The development of methods for the modification of peptides and proteins under benign aqueous conditions has received a great deal of attention from both industrial and academic laboratories.1 The ability to introduce a range of functional post-translational modifications, either natural or synthetic, to recombinantly expressed proteins, such as sugars, lipids, fluorophores, affinity tags or radionuclides, has been exploited to gain further insight into a plethora of biological process.2 A number of strategies for protein modification through manipulation of the amino or carboxy terminus have been developed.3 However, the vast majority of approaches rely on the reactivity of naturally occurring amino acid side chains such as lysine, tyrosine or cysteine.4
One bioconjugation approach exploits the elimination of cysteine to dehydroalanine, which can then be modified by adding suitable nucleophiles. A number of reagents have been developed to convert both peptide- and protein-based cysteines to dehydroalanine, for example O-mesitylenesulfonylhydroxylamine and hexamethylphosphorous triamide.4b, 5 However, the majority of currently used reagents require harsh conditions that limit their application. Recently, Davis has described the elegant use of 1,4-bishaloalkanes for the β-elimination of cysteine to effect protein modification under mild conditions.6 An illustrative example is the treatment of a single cysteine mutant of subtilisin from Bacillus lentus SBL(S156C) (1) with 2,5-dibromohexanediamide (2) at pH 8, which afforded intermediate dehydroalanine 3, presumably via the transient formation of sulfonium 4 (Scheme 1). Dehydroalanine 3 could be subsequently functionalised with a nucleophile to give modified protein 5.
Scheme 1.
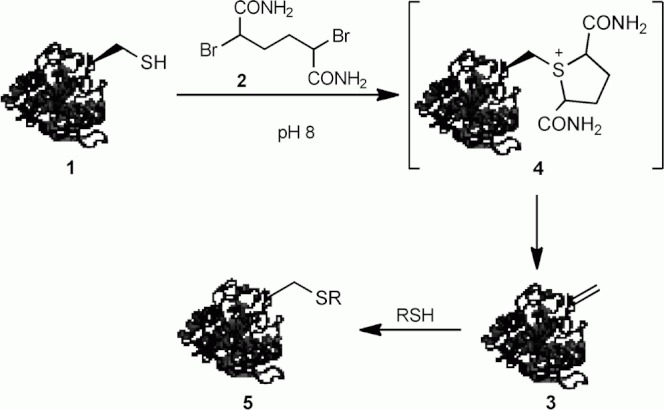
Modification of SBL(S156C) by cysteine β-elimination.
Intrigued by the potential application of the Davis double-alkylation methodology, we explored its applicability to a range of biological systems. Herein, we report preliminary studies on the modification of a cysteine mutant (S147C) of "superfolder" green fluorescent protein (GFP) bearing a single surface-accessible cysteine. We were able to synthesise unusually stable cyclic sulfonium species and to employ these in a novel bioconjugation that is complementary to current dehydroalanine-based technologies for substrates for which dehydroalanine formation is not facile. Although the presence of sulfonium ions in peptides and proteins derived from the alkylation of methionine is well established,7 to our knowledge this represents the first evidence for the formation of a stable cysteine-sulfonium on a protein.
We treated GFP(S147C) (6) with 2 under Davis' conditions (1500 equiv, 37 °C, 2 h, pH 8; Scheme 2).6 Unexpectedly the product was not the corresponding GFP dehydroalanine 7, rather electrospray (ES) MS indicated quantitative conversion to a species with a molecular weight equivalent to that of sulfonium 8 (29 486 calcd, 29 488 obs.). Indeed, throughout this study, we observed no evidence for the formation of dehydroalanine 7, or products derived therefrom, even upon incubation of sulfonium 8 for prolonged periods (vide infra). The isolation of this sulfonium species as a stable adduct and its reluctance to undergo elimination to give the corresponding dehydroalanine product 7 was noteworthy and we embarked upon further studies to understand and exploit this unexpected mode of reactivity.
Scheme 2.
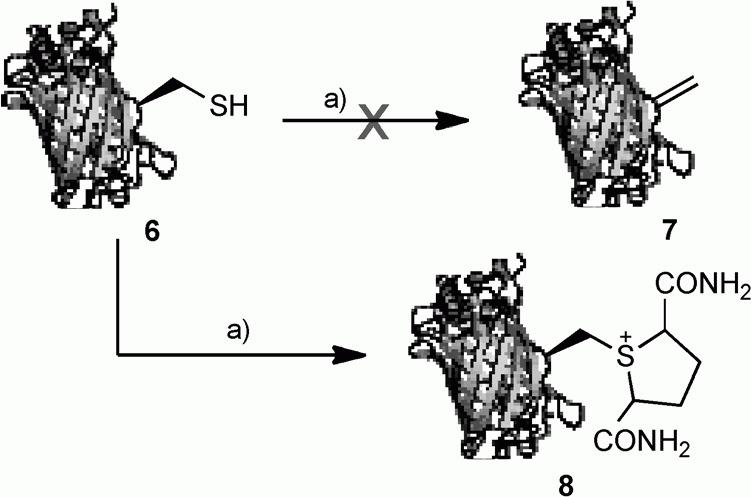
Reaction of GFP(S-147C) (100 μL, 1 mg mL−1 in H2O, pH 8, 100 mm phosphate) with dibromodiamide (2). a) 2 (1500 equiv; 10 μL, 340 mm in DMF), 37 °C, 2 h.
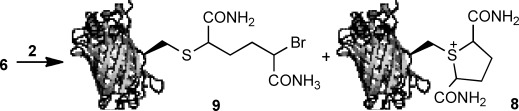
In order to gain further evidence for the site of reaction between GFP(S147C) and 2,5-dibromohexanediamide, sulfonium 8 was treated with N-methylbromomaleimide (1 equiv).4d No reaction was observed with 8, whereas 6 reacted cleanly, thus suggesting that 2 had reacted exclusively on cysteine to generate 8 (see the Supporting Information).
Optimisation of the formation of 8 was achieved by varying a number of parameters including the number of equivalents of 2, reaction temperature and reaction time (Table 1). Treatment of GFP(S147C) with 10 equivalents of 2 afforded no sulfonium 8 after 5 h; nevertheless, clear evidence for the formation of thioether 9 (29 566 calcd, 29 563 obs.) was apparent by LCMS (Table 1, entry 2). However, a prolonged incubation time, 20 h, afforded 8 as the only identifiable product in 53 % conversion (Table 1, entry 3). Increasing the concentration of 2,5-dibromohexanediamide afforded, as expected, more rapid formation of 8, with complete conversion of 6 at 21 °C with 25 or 50 equivalents of 2 within 20 h (Table 1, entries 4–9). At 4 °C, no sulfonium formation is apparent after 20 h even using 25 equivalents of 2,5-dibromohexanediamide (Table 1, entries 10 and 11). However, formation of sulfonium 8 at 4 °C is observed at higher concentrations of 2 (Table 1, entry 12). At 37 °C with 50 equivalents of 2 complete conversion of 6 to 8 could be rapidly achieved (Table 1, entry 13).
Table 1.
Optimisation of formation of sulfonium 8.[a]
 | ||||||
|---|---|---|---|---|---|---|
| 2 [equiv] | T [°C] | t [h] | 6 [%][b] | 9 [%][b] | 8 [%][b] | |
| 1 | 10 | 21 | 2 | >95 | 0 | 0 |
| 2 | 5 | 91 | 9 | 0 | ||
| 3 | 20 | 47 | 0 | 53 | ||
| 4 | 25 | 21 | 2 | 81 | 19 | 0 |
| 5 | 5 | 50 | 17 | 33 | ||
| 6 | 20 | 0 | 0 | >95 | ||
| 7 | 50 | 21 | 2 | 56 | 23 | 21 |
| 8 | 5 | 27 | 25 | 48 | ||
| 9 | 20 | 0 | 0 | >95 | ||
| 10 | 10 | 4 | 20 | >95 | 0 | 0 |
| 11 | 25 | 20 | 80 | 20 | 0 | |
| 12 | 50 | 20 | 56 | 24 | 20 | |
| 13 | 50 | 37 | 2 | 0 | 0 | >95 |
[a] Conditions: 6 (100 μL, 1 mg mL−1 in H2O) at pH 8 (100 mM phosphate), 2 (10 μL in DMF). [b] Determined by ratio of peak heights in deconvoluted mass spectrum.
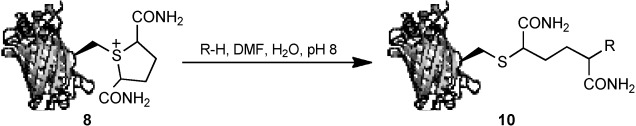
We then sought to establish the stability and reactivity profile of sulfonium 8. After preparation of sulfonium 8 under our optimised conditions (50 equiv 2, 37 °C, 2 h) excess 2 could be readily removed by repeated diafiltration into fresh buffer. Sulfonium 8 was found to be stable in the absence of nucleophiles at 21 °C for 24 h and at 4 °C for 1 month; although, significant decomposition was observed at 37 °C after 4 h (vide supra). Treatment of 8 with β-mercaptoethanol (1000 equiv, 37 °C, 2.5 h) afforded bisthioether 10 a (29 564 calcd, 29 566 obs.) in complete conversion, presumably through ring opening of the cyclic sulfonium (Scheme 3).
Scheme 3.
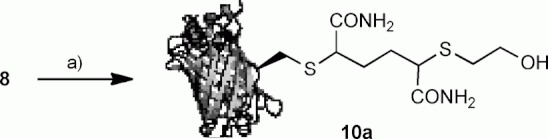
Reactivity of GFP(S147C) sulfonium 8 (100 μL, 0.9 mg mL−1 in H2O/DMF 10:1, pH 8, 100 mM phosphate) with β-mercaptoethanol (5 μL, 680 mM in H2O). a) HSCH2CH2OH (1000 equiv), 37 °C, 2.5 h, DMF, H2O, pH 8 (phosphate), >95 % conversion.
We then evaluated the reactivity of 8 with a range of simple nucleophiles. Treatment of 8 with the sodium salt of 1-thio-β-D-glucose (10 equiv) afforded the expected GFP–thioglucose conjugate 10 b as the sole product, with complete conversion after 5 h (Table 2, entry 1). Similarly, treatment of 8 with glutathione was also successful at both 21 and at 37 °C, generating the corresponding GFP–glutathione adduct 10 c in excellent conversion (entries 3–5). We also confirmed that these reactions could be carried out effectively at room temperature (21 °C) in 5 h, or more rapidly at 37 °C or with increased concentrations of the nucleophile (entries 2, 4 and 5). Treatment of sulfonium 8 with phenyl selenol (100 equiv) afforded the mixed thio/seleno bisether 10 d in excellent conversion (entry 6). Although the conversion from reaction between 8 and phthalimide to give 10 e was only modest (entry 7), we were delighted to observe clean reaction in high conversion upon treatment of 8 with sodium azide (1000 equiv) to generate azide-labelled GFP 10 f (entry 8).
Table 2.
Conjugation of nucleophiles with GFP sulfonium 8.[a]
 | ||||||
|---|---|---|---|---|---|---|
| R–H | Equiv | T [°C] | t [h] | Conv. 8 [%][b] | Product | |
| 1 2 | 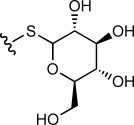 |
10 100 | 37 21 | 5 5 | >95 >95 | 10 b |
| 3 4 5 | 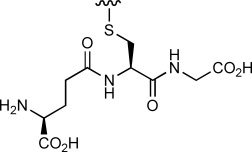 |
10 100 200 | 37 21 37 | 5 5 2 | >95 >95 >95 | 10 c |
| 6 | 100 | 37 | 5 | >95 | 10 d | |
| 7 | 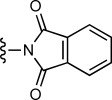 |
1000 | 37 | 2.5 | 40 | 10 e |
| 8 | 1000 | 37 | 2.5 | >95 | 10 f |
[a] Conditions: 8 (100 μL, 1 mg mL−1 in H2O) at pH 8 (100 mM phosphate), NuH (see the Supporting Information). [b] Determined by ratio of peak heights in deconvoluted mass spectrum.
In conclusion, we have shown that treatment of superfolder GFP with bis-alkylating agents can be used to prepare highly stable, yet synthetically useful cyclic protein sulfonium adducts. These species undergo clean addition reactions to allow the introduction of a variety of useful chemical motifs. We envisage that the stability of the generated cysteine-derived sulfonium is presumably strongly correlated to the chemical environment of the alkylated cysteine. This new bioconjugation technique could provide a useful entry into multiply functionalised proteins and further work will be directed toward extending these findings to other systems.
Acknowledgments
We gratefully acknowledge EPSRC (EP/E000754/1), UCL, the Wellcome Trust (090145/Z/09/Z), UCLB and GSK for support of our programme.
Supplementary material
References
- 1a.Antos JM, Francis MB. Curr. Opin. Chem. Biol. 2006;10:253–262. doi: 10.1016/j.cbpa.2006.04.009. [DOI] [PubMed] [Google Scholar]; Artner LM, Merkel L, Bohlke N, Beceren-Braun F, Weise C, Dernedde J, Budisa N, Hackenberger CPR. Chem. Commun. 2012;48:522–524. doi: 10.1039/c1cc16039g. [DOI] [PubMed] [Google Scholar]; Behrens CR, Hooker JM, Obermeyer AC, Romanini DW, Katz EM, Francis MB. J. Am. Chem. Soc. 2011;133:16398–16401. doi: 10.1021/ja2033298. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cohen L. Annu. Rev. Biochem. 1968;37:695–726. doi: 10.1146/annurev.bi.37.070168.003403. [DOI] [PubMed] [Google Scholar]; Glazer AN. Annu. Rev. Biochem. 1970;39:101–130. doi: 10.1146/annurev.bi.39.070170.000533. [DOI] [PubMed] [Google Scholar]; Holmes TJ, Jr, Lawton RG. J. Am. Chem. Soc. 1977;99:1984–1986. doi: 10.1021/ja00448a056. [DOI] [PubMed] [Google Scholar]; Walsh CT, Garneau-Tsodikova S, Gatto GJ. Angew. Chem. 117:7508–7539. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:7342–7372. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]
- 2.Song W, Wang Y, Qu J, Lin Q. J. Am. Chem. Soc. 2008;130:9654–9655. doi: 10.1021/ja803598e. [DOI] [PubMed] [Google Scholar]; Wang J, Zhang W, Song W, Wang Y, Yu Z, Li J, Wu M, Wang L, Zang J, Lin Q. J. Am. Chem. Soc. 2010;132:14812–14818. doi: 10.1021/ja104350y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li Q-F, Yang Y, Maleckis A, Otting G, Su X-C. Chem. Commun. 2012;48:2704–2706. doi: 10.1039/c2cc17900h. [DOI] [PubMed] [Google Scholar]; Moody P, Smith MEB, Ryan CP, Chudasama V, Baker JR, Molloy J, Caddick S. ChemBioChem. 2012;13:39–41. doi: 10.1002/cbic.201100603. [DOI] [PMC free article] [PubMed] [Google Scholar]; Qi DF, Tann CM, Haring D, Distefano MD. Chem. Rev. 2001;101:3081–3111. doi: 10.1021/cr000059o. [DOI] [PubMed] [Google Scholar]; Richter MPO, Holland-Nell K, Beck-Sickinger AG. Tetrahedron. 2004;60:7507–7513. [Google Scholar]; Stafforst T. ChemBioChem. 2012;13:505–507. doi: 10.1002/cbic.201100787. [DOI] [PubMed] [Google Scholar]
- 3a.Yi L, Sun H, Wu Y-W, Triola G, Waldmann H, Goody RS. Angew. Chem. 122:9607–9611. [Google Scholar]; Angew. Chem. Int. Ed. 2010;49:9417–9421. doi: 10.1002/anie.201003834. [DOI] [PubMed] [Google Scholar]; Lewinska M, Seitz C, Skerra A, Schmidtchen FP. Bioconjugate Chem. 2004;15:231–234. doi: 10.1021/bc034085f. [DOI] [PubMed] [Google Scholar]; Volkmann G, Liu X-Q. PLoS ONE. 2009;4:e8381. doi: 10.1371/journal.pone.0008381. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gilmore JM, Scheck RA, Esser-Kahn AP, Joshi NS, Francis MB. Angew. Chem. 118:5433–5437. doi: 10.1002/anie.200600368. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:5307–5311. doi: 10.1002/anie.200600368. [DOI] [PubMed] [Google Scholar]; Alam J, Keller TH, Loh T-P. Chem. Commun. 2011;47:9066–9068. doi: 10.1039/c1cc12926k. [DOI] [PubMed] [Google Scholar]; Zhang X, Li F, Lu X-W, Liu C-F. Bioconjugate Chem. 2009;20:197–200. doi: 10.1021/bc800488n. [DOI] [PubMed] [Google Scholar]
- 4a.Antos JM, Francis MB. J. Am. Chem. Soc. 2004;126:10256–10257. doi: 10.1021/ja047272c. [DOI] [PubMed] [Google Scholar]
- 4b.Bernardes GJL, Chalker JM, Errey JC, Davis BG. J. Am. Chem. Soc. 2008;130:5052–5053. doi: 10.1021/ja800800p. [DOI] [PubMed] [Google Scholar]; Chalker JM, Lercher L, Rose NR, Schofield CJ, Davis BG. Angew. Chem. 124:1871–1875. doi: 10.1002/anie.201106432. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012;51:1835–1839. doi: 10.1002/anie.201106432. [DOI] [PubMed] [Google Scholar]
- 4d.Smith MEB, Schumacher FF, Ryan CP, Tedaldi LM, Papaioannou D, Waksman G, Caddick S, Baker JR. J. Am. Chem. Soc. 2010;132:1960–1965. doi: 10.1021/ja908610s. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhu Y, van der Donk WA. Org. Lett. 2001;3:1189–1192. doi: 10.1021/ol015648a. [DOI] [PubMed] [Google Scholar]
- 5a.Bernardes GJL, Grayson EJ, Thompson S, Chalker JM, Errey JC, El Oualid F, Claridge TDW, Davis BG. Angew. Chem. 120:2276–2279. doi: 10.1002/anie.200704381. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:2244–2247. doi: 10.1002/anie.200704381. [DOI] [PubMed] [Google Scholar]; Miao Z, Tam JP. Org. Lett. 2000;2:3711–3713. doi: 10.1021/ol006624r. [DOI] [PubMed] [Google Scholar]
- 6.Chalker JM, Gunnoo SB, Boutureira O, Gerstberger SC, Fernández-González M, Bernardes GJL, Griffin L, Hailu H, Schofield CJ, Davis BG. Chem. Sci. 2011;2:1666–1676. [Google Scholar]
- 7a.Amunugama M, Roberts KD, Reid GE. J. Am. Soc. Mass Spectrom. 2006;17:1631–1642. doi: 10.1016/j.jasms.2006.07.013. [DOI] [PubMed] [Google Scholar]; Battistuzzi G, Stampler J, Bellei M, Vlasits J, Soudi M, Furtmüller PG, Obinger C. Biochemistry. 2011;50:7987–7994. doi: 10.1021/bi2008432. [DOI] [PubMed] [Google Scholar]; Lang S, Spratt DE, Guillemette JG, Palmer M. Anal. Biochem. 2006;359:253–258. doi: 10.1016/j.ab.2006.09.025. [DOI] [PubMed] [Google Scholar]; Metcalfe CL, Ott M, Patel N, Singh K, Mistry SC, Goff HM, Raven EL. J. Am. Chem. Soc. 2004;126:16242–16248. doi: 10.1021/ja048242c. [DOI] [PubMed] [Google Scholar]; Meza JE, Scott GK, Benz CC, Baldwin MA. Anal. Biochem. 2003;320:21–31. doi: 10.1016/s0003-2697(03)00296-3. [DOI] [PubMed] [Google Scholar]; Reid GE, Roberts KD, Simpson RJ, O'Hair RAJ. J. Am. Soc. Mass Spectrom. 2005;16:1131–1150. doi: 10.1016/j.jasms.2005.03.015. [DOI] [PubMed] [Google Scholar]; Rogers GA, Shaltiel N, Boyer PD. J. Biol. Chem. 1976;251:5711–5717. [PubMed] [Google Scholar]; Taylor KL, Pohl J, Kinkade JM. J. Biol. Chem. 1992;267:25282–25288. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


