Abstract
Cell surface chondroitin sulfate proteoglycan 4 (CSPG4) is an attractive target for antibody-based cancer immunotherapy because of its role in tumor cell biology, its high expression on malignant cells including cancer-initiating cells, and its restricted distribution in normal tissues. The clinical use of CSPG4 has been hampered by the lack of a CSPG4-specific chimeric, humanized, or fully human monoclonal antibody. To overcome this limitation, we generated a CSPG4-specific fully human single-chain antibody termed scFv-FcC21 and characterized its specificity and antitumor activity. Viable CSPG4+ melanoma cells were used in a screen of a human scFv phage display library that included CDR3 engineered to optimize antibody binding sites. The scFv antibody isolated was then recombinantly engineered with a human immunoglobulin G1 Fc region to construct the fully human antibody scFv-FcC21, which recognized tumors of neuroectodermal origin, various types of carcinomas, mesotheliomas, and sarcomas as well as myeloid leukemias. scFv-FcC21 inhibited in vitro growth and migration of tumor cells and in vivo growth of human tumor xenografts. These effects were mediated by inhibition of the activation of extracellular signal-regulated kinase and focal adhesion kinase signaling pathways that are critical for tumor cell growth and migration, respectively. Our findings define the CSPG4-specific fully human scFv-FcC21 antibody as a candidate therapeutic agent to target the many types of tumors that express CSPG4.
Introduction
The beneficial effects of tumor antigen-specific monoclonal antibody (mAb)-based immunotherapy on the clinical course of some hematologic and solid malignancies (1) have reinforced our interest in continuing our studies to optimize an mAb that targets the chondroitin sulfate proteoglycan 4 (CSPG4). This cell surface tumor antigen, also known as high-molecular-weight melanoma–associated antigen, is involved in the activation of several signaling pathways that play an important role in tumor cell proliferation, survival, and migration as well as in tumor progression (2–4).
CSPG4 is expressed on melanoma cells and on various types of carcinoma and sarcoma cells as well as on myeloid leukemic cells (5). Because of its high expression on tumor cells, including cancer-initiating cells; its restricted distribution in normal tissues; and its role in tumor cell biology, CSPG4 has been used as a target of antibody-based immunotherapy in patients with melanoma (6, 7). Induction of CSPG4-specific antibodies was associated with regression of metastases in a few patients (8) and statistically significant survival prolongation (6, 7, 9). This association is likely to reflect a cause–effect relationship between induction of CSPG4-specific antibodies and apparent clinical benefit, as administration of a CSPG4-specific mAb to immunodeficient mice grafted with CSPG4+ human cell lines inhibited their growth, recurrence of disease, and/or metastasis (4, 10, 11). The latter findings have prompted us to develop an immunotherapeutic strategy with a CSPG4-specific mAb for the treatment of tumors that express this antigen. However, the clinical application of CSPG4-specific mAb-based immunotherapy is hampered by the lack of a chimeric, humanized, or fully human CSPG4-specific mAb. To overcome this limitation, we have generated a CSPG4-specific fully human single chain of variable regions of heavy and light chain (scFv)-Fc antibody, which eliminates the mouse sequence-specific immune responses that patients may develop when injected with antibodies engineered from mouse mAb (12, 13). The scFv-Fc format with an approximate molecular weight of 100 kDa was preferred to a whole immunoglobulin G (IgG) with an approximate molecular weight of 150 kDa. The latter is expected to have a lower tumor-targeting/penetrating ability than the former, given the inverse relationship between an antibody's size and its tumor penetration (14). In this article, we describe the isolation of the CSPG4-specific human scFv C21 and the construction of the fully human scFv-FcC21 antibody. Furthermore, we characterize the specificity of this antibody and the mechanisms underlying its antitumor activity in vitro and in vivo.
Materials and Methods
Cell lines, cell lysates, and tissues
The melanoma cell lines Colo38, FO-1, M14, M21, Melur, MV3, and SK-MEL-28; the glioma cell line LN443; the head and neck squamous cell carcinoma (SCCHN) cell line PCI30; the breast carcinoma cell lines MDA-MB-231 and T47D; the mesothelioma cell line PPM-Mill; the bladder carcinoma cell line T24; the prostate carcinoma cell line PC3; the osteosarcoma cell line MG-63; the B-lymphoid cell lines JY, LG-2, LKT13, and Raji; and the myeloid leukemia cell line ML-2, which are all of human origin; the rat neural cell line B49; and the mouse myeloma cell line P3X63Ag8.653 were maintained in RPMI-1640 medium supplemented with 2 mmol/L -glutamine (Cell-gro) and 10% FBS (PAA Laboratories Inc). This medium is referred to as complete medium. The M14/CSPG4 cells, which express CSPG4 following transfection of the parental cells with a plasmid DNA of pcDNA 3.1 TM(+)/full length of CSPG4 DNA construct (2), were grown in complete medium supplemented with G418 (Promega; 0.4 mg/mL). The human primary chondrosarcoma cell line KC was cultured as described (15). Cells were cultured at 37° C in a 5% CO2 atmosphere. Cell lysates were prepared as described (16). The cell line Colo38 was obtained from G. Moore (Denver General Hospital, Denver, CO) in 1977; FO-1 from P.B. Fisher (Columbia University, New York, NY) in 1982; M14 and M21 from D.L. Morton (John Wayne Cancer Institute, Los Angeles, CA) in 1977; Melur from D.J. Ruiter (University Medical Center, Nijmegen, Nijmegen, Netherlands) in 1984; MV3 from K. Zanker (Witten/Herdecke University, Witten, Germany) in 1985; SK-MEL-28 from AN. Houghton (Memorial Sloan-Kettering Cancer Center, New York, NY) in 1992; LN443 from S.-Y Cheng (University of Pittsburgh Cancer Institute, Pittsburgh, PA) in 2011; PCI30 from A. Deleo (University of Pittsburgh Cancer Institute, Pittsburgh, PA) in 2010; MDA-MB-231 and T47D from T. Clay (Duke University Medical Center, Durham, NC) in 2007; PPM-Mill from M. Carbone (University of Hawaii Cancer Center, Honolulu, HI) in 2008; T24, PC3, and P3X63Ag8.653 from American Type Culture Collection in 1993, 1995, and 1985, respectively; MG-63 and KC from J.H. Schwab (Massachusetts General Hospital, Boston, MA) in 2009 and 2006, respectively; JY, LG-2, LKT13, and Raji from R.A Reisfeld (Scripps Research Institute, La Jolla, CA) in 1977; ML-2 from M. Wetzler (Roswell Park Cancer Institute, Buffalo, NY) in 2007; and B49 from W.B. Stallcup (Sanford-Burnham Medical Research Institute, La Jolla, CA) in 1998.
Cell lines were monitored at least every 3 months for expression of human leukocyte antigen (HLA) class I and II antigens, of tumor antigens, and of adhesion molecules, and for sensitivity to zeocin. Lesions of melanocytic origin were obtained from patients who had undergone surgery in the Department of Dermatology at Kumamoto University School of Medicine (Kumamoto, Japan). The diagnosis of melanoma lesions was based on histopathologic characteristics. Frozen and formalin-fixed melanoma tissue sections were prepared as described previously (16). Normal tissue microarray (Cat#FBN401) was purchased from US Biomax, Inc.
Monoclonal and polyclonal antibodies, scFv antibodies, and reagents
The CSPG4-specific mAb 149.53, 225.28, 763.74, TP61.5, VF1-TP34, and VF1-TP41.2 (5); the B7-H3–specific mAb 376.96 (17); the HLA class I antigen-specific mAb TP25.99 (18); the c-myc (all our scFv except scFv-Fc are c-myc–tagged) oncoprotein-specific mAb 9E10 (19); the anti-idiotypic (anti-id) mAb MK2-23 (20); and the calnexin-specific mAb TO-5 (21) were derived from immunized mice as described. The CSPG4-specific human scFv #28 (22), #61 (16), and #70 (22), and the anti– anti-idiotypic (anti–anti-id) scFv119 (23), which recognizes the anti-id mAb MK2-23, were isolated from the synthetic scFv library (#1; ref. 24) by panning with melanoma cells S5, purified CSPG4, melanoma cells SK-MEL-28, and anti-id mAb MK2-23, respectively. The human scFv F98 and W34, which recognize unrelated tumor cell surface antigens (unpublished data) were isolated from the semisynthetic scFv library (25) by panning with the melanoma cells FO-1 and WM1158, respectively. Mouse mAbs were purified from ascitic fluid as described (26). The purity and activity of mAb, scFv, and scFv-Fc preparations were assessed bySDS-PAGE andbytesting with each corresponding antigen in a binding assay, respectively. Purified mAb and scFv antibodies were biotinylated using NHS-LC-biotin (Pierce) according to the manufacturer's instructions. Purified scFv-Fc was labeled with Cy3, using the Cy3 mAb Labelling Kit (GE Healthcare) according to the manufacturer's instructions.
The CD20-specific rituximab (Hillman Pharmacy) antibodies specific for extracellular signal-regulated kinase (ERK)1/2, phosphorylated (p) ERK1/2(Thr202/Tyr204), and p-Histone H3(Ser10; Cell Signaling Technology); antibodies specific for focal adhesion kinase (FAK) and p-FAK (Tyr397; BD Bioscience), horseradish peroxidase (HRP)–anti-mouse IgG Fc antibodies and R-phycoerythrin (RPE)-F(ab′)2 fragments of goat anti-human IgG Fcγ antibody (Jackson Immu-noResearch Laboratories Inc.), RPE-F(ab′)2 fragments of goat anti-mouse Ig antibodies (BD Pharmingen), and Strep-tavidin–HRP conjugate (SA-HRP; Pierce) were purchased from the indicated companies. Short hairpin RNA (shRNA) lentivirus was provided by our in-house Vector Core Facility. The Enzymatic Protein Deglycosylation Kit was purchased from Sigma.
Animals
C.B-17 severe combined immunodeficient (SCID) mice (8– 10 weeks old) were purchased from Taconic Farms, Inc. All animal studies have been approved bythe Institutional Animal Care and Use Committee.
Phage display scFv library
A semisynthetic phage display scFv antibody library with designed CDR3 was constructed as described (25).
Selection of phage display scFv antibodies
Phage display scFv antibodies binding to m Sigma.elanoma cells were isolated from the phage display scFv antibody library (25) using the panning technique as described (22).
Preparation and purification of soluble scFv antibodies
Soluble scFv antibodies in supernatants or from the periplasmic space (periplasmic preparation) of individual bacterial colonies were produced as described (27). scFv antibodies were purified from supernatants or periplasmic preparation by affinity chromatography on a column, in which mAb 9E10 was immobilized on a HiTrap NHS-activated sepharose (Amersham) following the manufacturer's instructions.
Construction of fully human scFv-Fc genes
The procedure used is detailed in the Supplementary data.
Fully human scFv-Fc antibody expression and purification
The procedure used is detailed in the Supplementary data.
Binding assays
The binding assays with scFv C21/scFv-FcC21 antibodies were carried out as described (27). The procedures used are detailed in the Supplementary data.
shRNA knockdown of CSPG4 expression
The procedure used is detailed in the Supplementary data.
Indirect immunoprecipitation and SDS-PAGE
Solubilization of cells labeled with 125I (Na125I; Amersham), immunoprecipitation, SDS-PAGE, and autoradiography were carried out as described (16) except for the use of GammaBind plus sepharose (Amersham) instead of protein A sepharose coated with rabbit anti-mouse IgG antibodies. Labeling of cells with 35S-methionine (Trans-35S-label; ICN Biochemicals) in the presence of tunicamycin (Sigma) and fluorography were carried out as described (16).
Binding kinetics
Binding kinetics of scFv-FcC21 to CSPG4-expressing cells was analyzed as described (28). The procedure used is detailed in the Supplementary data.
Internalization assay
The procedure used to test the internalization of antibodies into melanoma cells is detailed in the Supplementary data.
CSPG4 deglycosylation
The procedure used to deglycosylate CSPG4 is detailed in the Supplementary data.
Immunohistochemical staining of tissue sections
The indirect immunoperoxidase staining of frozen and formalin-fixed tissue sections with scFvC21 and scFv-FcC21 was carried out as described previously (16) and in the Supplementary data.
Antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity assays
Antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity assays were carried out as described (29). The procedures used are detailed in the Supplementary data.
Cell growth and migration assays
These assays were carried out as described elsewhere (11).
Western blotting
Western blot assay for signaling-related proteins was carried out as described (11).
Antitumor activity of scFv-FcC21 in mice bearing established human melanoma cell-derived lung metastasis
The detailed protocol is described in the Supplementary data.
Statistical analysis
The statistical significance of the difference between the results obtained in the tested groups was analyzed using the Student t test. Survival statistics was analyzed using MedCalc software free trial (Mariakerke).
Results
Isolation of scFv C21 by panning the semisynthetic phage display scFv antibody library with CSPG4+ cells Colo38
Forty clones, which were isolated from the semisynthetic phage display scFv antibody library by panning 3 times with Colo38 cells, were screened in ELISA with CSPG4+ cells Colo38, and with CSPG4− B lymphoid cells LG2. The CSPG4-reactive clone scFv C21 was selected for additional studies because of its selective strong reactivity with Colo38 cells. Because scFv C21 tends to aggregate at a concentration of more than 1 mg/mL, we generated scFv-FcC21. The scFv C21 and scFv-FcC21 displayed superimposable reactivity patterns with cells; therefore, they have been used interchangeably in the experiments to be described.
Analysis of the specificity of scFv C21/scFv-FcC21
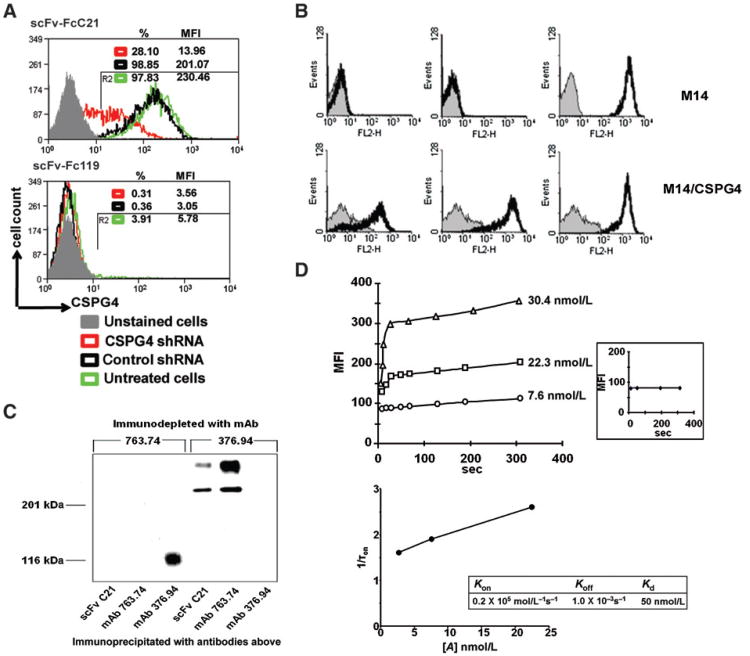
When tested in ELISA with a panel of human cell lines with differential CSPG4 expression, and with the rat neural cell line B49, which expresses the CSPG4 homolog NG2 (30), scFv C21 reacted only with the melanoma cell lines Colo38, FO-1, SK-MEL-28, and Melur. All of them are the only ones in the panel of the cell lines tested to express CSPG4, suggesting that scFv C21 is specific for CSPG4 (Supplementary Fig. S1). This possibility was proven by 4 lines of evidence. First, the reactivity of scFv-FcC21 with MV3 melanoma cells was reduced by at least 70% when CSPG4 expression was knocked down by transduction with CSPG4-specific shRNA lentivirus (Fig. 1A). Second, scFv C21 stained M14 cells, which had acquired CSPG4 expression following stable transfection with the full-length CSPG4 cDNA, but did not stain the parental M14 cells (Fig. 1B). Third, scFv C21 immunoprecipitated 2 moieties with an electrophoretic profile similar to that of CSPG4 components immunoprecipitated by CSPG4-specific mouse mAb from 125I-labeled Colo38 cells. Finally, in sequential immunoprecipitation experiments scFv C21 did not immunoprecipitate any components from a Colo38 cell lysate, which had been immunodepleted with the CSPG4-specific mouse mAb 763.74 (5). The immunodepletion is specific, as scFv C21 immunoprecipitated the CSPG4 components from a Colo38 cell lysate immunodepleted with the B7-H3-specific mAb 376.96 (ref. 17; Fig. 1C).
Figure 1.
CSPG4 specificity of scFvC21/scFv-FcC21. A, shRNA knockdown of CSPG4 expression resulting in reduced binding activity of scFv-FcC21. MV3 cells were transduced with either CSPG4-specific shRNA lentiviral particles or ABCB5-specific shRNA, which was used as a control. After 72 hours of lentiviral transduction, cells were harvested and stained with scFv-FcC21 (1 μg/100 μL) followed by incubation with RPE-goat anti-human IgG antibodies. scFv-Fc 119 was used as an isotype control. Cells were analyzed by flow cytometry. %, percentage of positive cells; MFI, mean fluorescence intensity. B, differential staining by scFv C21 of CSPG4+ M14/CSPG4 melanoma cells and CSPG4− parental M14 melanoma cells. M14/CSPG4 transfectants and parental CSPG4− M14 cells were incubated on ice with periplasmic preparation scFv C21 and mAb 9E10 (thick black line) and control periplasmic preparation scFv 119 and mAb 9E10 (thin gray line; left), with CSPG4-specific mouse mAb 763.74 (0.5 μg; thick black line) and isotype control mAb MK2-23 (thin gray line; middle) and with HLA class I antigen-specific mAb TP25.99 (thick black line) as a positive control and mAb MK2-23 as an isotype control (thin gray line; right). Binding of antibodies was detected using RPE-labeled F(ab′)2 fragments of goat anti-mouse Ig antibodies. Cells were analyzed with a FACScan flow cytometer. C, structural relationship between molecules recognized by scFv C21 and by CSPG4-specific mouse mAb 763.74 in a CSPG4+ Colo38 cell lysate. A 1% NP-40 extract of 125I-labeled Colo38 cells was immunodepleted with mAb 763.74. The immunodepleted cell extract was immunoprecipitated with insolubilized mAb 763.74 and scFv C21. Antigens were eluted and analyzed by SDS-PAGE in an 8% polyacrylamide gel. Gels were fixed, dried, and autoradiographed for up to 1 day at −80°C. A 1% NP-40 extract of 125I-labeled Colo38 cells immunodepleted with B7-H3-specific mouse mAb 376.94 was used as a specificity control. D, binding kinetics of scFv-FcC21 to MV3 cells measured by flow cytometry. MFI was measured at indicated times after mixing the complex of scFv-FcC21 (7.6, 22.3, and 30.4 nmol/L)/PE-secondary antibody with MV3 cells (top). Inset, MFIs were measured at indicated times after mixing the complex of scFv-Fc119 (30.4 nmol/L)/PE-secondary antibody with MV3 cells. The microscopic rate constants of the first-order binding reaction are related to the time constant of the fluorescence increase by 1/τon = koff + [A] × kon. The linear regression line shows the relationship between 1/τon (τon: time constant) and [A] (the concentration of antibody; bottom).
Binding kinetics and internalization of scFv-FcC21 to CSPG4+ living cells
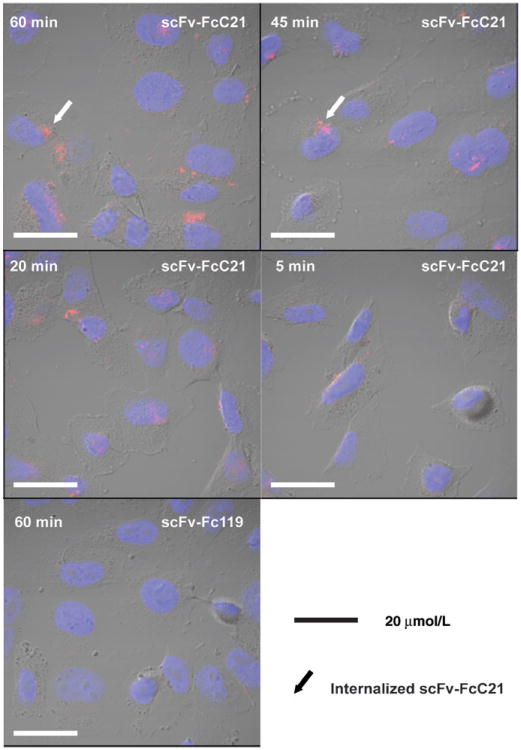
To obtain a physiologically relevant measurement of the binding kinetics of scFv-FcC21 to CSPG4, we used CSPG4+ living cells MV3 as the source of the antigen and flow cytometry. scFv-Fc119 was used as an isotype control. The dissociation constant of scFv-FcC21, kd (50 nmol/L) = koff (1.0 × 10−3 S−1)/kon (0.2 × 105 M−1 S−1), falls into a favorable range of an antibody for tumor percolation (ref. 31; Fig. 1D). Moreover, Cy3-labeled scFv-FcC21 was internalized following a 45-minute incubation with MV3 cells (Fig. 2).
Figure 2.
Internalization of scFv-FcC21 in melanoma cells. MV3 cells were incubated with Cy3-scFv-FcC21 for different times (5,20,45, or 60 minutes). Cy3-labeled scFv- Fc119 was used as an isotype control. Images were taken under a confocal fluorescence microscope (Olympus FV1000 confocal microscope; a ×60 objective was used plus a ×2 digital zoom). Arrows indicate internalized Cy3-scFv-FcC21 following a 45- and a 60-minute incubation. In contrast, Cy3-scFv-Fc119 was not nternalized at all the time points tested; the image taken following a 60-minute incubation is shown here.
Characterization of the scFv C21/scFv-FcC21 defined epitope
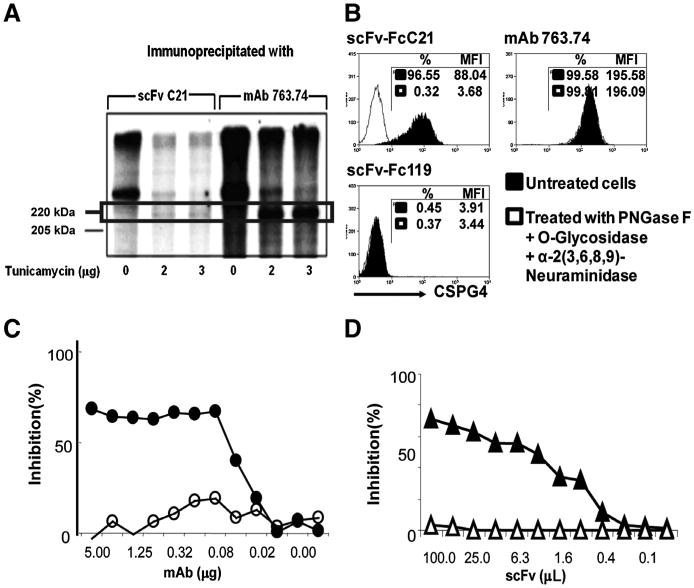
Carbohydrates appear to play a major role in the expression of the epitope defined by scFv C21 because of the marked reduction in the intensity of the CSPG4 components immunoprecipitated from Colo38 cells intrinsically labeled with 35S-methionine in the presence of the N-glycosylation inhibitor tunicamycin. The inhibition of N-glycosylation is indicated by the accumulation of the 220-kDa precursor recognized only by mAb 763.74 which defines a CSPG4 peptide epitope (Fig. 3A). In addition, the reactivity of scFv-FcC21 with MV3 cells was lost following incubation with deglycosylation enzymes (Fig. 3B).
Figure 3.
Characterization of the CSPG4 epitope recognized by scFv C21/scFv-FcC21. A, role of N-linked glycosylation in the expression of the epitope recognized by scFv C21 on CSPG4 isolated from a CSPG4+ Colo38 cell extract. A 1% NP-40 extract of Colo38 cells labeled with 35S-methionine in the presence of tunicamycin (0, 2, and 3 μg/mL) was immunoprecipitated with scFv C21. Antigens were eluted from the mmunoadsorbent and analyzed by SDS-PAGE in an 8% PAGE. Gels were fixed, dried, and processed for fluorography for up to 3 days at −80°C using Hyperfilm-ECL. CSPG4-specific mouse mAb 763.74 was used as a control. B, loss of reactivity of scFv-FcC21 with CSPG4+ cells incubated with deglycosylation enzymes. MV3 cells were treated with deglycosylation enzymes for 24 hours at 37°C. The deglycosylated cells were harvested and stained with scFv-FcC21 (1 μg/100μL). scFv-Fc119 was used as an isotype control. mAb 763.74 was used as a positive control, as it recognizes a peptide epitope on CSPG4; thereby its reactivity to CSPG4 is not affected by the treatment with deglycosylation enzymes. %, percentage of positive cells. Cand D, spatial proximity of the epitopes defined byscFvC21 and by mouse mAb VF1-TP34on CSPG4+melanoma cells SK-MEL-28. In C, the indicated amounts of CSPG4-specific mAb VF1-TP34 (—•—) were mixed with biotinylated scFv C21 (0.25 μg per well). In D, the indicated amounts of PPscFv C21 (— ▲—) were mixed with biotinylated mAb VF1-TP34 (40 μg per well). The mixtures were then transferred to wells containing CSPG4+ cells and incubated for 1 hour at 4°C. Binding of antibodies was detected using SA-HRP. Results are expressed as percentage of inhibition. The unrelated mAb TP25.99 (—○—) and the unrelated PP scFv 119 (—Δ—) were used as specificity controls.
Competition experiments showed that the epitopes recognized by scFv C21 and by mouse mAb VF1-TP34 are distinct, but spatially close, as they partially inhibited in a dose-dependent fashion the binding of each other to CSPG4+ SK-MEL-28 cells (Fig. 3C and D). In contrast, the scFv C21-defined epitope is distinct and spatially distant from those recognized by the mouse mAb 149.53,225.28, 763.74, TP61.5, and VF1-TP41.2 and by the human scFv #28, #61, and #70, as the latter antibodies did not inhibit the binding of scFv C21 to SK-MEL-28 cells (data not shown).
Staining of lesions of melanocytic origin by scFv C21/scFv-FcC21
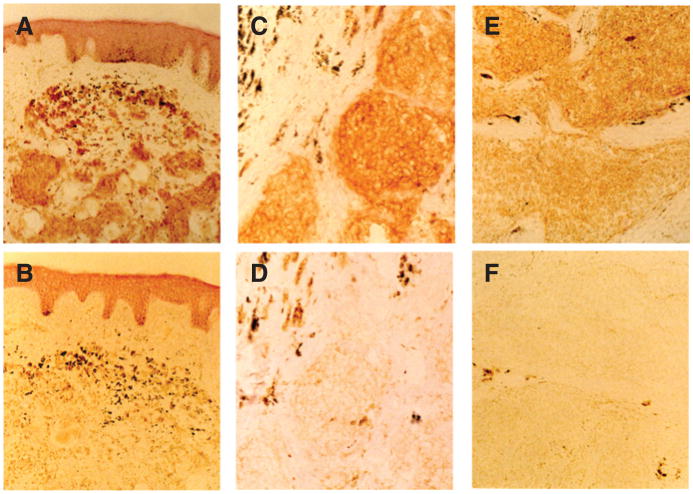
scFv C21 stained frozen melanocytic lesions in the immu-noperoxidase reaction, but did not stain formalin-fixed and paraffin-embedded (FFPE) melanocytic lesions. The staining was both membranous and cytoplasmic (Fig. 4A-F). scFv C21 and CSPG4-specific mouse mAb 763.74 stained homogeneously (>80% of cells were positively stained) 4 pigmented nevi with a membranous and a cytoplasmic pattern. Furthermore, mAb 763.74 stained 14 of 15 primary lesions with a homogeneous pattern, whereas scFv C21 stained 9 with a homogenous pattern and 2 with a heterogeneous (<80% of cells were positively stained) pattern. The staining was membranous in 1 lesion, membranous and cytoplasmic in 4, and cytoplasmic in 6. Finally, of the 6 metastatic lesions stained by mAb 763.74 with a homogeneous pattern, 3 were stained by scFv C21 with a homogeneous pattern and 3 with a heterogeneous pattern. The staining was membranous and cytoplasmic in 3 lesions and cytoplasmic in the remaining 3. The staining is specific as scFv C21 stained CSPG4+ M14/CSPG4 cell pellet–derived frozen sections, which served as a positive control, but did not stain CSPG4− M14 cell pellet–derived frozen sections, which serveds a negative control. In contrast, no staining of normal tissues was detected when a tissue microarray composed of 20 normal human tissues was stained with scFv-FcC21 (Supplementary Table S1).
Figure 4.
Immunohistochemical staining with scFv C21 of frozen sections of human melanocytic lesions. Benign pigmented nevus (A), primary melanoma (C), and metastatic melanoma (E) show homogeneous cytoplasmic and cytoplasmic membrane staining with scFv C21. No staining by scFv 119 (negative control) of corresponding sections of nevus (B), as well as primary (D), and metastatic melanoma (F) lesions was detected.
Reactivity of scFv-FcC21 with a panel of CSPG4+ malignant cell lines
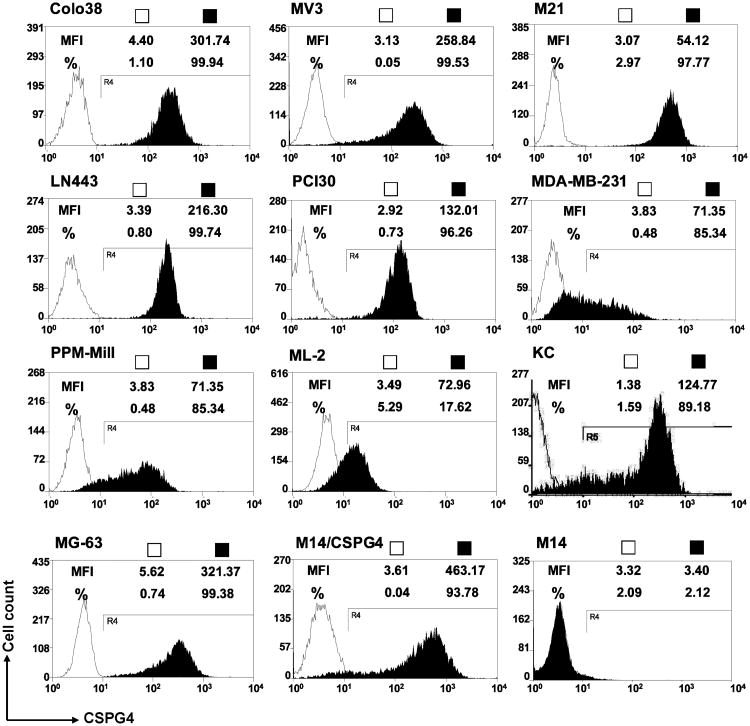
Flow cytometric analysis showed that scFv-FcC21 stained a panel of human malignant cell lines, all of which express CSPG4. Representative examples include the melanoma cell lines Colo38, MV3, and M21; the glioma cell line LN443; the SCCHN cell line PCI30; the breast carcinoma cell line MDA-MB-231; the mesothelioma cell line PPM-Mill; the primary chondrosarcoma cell line KC; the osteosarcoma cell line MG-63; and the myeloid leukemia cell line ML-2 (Fig. 5). The staining is specific for CSPG4 for the following reasons: (i) the reactivity pattern of scFv-FcC21 with the panel of cell lines is superimposable to that of the CSPG4-specific mouse mAb 225.28 (data not shown); (ii) scFv-FcC21 did not stain M14 cells which do not express CSPG4; and (iii) the isotype control scFv-Fc119 did not stain any of the cell lines tested.
Figure 5.
Staining by scFv-FcC21 of a panel of tumor cell lines. The human melanoma Colo38, MV3, and M21, glioma LN443, SCCHN PCI30, basal breast carcinoma MDA-MB-231, mesothelioma PPM-Mill, chondrosarcoma KC, osteosarcoma MG-63, and myeloid leukemia ML-2 were sequentially incubated with scFv-FcC21 (■) or isotype control scFv-Fc119 (□) and RPE-labeled anti-human IgGFcγ antibody.The stained cells were analyzed by flow cytometry. The percentages of cells stained by scFv-FcC21 or scFv-Fc119 in each cell line and the MFI are shown in each histogram. The specificity of the staining was monitored using the human melanoma cell line M14, which acquires CSPG4expression following transfection with CSPG4 cDNA, and the parental melanoma cells M14 which do not express CSPG4.
Functional in vitro properties of scFv-FcC21
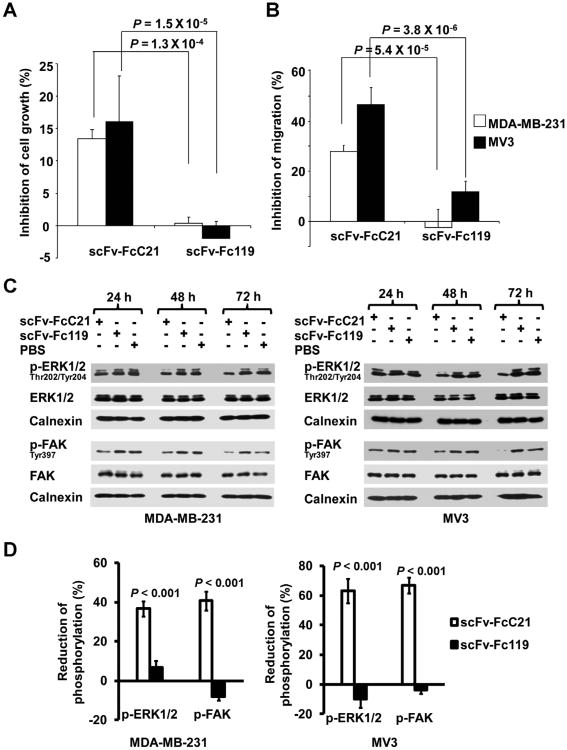
Comparison of the functional properties of scFv-FcC21 and of scFv C21 showed that the latter is markedly less active than the former one (data not shown). Therefore, only scFv-FcC21 was used in functional assays. scFv-FcC21 mediated neither antibody-dependent cell-mediated cytotoxicity nor complement-dependent cytotoxicity of target cells (Supplementary Fig. S2). In contrast, scFv-FcC21 inhibited the growth in vitro of CSPG4+ MDA-MB-231, and MV3 cells by 13% and 17%, respectively, when they were cultured in 3-dimensional Matrigel matrices (Fig. 6A and B). Furthermore, scFv-FcC21 inhibited the migration of MDA-MB-231 and MV3 cells toward fibro-nectin in a Boyden chamber assay by 28% and 50%, respectively. The inhibitory effects of scFv-FcC21 are likely to reflect its ability to interfere with the CSPG4 role in ERK and FAK signaling pathways, which are associated with tumor cell growth and motility. Incubation with scFv-FcC21 for up to 72 hours inhibited, in a time-dependent manner, the activation of p-ERK1/2 by 37% and 63% and of FAK by 40% and 67% in MDA-MB-231 and MV3 cells, respectively (Fig. 6C and D).
Figure 6.
Inhibition by scFv-FcC21 of MDA-MB-231 and MV3 cell growth and migration in vitro. A, cells (5 × 104 per well) were seeded in a 96-well plate containing Matrigel (growth factor-reduced Matrigel-CB-40230; BD Biosciences) diluted 4 times in serum-free RPMI-1640 medium. Then, either PBS-diluted scFv-FcC21 (0.25 mg/mL), control scFv-Fc119 (0.25 mg/mL), or PBS were added (total volume 200 μL per well). Following a 6-day incubation at 37°C in a 5% CO2 atmosphere, cells in each well were harvested from Matrigel and counted by 2 individuals using Trypan Blue. The results are expressed as percentage inhibition of cell growth, using the values obtained in PBS only as a reference. The values shown are the mean of the results obtained in 3 independent experiments. B, cells (5 × 104 per well) were seeded in a 24-Transwell plate with scFv-FcC21 (0.25 mg/mL), control scFv-Fc1 19(0.25 mg/mL), or PBS. Cells migrated toward serum-free RPMI-1640 medium containing 10 μg/mL fibronectin. After a 48-hour incubation at 37°C, migrated cells were stained with HEMA 3 stain set, images were taken and counted under a Zeiss Inverted Fluorescence Microscope (AxioVision Software). The means of 6 independent fields(×200) were determined. The results are expressed as percentage of inhibition of cell migration, using the values obtained in PBS only as a reference. The values shown are the mean of the results obtained in 3 independent experiments. C, MDA-MB-231 and MV3 cells were treated with scFv-FcC21 (0.1 mg/mL), the control scFv-Fc1 19(0.1 mg/mL), or PBS at 37°C for 24, 48, and 72 hours. Representative immunoblot analyses of p-ERK1/2(Thr202/Tyr204), ERK1/2, p-FAK(Tyr397), and FAK are shown. Calnexin was used as the loading control. D, densitometric analyses (NIH ImageJ software) of p-ERK1/2 and p-FAK in MDA-MB-231 and MV3 cells treated with scFv-FcC21 for 72 hours are shown. The results are expressed as percentage of reduction of the phosphorylated proteins using the values obtained from PBS-treated cells as a 100% reference. The experiment was carried out 3 independent times. p, phosphorylated.t1
Inhibition by scFv-FcC21 of established human melanoma cell–derived lung metastases in vivo
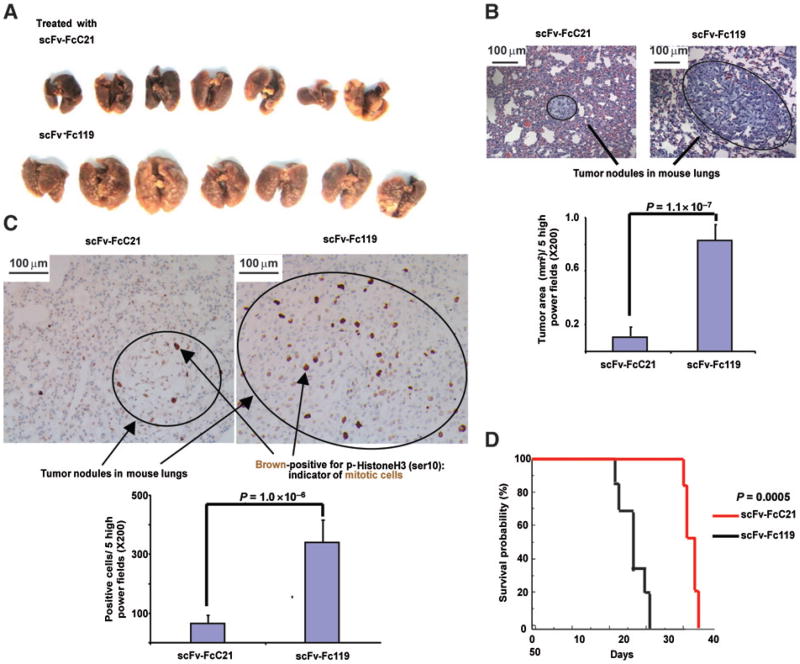
scFv-FcC21 inhibited the growth of established experimental lung metastases in SCID mice that had been injected i.v. with the human melanoma cell lines MV3 or M21. The lungs harvested from the mice injected i.v. 3 times with scFv-FcC21 were smaller and had a much smoother surface than those harvested from the mice treated with an isotype control (Fig. 7A). Furthermore, quantitative histologic examination revealed that scFv-FcC21 had caused an 87% and a 95% reduction in the size of established lung metastatic nodules in the mice injected with the cell lines MV3 and M21, respectively, as compared with the mice treated with the isotype control scFv-Fc119 (Fig. 7B and Supplementary Fig. S3). Metastases were also evaluated for the rate of tumor cell proliferation using the surrogate marker p-Histone H3. scFv-FcC21 reduced by 80% and 89% the number of mitotic cells in the established lung metastatic nodules in the mice injected with the cell lines MV3 and M21, respectively, as compared with the mice treated with the isotype control scFv-Fc119 (Fig. 7C and Supplementary Fig. S3). In addition, scFv-FcC21 significantly prolonged the survival of mice bearing MV3 cell–derived lung metastases as compared with the mice treated with the isotype control antibody (Fig. 7D).
Figure 7.
Reduction by scFv-FcC21 of established experimental lung metastases in vivo. Human melanoma cells MV3 (1.4 × 106 per mouse) were injected intravenously on day 0 to 26 mice which were randomized into 2 groups (13 per group). Starting on day 15, one group was injected intravenously with scFv-FcC21 (100 μg per mouse) and the other group was injected i.v. with the control antibody scFv-Fc1 19 every 48 hours for a total of 3 injections. On day 20, 7 mice per group were sacrificed and the lungs were collected and fixed in 10% formalin (A) and paraffin-embedded. The sections of FFPE tissue (5 sections per slide per mouse) were subjected to the following analysis: the sizes/areas of metastatic nodules [total nodules examined: 37 in scFv-FcC21-treated group and 45 in scFv-Fc119-treated group using 5 randomly selected high-power fields (×200) per each section; OLYMPUS BX51 microscope; OLYMPUS UK Ltd.] were measured and calculated by the SPOT IMAGING SOFTWARE Advanced (Diagnostic Instruments, Inc.) The values shown are the mean tumor area of each group (B); the number of proliferating tumor cells was detected by staining p-Histone H3 protein in lung FFPE sections and determined by counting 5 fields per slide per mouse (× 200; C).D, the values shown are the mean number of mitotic tumor cells in each group. The remaining 6 mice per group were monitored for survival.
Discussion
The specific reactivity with CSPG4+ cells, the marked reduction of reactivity with cells caused by shRNA knock-down of CSPG4 expression, and the molecular profile of the moieties immunoprecipitated from CSPG4+ cells indicates the CSPG4 specificity of scFv C21. This scFv was isolated from a semisynthetic phage display scFv antibody library by panning with the cultured CSPG4+ human melanoma cells Colo38. CSPG4 is an attractive target to implement antibody-based immunotherapy of malignant diseases for several reasons. First, CSPG4 is highly expressed on malignant cells, but has a restricted distribution in normal tissues (32). This distribution accounts for the selective targeting of malignant lesions when radiolabeled CSPG4-specific mAbs have been injected in patients with CSPG4+ melanoma lesions (33, 34). Furthermore, these results altogether provide an explanation for the lack of side effects in patients with melanoma who developed CSPG4-specific antibodies following immunization with CSPG4 mimics, in spite of an apparent antitumor effect (7, 8). Second, CSPG4 has a higher expression on activated pericytes in the tumor microenvi-ronment than on resting pericytes in other anatomic sites (4, 11). This differential distribution of CSPG4 accounts for the lack of association of the antiangiogenic effects mediated by immunotargeting of CSPG4 in the tumor microen-vironment with side effects associated with blood vessel damage such as delay in wound healing (35). Third, the distribution of CSPG4 on tumors of different embryologic origin is broader than what we originally reported (5). Therefore, immunotherapeutic strategies targeting CSPG4 may be applicable to a variety of malignant diseases. Fourth, at least in HNSCC and in triple-negative breast carcinoma, CSPG4 is expressed on cancer-initiating cells (4, 11). As a result, CSPG4 can mediate immunotargeting of these cells, which, according to the cancer stem cell theory (36, 37), have to be eradicated in order for immunotherapy to be effective. Finally, scFv-FcC21 is able to internalize into tumor cells, thereby providing its potential, when conjugated with a cytotoxic agent, as a CSPG4+ tumor-selective antibody–drug conjugate.
Carbohydrates play a role in the expression of the scFv C21 defined epitope, as scFv C21 immunoprecipitated a markedly reduced level of CSPG4 from melanoma cells which synthesize the antigen in the presence of tunicamycin, an inhibitor of N-glycosylation of glycoproteins (38). This conclusion was corroborated by the lack of reactivity of scFv-FcC21 with CSPG4+ MV3 cells treated with a pool of endoglycosidases. Regardless of its chemical nature, the scFv C21 defined epitope, like those recognized by CSPG4-specific mouse mAb (5, 39), is expressed not only on cell lines but also in surgically removed lesions of melanocytic origin and has a heterogeneous distribution at the cellular and at the molecular level.
scFv C21 shares some characteristics with the CSPG4-specific human scFv antibodies we have previously described (16, 22). Like scFv#70 (22), scFv C21 immunoprecipitates both subunits of CSPG4 from melanoma cells. This finding may reflect the expression of the epitope recognized by scFv C21 on both CSPG4 components, provided that the latter are not noncovalently associated. Furthermore, like scFv #70 (22), scFv C21 recognizes an epitope that is not shared with xenogeneic CSPG4 counterparts and is distinct, but spatially close to that recognized by one CSPG4-specific mouse mAb. The latter finding is compatible with similarities in the recognition of CSPG4 by the human and mouse immune systems, provided that the specificities of the human scFv antibodies isolated from the semisynthetic phage display scFv antibody library are representative of the repertoire of the human immunesystem. Finally, like scFv #28 (22) and #61 (16), scFv C21 recognizes an epitope which is carbohydrate in nature. However, scFv C21 isolated from the semisynthetic phage display scFv antibody library, which has highly desir-ableantibody binding sites because of designed CDR3 (25), has a higher association constant than the CSPG4-specific scFv #28 (22), #70 (22), and #60 (16) isolated by us previously from the synthetic scFv library (#1; data not shown; ref. 24). At variance with other scFv antibodies described in the literature (40), scFv C21 is stable even at low concentrations but forms aggregates at higher concentrations. However, like other scFv antibodies, scFv C21, which has an approximate molecular weight of 27 kDa, has the limitation to have rapid in vivo clearance with a short half-life (41) and lacks immunologic effector functions contributed by the Fc region of IgG. To overcome these limitations, we have generated a fully human scFv-Fc antibody by fusing scFv C21 to a human IgG1Fc region. This molecule, which, as a monomer, has an approximatesize of 50 kDa, forms dimersas shown by SDS-PAGE and Western blot analysis (data not shown). Human IgG1Fc region was selected to generate the fully human scFv-Fc antibody, as among the human Fc isotypes it is the most effective in activating complement and has the highest affinity to Fc receptors (42). Nevertheless, scFv-FcC21 mediated neither cell-dependent cytotoxicity nor complement-dependent cytotoxicity of CSPG4+ cells in 51Cr release assays. This finding may reflect the location of the epitope recognized by scFv-FcC21 at a site of CSPG4 distant from cell membrane (43–46).
As shown by others and by us, CSPG4 is involved in the activation of several signaling pathways, including ERK and FAK, which are known to play an important role in tumor cell proliferation and migration as well as in tumor progression (2–4). As a result, blocking, by scFv-FcC21 of CSPG4 related ERK and FAK pathway signaling, inhibited effectively tumor cell in vitro growth and migration as well as in vivo proliferation. In addition, the desirable affinity of scFv-FcC21 (10−8 mol/L) for tumor percolation and retention (31, 47) may also play a role in its marked in vivo antitumor activity. However, the dose of scFv-FcC21 to be used for the treatment of solid tumors has to be optimized. It is noteworthy that the antitumor activity of scFv-FcC21 is not markedly influenced by the CSPG4 expression level on target cells, as the extent of inhibition of tumor growth was similar for MDA-MB-231 and MV3 cells, which have medium to low [mean fluorescence intensity (MFI): 71] and high (MFI: 258) CSPG4 expression, respectively. Finally, repeated (2 injections/week) i.v. administrations of scFv-FcC21 (100 μg/injection) for 2 months caused no detectable side effects in mice, such as loss of body weight and delay in wound healing (unpublished results). Nevertheless, the latter data should be interpreted with caution, as the available evidence suggests that the scFv-FcC21 defined epitope is not expressed by mouse cells (data not shown).
In conclusion, by combining phage display scFv library technology and recombinant DNA cloning technology we have generated the first CSPG4-specific fully human scFv-Fc antibody. Furthermore, we have characterized the spectrum of reactivity of scFv-FcC21 with a panel of malignant tumor cell lines. Finally, we have analyzed the in vitro and in vivo antitumor activities and underlying mechanisms of action of this human antibody. Collectively, our results suggest that scFv-FcC21 antibody holds promise to develop immunotherapeutic strategies for the clinical treatment of CSPG4-expressing tumors.
Supplementary Material
Acknowledgments
The authors thank Xiaojuan Deng for the preparation of mouse mAb and human scFv-Fc antibodies; Dr. Alain Trautmann, Institut Cochin, Paris, France, for his generous help in determining the binding kinetics of scFv-FcC21; and Dr. Can Fang, Department of Medicine, Division of Gastroenterology, Hepatology, Nutrition, University of Pittsburgh, for using the MATLAB software to calculate the rate constants kon and koff.
Grant Support: This study was supported by The Elsa U Pardee Foundation (X. Wang), Hillman Foundation (X. Wang, S. Ferrone), RO3RFAPA-08-209 (X. Wang), RO1 CA105500 (S. Ferrone), RO1 CA138188 (S. Ferrone), and 5P30CA047904 (University of Pittsburgh Cancer Institute).
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Campoli M, Ferrone S. Immunotherapy of malignant disease: the coming age of therapeutic monoclonal antibodies. In: DeVita V, Hellman S, Rosenberg SA, editors. Cancer: principles & practice of oncology. New York: Lippincott Williams & Wilkins; 2009. pp. 1–18. [Google Scholar]
- 2.Yang J, Price MA, Neudauer CL, Wilson C, Ferrone S, Xia H, et al. Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms. J Cell Biol. 2004;165:881–91. doi: 10.1083/jcb.200403174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Price MA, Li GY, Bar-Eli M, Salgia R, Jagedeeswaran R, et al. Melanoma proteoglycan modifies gene expression to stimulate tumor cell motility, growth, and epithelial-to-mesenchymal transition. Cancer Res. 2009;69:7538–47. doi: 10.1158/0008-5472.CAN-08-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Wang Y, Yu L, Sakakura K, Visus C, Schwab JH, et al. CSPG4 in cancer: multiple roles. Curr Mol Med. 2010;10:419–29. doi: 10.2174/156652410791316977. [DOI] [PubMed] [Google Scholar]
- 5.Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteogly-can (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24:267–96. doi: 10.1615/critrevimmunol.v24.i4.40. [DOI] [PubMed] [Google Scholar]
- 6.Mittelman A, Chen ZJ, Kageshita T, Yang H, Yamada M, Baskind P, et al. Active specific immunotherapy in patients with melanoma. A clinical trial with mouse antiidiotypic monoclonal antibodies elicited with syngeneic anti-high-molecular-weight-melanoma-associated antigen monoclonal antibodies. J Clin Invest. 1990;86:2136–44. doi: 10.1172/JCI114952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittelman A, Chen ZJ, Yang H, Wong GY, Ferrone S. Human high molecular weight melanoma-associated antigen (HMW-MAA) mimicry by mouse anti-idiotypic monoclonal antibody MK2-23: induction of humoral anti-HMW-MAA immunity and prolongation of survival in patients with stage IV melanoma. Proc Natl Acad Sci U S A. 1992;89:466–70. doi: 10.1073/pnas.89.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittelman A, Chen ZJ, Liu CC, Hirai S, Ferrone S. Kinetics of the immune response and regression of metastatic lesions following development of humoral anti-high molecular weight-melanoma associated antigen immunity in three patients with advanced malignant melanoma immunized with mouse antiidiotypic monoclonal antibody MK2-23. Cancer Res. 1994;54:415–21. [PubMed] [Google Scholar]
- 9.Mittelman A, Chen GZ, Wong GY, Liu C, Hirai S, Ferrone S. Human high molecular weight-melanoma associated antigen mimicry by mouse anti-idiotypic monoclonal antibody MK2-23: modulation of the immu-nogenicity in patients with malignant melanoma. Clin Cancer Res. 1995;1:705–13. [PubMed] [Google Scholar]
- 10.Hafner C, Breiteneder H, Ferrone S, Thallinger C, Wagner S, Schmidt WM, et al. Suppression of human melanoma tumor growth in SCID mice bya human high molecular weight-melanoma associated antigen (HMW-MAA) specific monoclonal antibody. Int J Cancer. 2005;114:426–32. doi: 10.1002/ijc.20769. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Osada T, Wang Y, Yu L, Sakakura K, Katayama A, et al. CSPG4 protein asa new target for the antibody-based immunotherapy of triple-negative breast cancer. J Natl Cancer Inst. 2010;102:1496–512. doi: 10.1093/jnci/djq343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzales NR, Padlan EA, De Pascalis R, Schuck P, Schlom J, Kashmiri SV. SDR grafting of a murine antibody using multiple human germline templates to minimize its immunogenicity. Mol Immunol. 2004;41:863–72. doi: 10.1016/j.molimm.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Presta LG. Engineering of therapeutic antibodies to minimize immu-nogenicity and optimize function. Adv Drug Deliv Rev. 2006;58:640–56. doi: 10.1016/j.addr.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 14.Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52:3402–8. [PubMed] [Google Scholar]
- 15.Schwab JH, Boland PJ, Agaram NP, Socci ND, Guo T, O'Toole GC, et al. Chordoma and chondrosarcoma gene profile: implications for immunotherapy. Cancer Immunol Immunother. 2009;58:339–49. doi: 10.1007/s00262-008-0557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai SA, Wang X, Noronha EJ, Kageshita T, Ferrone S. Characterization of human anti-high molecular weight-melanoma-associated antigen single-chain Fv fragments isolated from a phage display antibody library. Cancer Res. 1998;58:2417–25. [PubMed] [Google Scholar]
- 17.Imai K, Wilson BS, Bigotti A, Natali PG, Ferrone S. A 94,000-dalton glycoprotein expressed by human melanoma and carcinoma cells. J Natl Cancer Inst. 1982;68:761–9. [PubMed] [Google Scholar]
- 18.Desai SA, Wang X, Noronha EJ, Zhou Q, Rebmann V, Grosse-Wilde H, et al. Structural relatedness of distinct determinants recognized by monoclonal antibody TP25.99 on beta 2-microglobulin-associated and beta 2-microglobulin-free HLA class I heavy chains. J Immunol. 2000;165:3275–83. doi: 10.4049/jimmunol.165.6.3275. [DOI] [PubMed] [Google Scholar]
- 19.Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–6. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusama M, Kageshita T, Chen ZJ, Ferrone S. Characterization of syngeneic antiidiotypic monoclonal antibodies to murine anti-human high molecular weight melanoma-associated antigen monoclonal antibodies. J Immunol. 1989;143:3844–52. [PubMed] [Google Scholar]
- 21.Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens. 2003;62:385–93. doi: 10.1034/j.1399-0039.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 22.Noronha EJ, Wang X, Desai SA, Kageshita T, Ferrone S. Limited diversity of human scFv fragments isolated by panning a synthetic phage-display scFv library with cultured human melanoma cells. J Immunol. 1998;161:2968–76. [PubMed] [Google Scholar]
- 23.Sharma S, Tammela J, Wang X, Arnouk H, Driscoll D, Mhawech-Fauceglia P, et al. Characterization of a putative ovarian oncogene, elongation factor 1alpha, isolated by panning a synthetic phage display single-chain variable fragment library with cultured human ovarian cancer cells. Clin Cancer Res. 2007;13:5889–96. doi: 10.1158/1078-0432.CCR-07-0703. [DOI] [PubMed] [Google Scholar]
- 24.Nissim A, Hoogenboom HR, Tomlinson IM, Flynn G, Midgley C, Lane D, et al. Antibody fragments from a ‘single pot’ phage display library as immunochemical reagents. EMBO J. 1994;13:692–8. doi: 10.1002/j.1460-2075.1994.tb06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Kruif J, Boel E, Logtenberg T. Selection and application of human single chain Fv antibody fragments from a semi-synthetic phage antibody display library with designed CDR3 regions. J Mol Biol. 1995;248:97–105. doi: 10.1006/jmbi.1995.0204. [DOI] [PubMed] [Google Scholar]
- 26.Temponi M, Kageshita T, Perosa F, Ono R, Okada H, Ferrone S. Purification of murine IgG monoclonal antibodies by precipitation with caprylic acid: comparison with other methods of purification. Hybridoma. 1989;8:85–95. doi: 10.1089/hyb.1989.8.85. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Campoli M, Ko E, Luo W, Ferrone S. Enhancement of scFv fragment reactivity with target antigens in binding assays following mixing with anti-tag monoclonal antibodies. J Immunol Methods. 2004;294:23–35. doi: 10.1016/j.jim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Boulla G, Randriamampita C, Raposo G, Trautmann A. Binding kinetics of soluble ligands to transmembrane proteins: comparing an optical biosensor and dynamic flow cytometry. Cytometry. 2000;40:76–80. doi: 10.1002/(sici)1097-0320(20000501)40:1<76::aid-cyto10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006;176:346–56. doi: 10.4049/jimmunol.176.1.346. [DOI] [PubMed] [Google Scholar]
- 30.Stallcup WB, Dahlin K, Healy P. Interaction of the NG2 chondroitin sulfate proteoglycan with type VI collagen. J Cell Biol. 1990;111:3177–88. doi: 10.1083/jcb.111.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujimori K, Covell DG, Fletcher JE, Weinstein JN. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J Nucl Med. 1990;31:1191–8. [PubMed] [Google Scholar]
- 32.Ferrone S, Temponi M, Gargiulo D, Scassellati GA, Cavaliere R, Natali PG. Selection and utilization of monoclonal antibody defined melanoma associated antigens for immunoscintigraphy in patients with melanoma. In: Srivastava SC, editor. Radiolabeled monoclonal antibodies for imaging and therapy. Vol. 152. New York and London: Plenum Press; 1988. pp. 55–73. (NATO ASI Series). [Google Scholar]
- 33.Buraggi GL, Callegaro L, Mariani G, Turrin A, Cascinelli N, Attili A, et al. Imaging with 131I-labeled monoclonal antibodies to a high-molecular-weight melanoma-associated antigen in patients with melanoma: efficacy of whole immunoglobulin and its F(ab′)2 fragments. Cancer Res. 1985;45:3378–87. [PubMed] [Google Scholar]
- 34.Siccardi AG, Buraggi GL, Natali PG, Scassellati GA, Viale G, Ferrone S. European multicentre study on melanoma immunoscintigraphy by means of 99mTc-labelled monoclonal antibody fragments. The European Multicentre Study Group Eur J Nucl Med. 1990;16:317–23. doi: 10.1007/BF00842787. [DOI] [PubMed] [Google Scholar]
- 35.Maciag PC, Seavey MM, Pan ZK, Ferrone S, Paterson Y. Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer Res. 2008;68:8066–75. doi: 10.1158/0008-5472.CAN-08-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 37.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 38.Duksin D, Bornstein P. Changes in surface properties of normal and transformed cells caused by tunicamycin, an inhibitor of protein glycosylation. Proc Natl Acad Sci U S A. 1977;74:3433–7. doi: 10.1073/pnas.74.8.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Natali PG, Cavaliere R, Bigotti A, Nicotra MR, Russo C, Ng AK, et al. Antigenic heterogeneity of surgically removed primary and autologous metastatic human melanoma lesions. J Immunol. 1983;130:1462–6. [PubMed] [Google Scholar]
- 40.Glockshuber R, Malia M, Pfitzinger I, Pluckthun A. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry. 1990;29:1362–7. doi: 10.1021/bi00458a002. [DOI] [PubMed] [Google Scholar]
- 41.Huston JS, George AJ, Adams GP, Stafford WF, Jamar F, Tai MS, et al. Single-chain Fv radioimmunotargeting. Q J Nucl Med. 1996;40:320–33. [PubMed] [Google Scholar]
- 42.Bruggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, et al. Comparison of the effector functions of human immunoglo-bulins using a matched set of chimeric antibodies. J Exp Med. 1987;166:1351–61. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bindon CI, Hale G, Waldmann H. Importance of antigen specificity for complement-mediated lysis by monoclonal antibodies. Eur J Immunol. 1988;18:1507–14. doi: 10.1002/eji.1830181006. [DOI] [PubMed] [Google Scholar]
- 44.Xia MQ, Hale G, Waldmann H. Efficient complement-mediated lysis of cells containing the CAMPATH-1 (CDw52) antigen. Mol Immunol. 1993;30:1089–96. doi: 10.1016/0161-5890(93)90155-5. [DOI] [PubMed] [Google Scholar]
- 45.Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–71. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 46.Bluemel C, Hausmann S, Fluhr P, Sriskandarajah M, Stallcup WB, Baeuerle PA, et al. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol Immunother. 2010;59:1197–209. doi: 10.1007/s00262-010-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001;61:4750–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.