grossly, hemangioblastomas appear bright red or orange/yellow in color and are invariably associated with intense vascularity (Figure 3). Typically, abnormal tortuous feeding arteries and arterialized draining veins can be found entering and exiting the tumor. Histologically, hemangioblastomas have characteristic features that include proliferation of stromal cells and endothelial cells (WHO grade 1; Figure 3).34 The endothelial cells form vascular channels around the neoplastic stromal cells.35 The stromal cells have numerous lipid-containing vacuoles that result in the clear cell morphology similar to RCC. Because VHL patients often have contemporaneous RCCs that can metastasize to the CNS or metastasize to hemangioblastomas,34,36 immunohistochemical differentiation between renal cell carcinoma and/or hemangioblastoma may be necessary.
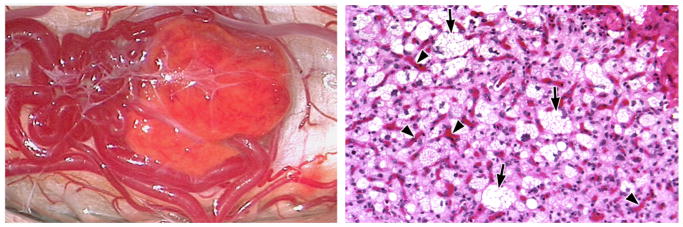
Figure 3. Hemangioblastoma gross appearance and histology.
(Left) Intraoperative view of a hemangioblastoma of the cervical spinal cord. These tumors appear bright red or orange/yellow (due to high lipid content of stromal cells) in color and are invariably associated with large tortuous feeding arteries and arterialized draining veins. (Right) Corresponding histology (hematoxylin and eosin staining; original magnification 20 X) demonstrating the vacuolated (clear cell) hemangioblastoma stromal cells (arrows) surrounded by endothelial cells (arrowheads).
Developmental origin
Based on embryologic findings and evidence of intratumoral hematopoiesis and endothelial cell formation, it was hypothesized that hemangioblastomas may derive from an embryologic cell capable of blood and vessel formation. This theory was not directly testable until 1998, when Choi and colleagues characterized a common embryologic precursor cell that was transiently present during mesoderm development and was capable of blood and endothelial cell formation.37 They defined this multipotent embryologic cell as a “hemangioblast”. Subsequent investigations uniquely identified the embryonic hemangioblast by co-expression of brachyury, Flk-1 (VEGF receptor-2) and Scl (stem cell leukemia).38
To determine if the neoplastic cell of origin in hemangioblastomas is the embryologically-arrested hemangioblast, the expression of brachyury, Flk-1 and Scl was recently characterized from neoplastic stromal cells of CNS hemangioblastomas from VHL patients.39 Similar to the embryologic hemangioblast and consistent with a cell of mesodermal origin, the neoplastic stromal cells in hemangioblastomas co-expressed brachyury (only expressed during mesoderm development), Flk-1 and Scl. Analogous to embryologic hemangioblasts, the hemangioblastoma-derived hemangioblasts demonstrated self-renewal and could be differentiated into hematopoietic and endothelial lineages in variable microenvironments. The hematopoietic and endothelial progeny were confirmed to derive from tumor hemangioblasts by loss of heterozygosity analysis.
Treatment
Complete resection of hemangioblastomas is curative and most craniospinal hemangioblastomas can be resected safely.26,27,40 While mild transient neurologic deficits can occur after CNS hemangioblastoma resection, these are typically not function limiting and patients will return to their baseline neurologic status within 6 weeks.5,26 Due to the multiplicity of CNS hemangioblastomas and because their growth rate is unpredictable, surgery is generally reserved until associated symptoms arise.25,28 Using this strategy, most VHL patients can maintain excellent neurologic function and unnecessary surgical resection can be avoided.28
Because of the potential morbidity that can be associated with resection of large craniospinal hemangioblastomas, stereotactic radiosurgery has been investigated as a potential therapeutic option.33,41 Small hemangioblastomas not associated with peritumoral cysts may respond best to radiotherapy. While some studies have established the successful use of radiosurgery based on stability of tumor size, lack of hemangioblastoma progression in these cases may represent a quiescent period and not a response to treatment.26,28 Longer-term assessment of more patients will be necessary to determine the effectiveness of this treatment and the potential risk of developing new neoplasms in patients with a constitutional haploinsufficiency.
ELSTs
Epidemiology
ELSTs were first established as a distinct pathologic entity by Heffner, in 1989.42 While ELSTs may occur sporadically, they were recognized as a phenotypic expression of VHL by Manski and colleagues43 in 1997 and were later genetically confirmed to be part of the VHL syndrome.44 Imaging evidence of an ELST can be found in approximately 10 to 15% of VHL patients and 30% of VHL patients with an ELST will develop bilateral ELSTs (the only clinical circumstance in which bilateral ELSTs have been reported).43 Despite their benign histology, ELSTs are locally invasive tumors that most often cause audiovestibular morbidity including, hearing loss, vertigo, tinnitus and/or aural pain.43,45,46
Imaging findings
Optimal identification of ELSTs requires high-resolution CT-and MR-imaging. ELSTs on CT-imaging are soft tissue density tumors that expand, destroy and incorporate the adjacent temporal bone. Small bony erosions adjacent to the vestibular aqueduct may be evident on CT-scanning as an initial radiographic finding of an ELST. ELSTs on MR-imaging can have considerable heterogeneity, as internal hemorrhage and cysts, incorporated temporal bone, cholesterol granuloma and vascular flow voids may be present to varying degrees. Intralabyrinthine hemorrhage, a common imaging feature associated with ELSTs, appears as a T1-weighted hyperintensity within the vestibule, cochlea or semicircular canals (Figure 1) distinct from the site of tumor.
Presenting signs and symptoms
VHL patients with imaging evidence of an ELST present with hearing loss (95%), tinnitus (90%), vertigo or disequilibrium (66%), aural fullness (30%) and facial paresis (8%) attributable to the imaged lesion.43 Hearing loss can occur either acutely (86% of patients) or gradually over several years (14%).43 Generally, once hearing loss occurs it is irreversible and it typically occurs early in life (Table 1).43 Similar to our case, patients usually report that hearing loss coincides with the exacerbation of vestibular symptoms.47 A significant fraction (59%) of VHL patients with vestibulocochlear symptoms have no imaging evidence of ELSTs.43,46 Recent evidence suggests that the etiology of these clinical manifestations may be due to a microscopic ELST or hyperplasia of the endolymphatic epithelium in VHL patients.45,48
Mechanisms of hearing loss
To define ELST-associated mechanisms underlying audiovestibular pathophysiology, a prospective study of VHL patients with ELSTs was performed.49 Clinical and audiologic data were correlated with serial CT- and MR-imaging to elucidate the mechanisms underlying audiovestibular dysfunction. While tumor invasion of the otic capsule (bony covering of the inner ear apparatus) (18% of patients) was associated with large ELSTs and caused hearing loss (100% of patients with otic capsule invasion), the majority of patients (82%) had small ELSTs that did not invade the otic capsule. In this group of patients, hearing loss was still present (91% of affected ears) and either developed suddenly (43%) or gradually (48%). Sudden hearing loss in these patients correlated with MR-imaging evidence of intralabyrinthine hemorrhage but hemorrhage was not seen in patients with gradual or normal hearing. Tumor size was not associated with the development of audiovestibular symptoms.
The imaging and clinical findings from this study support 3 distinct mechanisms of ELST-associated audiovestibular morbidity including, direct invasion of the otic capsule by tumor, intralabyrinthine hemorrhage and/or endolymphatic hydrops. First, direct ELST erosion into the inner ear can result in membranous labyrinth destruction that disrupts endolymphatic flow causing hearing loss and vestibulopathy. Second, acute intralabyrinthine hemorrhage, as seen in patients with sudden hearing loss without otic capsule invasion (Figure 1), indicates that ELST-associated spontaneous hemorrhage conducted into the labyrinth via the endolymphatic duct may underlie the sudden hearing loss in these cases. This mechanism is further supported by the presence of hemosiderin in the membranous labyrinth of a deaf VHL patient with an ELST at autopsy.45 Finally, in patients with gradual hearing loss, no hemorrhage was identified and the symptom complex was similar to our patient and mimicked that of Meniere’s disease (idiopathic endolymphatic hydrops). Development of endolymphatic hydrops is another potential consequence of a small ELST. Hydrops represents an increase in endolymph volume, the regulation of which is thought to be the primary function of the endolymphatic sac. As endolymph is primarily produced within the cochlea, hydrops in ELST patients could develop via impaired endolymph resorption or due to excess production of fluid into the membranous labyrinth. Excess production of fluid by the ELST could be analogous to the formation of peritumoral edema/cysts associated with CNS hemangioblastomas and visceral tumors in VHL.5,45
Tumor features
Grossly ELSTs can appear bright or dark red in color and are associated with intense vascularity of the tumor mass and surrounding endolymphatic sac. ELSTs can often be found in association with cholesterol granuloma formation. Histologically, ELSTs are papillary-cystic neoplasms that are well vascularized. They are typically lined by a row of cuboidal cells and mitosis are typically absent. Recently, high-resolution imaging and histologic analyses demonstrate that ELSTs originate in the vestibular aqueduct portion of the endolymphatic sac/duct system. 50
Developmental origin
Studies have revealed multifocal, VHL-deficient epithelial cell proliferations throughout the endolymphatic duct and sac in VHL patients. These proliferations may represent potential precursor structures for the development of frank tumor.48 The abundance of precursor structures detected in the endolymphatic sac and duct in a VHL patient without ELST at autopsy suggested that most precursor structures do not develop into frank tumor during the lifetime of an individual patient.51 In visceral VHL target sites, evidence is emerging that tumors develop coincident with activation of distinct proteins, which are undetectable in potential precursor lesions. As yet, no such “activating” mechanism is known that could differentiate early tumor from precursor state in the endolymphatic sac or duct.
Treatment
Previously, the management of ELSTs has been conservative, as they grow slowly and are not considered malignant.43,46 The demonstration that significant audiovestibular morbidity is frequently associated with very small tumors and the potential of irreversible hearing loss to occur suddenly independent of tumor size, suggests that early surgical intervention may be warranted. Complete resection of ELSTs is curative, can alleviate vestibular symptomatology and can be performed with hearing preservation and minimal morbidity.47,52,53 To intervene early in VHL patients, prompt diagnosis based on clinical findings and supported by high-resolution CT- and MR-imaging to detect small ELSTs and/or intralabyrinthine hemorrhage is warranted. Detection of an ELST may, after weighing potential risks, prompt surgery to ameliorate symptoms and prevent progression of hearing loss.
Conclusions
Recent investigations into VHL-associated CNS lesions have given insight into the origin and development of these tumors. Emerging data from imaging and clinical surveillance protocols have provided insights into the natural history of VHL-associated ELSTs and hemangioblastomas. Because of their differing natural histories, the optimal management strategies for the 2 neurologic manifestations of VHL are different.
Footnotes
Financial Disclosures
The authors declare no financial interests, relationships or affiliations relevant to the subject of this manuscript.
References
- 1.Neumann HP, Wiestler OD. Clustering of features of von Hippel-Lindau syndrome: evidence for a complex genetic locus. Lancet. 1991;337(8749):1052–1054. doi: 10.1016/0140-6736(91)91705-y. [DOI] [PubMed] [Google Scholar]
- 2.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 3.Wait SD, Vortmeyer AO, Lonser RR, et al. Somatic mutations in VHL germline deletion kindred correlate with mild phenotype. Ann Neurol. 2004 Feb;55(2):236–240. doi: 10.1002/ana.10807. [DOI] [PubMed] [Google Scholar]
- 4.Maher ER, Iselius L, Yates JR, et al. Von Hippel-Lindau disease: a genetic study. J Med Genet. 1991;28(7):443–447. doi: 10.1136/jmg.28.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003 Jun 14;361(9374):2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 6.Richard S, Campello C, Taillandier L, Parker F, Resche F. Haemangioblastoma of the central nervous system in von Hippel-Lindau disease. French VHL Study Group. J Intern Med. 1998;243(6):547–553. doi: 10.1046/j.1365-2796.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 7.Lamiell JM, Salazar FG, Hsia YE. von Hippel-Lindau disease affecting 43 members of a single kindred. Medicine. 1989;68(1):1–29. doi: 10.1097/00005792-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Melmon KL, Rosen SW. Lindau’s disease. Am J Med. 1964;36:595–617. doi: 10.1016/0002-9343(64)90107-x. [DOI] [PubMed] [Google Scholar]
- 9.Woodward ER, Wall K, Forsyth J, Macdonald F, Maher ER. VHL mutation analysis in patients with isolated central nervous system haemangioblastoma. Brain. 2007;130:836–842. doi: 10.1093/brain/awl362. [DOI] [PubMed] [Google Scholar]
- 10.Stolle C, Glenn G, Zbar B, et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum Mutat. 1998;12(6):417–423. doi: 10.1002/(SICI)1098-1004(1998)12:6<417::AID-HUMU8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 11.Sgambati MT, Stolle C, Choyke PL, et al. Mosaicism in von Hippel-Lindau disease: lessons from kindreds with germline mutations identified in offspring with mosaic parents. Am J Hum Genet. 2000;66(1):84–91. doi: 10.1086/302726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seizinger BR, Rouleau GA, Ozelius LJ, et al. Von Hippel-Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988 Mar 17;332(6161):268–269. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- 13.Knudson AGJ, Strong LC. Mutation and cancer: neuroblastoma and pheochromocytoma. Am J Hum Genet. 1972;24:514–532. [PMC free article] [PubMed] [Google Scholar]
- 14.Kaelin WG., Jr Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002 Sep;2(9):673–682. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 15.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 16.Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394(6692):485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 17.Reifenberger G, Reifenberger J, Bilzer T, Wechsler W, Collins VP. Coexpression of transforming growth factor-alpha and epidermal growth factor receptor in capillary hemangioblastomas of the central nervous system. Am J Pathol. 1995;147(2):245–250. [PMC free article] [PubMed] [Google Scholar]
- 18.Pause A, Lee S, Lonergan KM, Klausner RD. The von Hippel-Lindau tumor suppressor gene is required for cell cycle exit upon serum withdrawal. Proc Natl Acad Sci U S A. 1998;95(3):993–998. doi: 10.1073/pnas.95.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gnarra JR, Zhou S, Merrill MJ, et al. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc Natl Acad Sci U S A. 1996;93(20):10589–10594. doi: 10.1073/pnas.93.20.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohh M, Yauch RL, Lonergan KM, et al. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol Cell. 1998;1(7):959–968. doi: 10.1016/s1097-2765(00)80096-9. [DOI] [PubMed] [Google Scholar]
- 21.Walther MM, Choyke PL, Glenn G, et al. Renal cancer in families with hereditary renal cancer: prospective analysis of a tumor size threshold for renal parenchymal sparing surgery. J Urol. 1999;161(5):1475–1479. doi: 10.1016/s0022-5347(05)68930-6. [DOI] [PubMed] [Google Scholar]
- 22.Blansfield JA, Choyke L, Morita SY, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs) Surgery. 2007 Dec;142(6):814–818. doi: 10.1016/j.surg.2007.09.012. discussion 818 e811–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walther MM, Reiter R, Keiser HR, et al. Clinical and genetic characterization of pheochromocytoma in von Hippel-Lindau families: comparison with sporadic pheochromocytoma gives insight into natural history of pheochromocytoma. J Urol. 1999;162(3 Pt 1):659–664. doi: 10.1097/00005392-199909010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Browne TR, Adams RD, Roberson GH. Hemangioblastoma of the spinal cord: review and report of five cases. Arch Neurol. 1976;33:435–441. doi: 10.1001/archneur.1976.00500060041009. [DOI] [PubMed] [Google Scholar]
- 25.Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg. 2003 Jan;98(1):82–94. doi: 10.3171/jns.2003.98.1.0082. [DOI] [PubMed] [Google Scholar]
- 26.Jagannathan J, Lonser RR, Smith R, DeVroom HL, Oldfield EH. Surgical management of cerebellar hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2008 Feb;108(2):210–222. doi: 10.3171/JNS/2008/108/2/0210. [DOI] [PubMed] [Google Scholar]
- 27.Lonser RR, Weil RJ, Wanebo JE, DeVroom HL, Oldfield EH. Surgical management of spinal cord hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2003 Jan;98(1):106–116. doi: 10.3171/jns.2003.98.1.0106. [DOI] [PubMed] [Google Scholar]
- 28.Ammerman JM, Lonser RR, Dambrosia J, Butman JA, Oldfield EH. Long-term natural history of hemangioblastomas in patients with von Hippel-Lindau disease: implications for treatment. J Neurosurg. 2006 Aug;105(2):248–255. doi: 10.3171/jns.2006.105.2.248. [DOI] [PubMed] [Google Scholar]
- 29.Lonser RR, Butman JA, Oldfield EH. Pathogenesis of tumor-associated syringomyelia demonstrated by peritumoral contrast material leakage. Case illustration. J Neurosurg Spine. 2006 May;4(5):426. doi: 10.3171/spi.2006.4.5.426. [DOI] [PubMed] [Google Scholar]
- 30.Lohle PN, van Mameren H, Zwinderman KH, Teepen HL, Go KG, Wilmink JT. On the pathogenesis of brain tumour cysts: a volumetric study of tumour, oedema and cyst. Neuroradiology. 2000 Sep;42(9):639–642. doi: 10.1007/s002340000363. [DOI] [PubMed] [Google Scholar]
- 31.Aiello LP, George DJ, Cahill MT, et al. Rapid and durable recovery of visual function in a patient with von hippel-lindau syndrome after systemic therapy with vascular endothelial growth factor receptor inhibitor su5416. Ophthalmology. 2002;109(9):1745–1751. doi: 10.1016/s0161-6420(02)01159-4. [DOI] [PubMed] [Google Scholar]
- 32.Girmens JF, Erginay A, Massin P, Scigalla P, Gaudric A, Richard S. Treatment of von Hippel-Lindau retinal hemangioblastoma by the vascular endothelial growth factor receptor inhibitor SU5416 is more effective for associated macular edema than for hemangioblastomas. Am J Ophthalmol. 2003 Jul;136(1):194–196. doi: 10.1016/s0002-9394(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 33.Niemela M, Lim YJ, Soderman M, Jaaskelainen J, Lindquist C. Gamma knife radiosurgery in 11 hemangioblastomas. J Neurosurg. 1996 Oct;85(4):591–596. doi: 10.3171/jns.1996.85.4.0591. [DOI] [PubMed] [Google Scholar]
- 34.Aldape KD, Plate KH, Vortmeyer AO, Zagzag D, Neumann HPH. Haemangioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. 4. Lyon: International Agency for Research on Cancer (IARC); 2007. pp. 184–186. [Google Scholar]
- 35.Vortmeyer AO, Gnarra JR, Emmert-Buck MR, et al. von Hippel-Lindau gene deletion detected in the stromal cell component of a cerebellar hemangioblastoma associated with von Hippel-Lindau disease. Hum Pathol. 1997;28(5):540–543. doi: 10.1016/s0046-8177(97)90075-7. [DOI] [PubMed] [Google Scholar]
- 36.Jarrell ST, Vortmeyer AO, Linehan WM, Oldfield EH, Lonser RR. Metastases to hemangioblastomas in von Hippel-Lindau disease. J Neurosurg. 2006 Aug;105(2):256–263. doi: 10.3171/jns.2006.105.2.256. [DOI] [PubMed] [Google Scholar]
- 37.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998 Feb;125(4):725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 38.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004 Dec 2;432(7017):625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 39.Park DM, Zhuang Z, Chen L, et al. von Hippel-Lindau Disease-Associated Hemangioblastomas Are Derived from Embryologic Multipotent Cells. PLoS Med. 2007 Feb 13;4(2):e60. doi: 10.1371/journal.pmed.0040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weil RJ, Lonser RR, DeVroom HL, Wanebo JE, Oldfield EH. Surgical management of brainstem hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2003 Jan;98(1):95–105. doi: 10.3171/jns.2003.98.1.0095. [DOI] [PubMed] [Google Scholar]
- 41.Patrice SJ, Sneed PK, Flickinger JC, et al. Radiosurgery for hemangioblastoma: results of a multiinstitutional experience. Int J Radiat Oncol Biol Phys. 1996;35(3):493–499. doi: 10.1016/s0360-3016(96)80011-3. [DOI] [PubMed] [Google Scholar]
- 42.Heffner DK. Low-grade adenocarcinoma of probable endolymphatic sac origin. A clinicopathologic study of 20 cases. Cancer. 1989 Dec 1;64(11):2292–2302. doi: 10.1002/1097-0142(19891201)64:11<2292::aid-cncr2820641119>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Manski TJ, Heffner DK, Glenn GM, et al. Endolymphatic sac tumors. A source of morbid hearing loss in von Hippel-Lindau disease. JAMA. 1997 May 14;277(18):1461–1466. doi: 10.1001/jama.277.18.1461. [DOI] [PubMed] [Google Scholar]
- 44.Vortmeyer AO, Choo D, Pack S, Oldfield E, Zhuang Z. VHL gene inactivation in an endolymphatic sac tumor associated with von Hippel-Lindau disease. Neurology. 2000 Aug 8;55(3):460. doi: 10.1212/wnl.55.3.460. [DOI] [PubMed] [Google Scholar]
- 45.Lonser RR, Kim HJ, Butman JA, Vortmeyer AO, Choo DI, Oldfield EH. Tumors of the endolymphatic sac in von Hippel-Lindau disease. N Engl J Med. 2004 Jun 10;350(24):2481–2486. doi: 10.1056/NEJMoa040666. [DOI] [PubMed] [Google Scholar]
- 46.Choo D, Shotland L, Mastroianni M, et al. Endolymphatic sac tumors in von Hippel-Lindau disease. J Neurosurg. 2004 Mar;100(3):480–487. doi: 10.3171/jns.2004.100.3.0480. [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ, Butman JA, Brewer C, et al. Tumors of the endolymphatic sac in patients with von Hippel-Lindau disease: implications for their natural history, diagnosis, and treatment. J Neurosurg. 2005 Mar;102(3):503–512. doi: 10.3171/jns.2005.102.3.0503. [DOI] [PubMed] [Google Scholar]
- 48.Glasker S, Lonser RR, Tran MG, et al. Effects of VHL deficiency on endolymphatic duct and sac. Cancer Res. 2005 Dec 1;65(23):10847–10853. doi: 10.1158/0008-5472.CAN-05-1104. [DOI] [PubMed] [Google Scholar]
- 49.Butman JA, Kim HJ, Baggenstos M, et al. Mechanisms of morbid hearing loss associated with tumors of the endolymphatic sac in von Hippel-Lindau disease. JAMA. 2007 Jul 4;298(1):41–48. doi: 10.1001/jama.298.1.41. [DOI] [PubMed] [Google Scholar]
- 50.Lonser RR, Baggenstos M, Kim HJ, Butman JA, Vortmeyer AO. The vestibular aqueduct: site of origin of endolymphatic sac tumors. J Neurosurg. 2008 Apr;108(4):751–756. doi: 10.3171/JNS/2008/108/4/0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glasker S, Li J, Xia JB, et al. Hemangioblastomas share protein expression with embryonal hemangioblast progenitor cell. Cancer Res. 2006 Apr 15;66(8):4167–4172. doi: 10.1158/0008-5472.CAN-05-3505. [DOI] [PubMed] [Google Scholar]
- 52.Megerian CA, Haynes DS, Poe DS, Choo DI, Keriakas TJ, Glasscock ME., 3rd Hearing preservation surgery for small endolymphatic sac tumors in patients with von Hippel-Lindau syndrome. Otol Neurotol. 2002 May;23(3):378–387. doi: 10.1097/00129492-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues S, Fagan P, Turner J. Endolymphatic sac tumors: a review of the St. Vincent’s hospital experience. Otol Neurotol. 2004 Jul;25(4):599–603. doi: 10.1097/00129492-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 54.Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995;194(3):629–642. doi: 10.1148/radiology.194.3.7862955. [DOI] [PubMed] [Google Scholar]
Reference List
- 1.Duan DR, Humphrey JS, Chen DY, Weng Y, Sukegawa J, Lee S, et al. Characterization of the VHL tumor suppressor gene product: localization, complex formation, and the effect of natural inactivating mutations. Proc Natl Acad Sci U S A. 1995;92:6459–63. doi: 10.1073/pnas.92.14.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pause A, Lee S, Worrell RA, Chen DY, Burgess WH, Linehan WM, et al. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci U S A. 1997;94:2156–61. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]