Summary
Understanding disease distributions is of fundamental and applied importance, yet few studies benefit from integrating broad sampling with ecological and phylogenetic data. Here, anther-smut disease, caused by the fungus Microbotryum, was assessed using herbarium specimens of Silene and allied genera of the Caryophyllaceae.
A total of 42 000 herbarium specimens were examined, and plant geographical distributions and morphological and life history characteristics were tested as correlates of disease occurrence. Phylogenetic comparative methods were used to determine the association between disease and plant life-span.
Disease was found on 391 herbarium specimens from 114 species and all continents with native Silene. Anther smut occurred exclusively on perennial plants, consistent with the pathogen requiring living hosts to overwinter. The disease was estimated to occur in 80% of perennial species of Silene and allied genera. The correlation between plant life-span and disease was highly significant while controlling for the plant phylogeny, but the disease was not correlated with differences in floral morphology.
Using resources available in natural history collections, this study illustrates how disease distribution can be determined, not by restriction to a clade of susceptible hosts or to a limited geographical region, but by association with host life-span, a trait that has undergone frequent evolutionary transitions.
Keywords: anther smut, Caryophyllaceae, disease risk, herbarium, life history evolution, Microbotryum, Silene, Ustilago violacea
Introduction
Theoretical studies have emphasized that epidemiological and evolutionary dynamics may be heavily influenced by details of the pathogen’s and the host’s life cycles (Anderson & May, 1981; Thrall & Burdon, 2004; Barrett et al., 2008). However, surprisingly few examples are available of diseases in natural populations where broad geographical sampling can be combined with ecological and phylogenetic data to understand pathogen distributions. Because endemic pathogens and their hosts are affected by long-term co-evolutionary processes, phylogenetic analyses can be combined with ecological and geographical data to identify the population, life history, and genetic traits that contribute to disease spread and persistence. Such an integrated approach can also be used to evaluate ecological factors and life history traits that put hosts at risk for the emergence of new diseases.
With the objective of assessing host characteristics that may influence the distribution of disease, we present here the results of a world-wide survey of the incidence of anther-smut disease using a survey of over 42 000 herbarium specimens of plant species in the genus Silene and allied genera of the Caryophyllaceae. The disease is vectored by insect pollinators and replaces the pollen in anthers of infected plants with fungal spores. The dark fungal spores in the anthers of infected plants can be recognized on herbarium specimens and confirmed by simple microscopy. However, the disease is sufficiently inconspicuous that plant collections appear to have been made without awareness of the disease (Antonovics et al., 2003; Hood & Antonovics, 2003). Similar surveys of herbarium specimens have proven increasingly useful for understanding a variety of other naturally occurring diseases (Evans, 1987; Alexander et al., 2007; Malmstrom et al., 2007).
Anther-smut disease is caused by the basidiomycete fungus Microbotryum violaceum sensu lato. Although relatively uniform morphologically, M. violaceum on the Caryophyllaceae forms a large species complex with many specialized and evolutionarily independent lineages, some of which have been elevated recently to the level of distinct species (Lutz et al., 2005; Le Gac et al., 2007; Giraud et al., 2008). Anther smut is one of the best studied nonagricultural diseases of plants, particularly in the context of pathogen ecology, phylogeny, and genetics (Antonovics et al., 2002; Garber & Ruddat, 2002; Giraud et al., 2008; Bernasconi et al., 2009).
Anther-smut epidemiology can be driven by the host’s physiological resistance and by traits such as host life-span, mating system, floral displays, and pollinator specificity (Thrall et al., 1993; Marr & Delph, 2005). Such fundamental plant traits are known to be under intense selection across diverse environmental and ecological conditions (Crawford & Abbott, 1994; Giles et al., 2006), and they vary to a large degree among caryophyllaceous plants (Desfeux et al., 1996; Kephart et al., 2006). Combined with theoretical models on how this disease interacts with host life history traits, Thrall et al. (1993) showed that published reports of anther smut on the Caryophyllaceae were most frequent for perennial plant species and outcrossing subfamilies, with a trend toward species with larger flowers being more likely to be diseased. The survey by Thrall et al. (1993) included species from Europe and North America, which was the recognized range of the pathogen at the time. While the Caryophyllaceae has a world-wide distribution, it has been commonly accepted that anther-smut fungi are largely limited to temperate and subarctic regions of the Northern Hemisphere, although targeted surveys have not yet been conducted in other regions (but see Piepenbring, 2001; Marr & Delph, 2005). A tacit assumption has been that the pathogen’s geographical range is limited by climate, with cooler temperatures favoring transmission, or by latitudinal changes in the proportion of annual to perennial species.
To test the generality of the patterns described above, data on the presence of anther-smut disease in herbarium specimens were analyzed for associations with plant life-span, floral characteristics, and geographical distributions. This research greatly expands upon the previous study by Thrall et al. (1993), where the incidence of anther smut in relation to floral and life history characters was largely based on a literature survey, and geographical distributions and host phylogeny were not explicitly considered. Our goals were: first, to determine the degree to which the disease is confined to perennial plant species; secondly, to predict the proportion of perennial species expected to harbor the disease in nature; thirdly, to assess whether disease frequencies among perennials were associated with the plants’ geographical distributions or floral characteristics; and fourthly, to establish whether these relationships had evolved independently with regard to the host phylogeny. To establish whether anther-smut disease observed on specimens from distant hosts or geographical regions could be included in the discussion of fungi in the genus Microbotryum, we also carried out a limited phylogenetic study on a subset of pathogen samples from the herbaria using DNA sequencing. The current study covers a much larger geographical area and a more inclusive list of plant species than previous investigations (Thrall et al., 1993; Antonovics et al., 2003). The study reveals the nearly global distribution of anther-smut disease on the Caryophyllaceae while also assessing the relationships between infection and host traits in a quantitative and phylogenetic context.
Materials and Methods
Natural history
Anther-smut disease on the Caryophyllaceae is caused by fungal species of the genus Microbotryum (Basidiomycetes: Microbotryales). Related fungal pathogens, often classified in separate genera, are found on other plant families, including the Dipsacaceae, Lamiaceae, Polygonaceae, and Portulacaceae (Kemler et al., 2006, 2009). Although sometimes referred to as a single species, the name Microbotryum violaceum represents a suite of host-specific species that remain incompletely resolved taxonomically (Le Gac et al., 2007; Lutz et al., 2008; Denchev et al., 2009); therefore the genus name Microbotryum is used hereafter. Many species in the Caryophyllaceae are hosts to anther smut, particularly within the genus Silene (Thrall et al., 1993). Anther-smut disease sterilizes infected hosts by causing female structures to abort and replacing pollen with powdery, dark-colored fungal spores. The disease is transmitted primarily by pollinators visiting infected flowers (Antonovics & Alexander, 1992; Biere & Honders, 1998).
The Caryophyllaceae consists almost exclusively of herbaceous plant species, representing a diverse range of ecologies and life histories from annuals to extremely long-lived perennials (e.g. Desfeux et al., 1996; Forbis & Doak, 2004; Kephart et al., 2006). Within this family, anther-smut disease is most common on the tribe Sileneae (Oxelman et al., 2001; Table 2; Thrall et al., 1993). Europe and Asia contain the largest numbers of species in the Sileneae, with smaller numbers found in Africa and North America and yet fewer in South America (Heywood, 1978). A suggested phylogeographical history of the genus Silene is a Eurasian origin followed by migration into the Americas via the Beringian region (Popp et al., 2005; Popp & Oxelman, 2007). There are no native members of the Sileneae in Australia, although some species have become naturalized following introduction.
Table 2.
Number of diseased specimens found in herbaria, classified by plant genus and life-span
| Family Caryophyllaceae | |||||
|---|---|---|---|---|---|
| Subfamily Caryophylloideae | |||||
| Total | Perennial | Annual | Undetermined | ||
| Tribe Sileneae | Silene | 316 (95) | 316 (95) | 0 (0) | 0 (0) |
| Lychnis | 32 (7) | 32 (7) | 0 (0) | 0 (0) | |
| Heliosperma | 1 (1) | 1 (1) | 0 (0) | 0 (0) | |
| Viscaria | 10 (2) | 10 (2) | 0 (0) | 0 (0) | |
| Atocion | 3 (2) | 3 (2) | 0 (0) | 0 (0) | |
| Eudianthe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Agrostemma | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Remaining Caryophylloideae | Saponaria | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Dianthus | 9 (2) | 9 (2) | 0 (0) | 0 (0) | |
| Petrorhagia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Remaining Caryophyllaceae | Stellaria | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Family Portulaceae | Calandrinia | 18 (3) | 18 (3) | 0 (0) | 0 (0) |
| Cistanthe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Ceraria | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Calyptrotheca | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Total | 391 (114) | 391 (114) | 0 (0) | 0 (0) | |
Numbers of species with disease per genus are shown in parentheses.
Herbarium surveys
Specimens were examined from the following plant herbaria (with herbarium abbreviations according to Holmgren & Holmgren, 1998): European: Centro di Ricerche Floristiche dell’Appennino (APP); Botánico de Barcelona (BC); Instituto Museo di Storia Naturale dell’Università, Firenze (FI); Royal Botanical Gardens, Kew (K); Real Jardín Botánico de Madrid (MA); Muséum National d’Histoire Naturelle, Paris (P); North American: Gray Herbarium, Harvard University (GH); Missouri Botanical Garden (MO); New York Botanical Garden (NY); Smithsonian Institution, Washington, DC (US); African: Bolus Herbarium (BOL); South American: Museo Nacional de Historía Natural, Santiago, Chile (SGO); Asian: Jiangsu Institute of Botany, Nanjing (NAS). The survey focused on the genus Silene and other genera in the tribe Sileneae. Some members of the subfamilies Caryophylloideae (i.e. Saponaria, Dianthus, and Petrorhagia) and Alsinoideae (i.e. Stellaria) were also examined (Table 1), as were North and South American members of the genus Calandrinia in the Portulacaceae as a result of the recent discovery of an anther smut on Calandrinia that is related to Microbotryum on Silene (Le Gac et al., 2007). Each herbarium sheet was examined for specimens with diseased flowers, and counts were based on total number of sheets with or without diseased specimens; no attempt was made to distinguish individual plants (see Hood & Antonovics, 2003). Locality information from herbarium labels of the diseased specimens was recorded and used to generate a distribution map of anther-smut disease found in this survey. In no case did we find any annotation indicating that the collector might have noticed that specimens on the sheets were diseased.
Table 1.
Number of herbarium specimens examined for anther-smut disease classified by plant genus and life-span
| Family Caryophyllaceae | |||||
|---|---|---|---|---|---|
| Subfamily Caryophylloideae | |||||
| Total | Perennial | Annual | Undetermined | ||
| Tribe Sileneae | Silene | 37 275 (728) | 24 136 (514) | 13 008 (141) | 131 (73) |
| Lychnis | 1471 (31) | 1469 (29) | 1 (1) | 1 (1) | |
| Heliosperma | 275 (7) | 275 (7) | 0 (0) | 0 (0) | |
| Viscaria | 501 (3) | 501 (3) | 0 (0) | 0 (0) | |
| Atocion | 542 (4) | 542 (4) | 0 (0) | 0 (0) | |
| Eudianthe | 530 (2) | 0 (0) | 530 (2) | 0 (0) | |
| Agrostemma | 6 (1) | 0 (0) | 6 (1) | 0 (0) | |
| Remaining Caryophylloideae | Saponaria | 336 (67) | 282 (38) | 8 (3) | 46 (26) |
| Dianthus | 482 (24) | 431 (8) | 36 (8) | 15 (8) | |
| Petrorhagia | 21 (2) | 11 (1) | 10 (1) | 0 (0) | |
| Remaining Caryophyllaceae | Stellaria | 398 (4) | 398 (4) | 0 (0) | 0 (0) |
| Family Portulaceae | Calandrinia | 772 (72) | 311 (21) | 425 (40) | 36 (11) |
| Cistanthe | 3 (2) | 3 (2) | 0 (0) | 0 (0) | |
| Ceraria | 20 (4) | 20 (4) | 0 (0) | 0 (0) | |
| Calyptrotheca | 77 (3) | 0 (0) | 77 (3) | 0 (0) | |
| Total | 42 709 (955) | 28 379 (636) | 14 101 (200) | 229 (119) | |
Numbers of species per genus are shown in parentheses. The tribe Sileneae is as defined in Oxelman et al. (2001).
To determine how representative the herbarium survey was of the species in the tribe Sileneae, a simulation analysis was performed based upon resampling specimens from the compiled data set. Entries were chosen from the data set of over 40 000 Sileneae specimens at random and with replacement, and each was assessed for whether the plant species had been sampled previously in the simulation. The expected number of new plant species added for each additional herbarium specimen was determined. After the first 20 000 specimens the rate of new plant species per specimen had reached a relatively constant value of c. 0.003. We therefore concluded that the database was sufficiently large and representative to provide a thorough survey of the Sileneae.
Plant characteristics
To assess factors that may explain the disease frequencies, information was compiled for the life-span (annual or perennial), petal limb length, and flower color of each species (see Supporting Information Table S1). Species listed as biennial were included with annuals, and species listed variably as annual/perennial or biennial/perennial were included with perennials. Biennial species were included with annuals because, like annuals, individuals do not exhibit repeated flowering which is necessary for the persistence of this pollinator-transmitted disease over successive seasons (Thrall et al., 1993); moreover, species with a winter-annual habit are sometimes described in the literature as biennial. The floral trait of petal limb length was used as a proxy for flower size; a previous study had suggested a positive relationship between flower size and disease incidence (Thrall et al., 1993). The darkness of flower color was also investigated because we were concerned that it might bias collection of diseased plants by collectors, the disease being more conspicuous on white-colored flowers. The darkness of flower color was assessed on a scale from 1 to 4: 1, white; 2, white-yellow, white-green, or white-pink; 3, pink, violet, red-white, or lilac; 4, red, red-violet, dark red, or dark violet.
Data on plant traits were gathered from various sources including floras (Flora of North America,Rabeler & Hartman, 2005; Flora of China, Zhou et al., 2001; Atlas Florae Europaeae, Jalas & Suominen, 1988; Flora iberica, online at http://www.bioscripts.net/flora/) and published articles (Desfeux et al., 1996; Jürgens et al., 1996, 2002a,b; Burleigh & Holtsford, 2003; Popp et al., 2005; Jürgens, 2006; Popp & Oxelman, 2007). Particular efforts were made to confirm species identifications and avoid cases of taxonomic synonymy, principally using the Sileneae database (http://www.sileneae.info/boxweb), International Plant Names Index (http://www.ipni.org), and the Atlas Florae Europaeae (Jalas & Suominen, 1988). While systematic revisions are ongoing, this study used the current state of species identifications as operational taxonomic units for the analysis of disease distributions.
Rates of disease
The frequency of disease within a species was assessed using the proportion of herbarium sheets with plants showing anther-smut symptoms.
To identify species that showed higher or lower disease frequencies than expected, while accounting for differences in the number of specimens examined, we used the probability of deviation from random expectations (i.e. binomial distribution probabilities) based on the diseased proportion of all perennial specimens. This analysis was restricted to perennial species because the disease is absent from annuals (see Results section). Perennial species likely to be disease-free in nature were identified as those where no disease was found despite a binomial distribution probability of < 0.05 for finding zero diseased specimens out of the number of specimens examined based on the diseased proportion of all perennial specimens and employing a Bonferroni correction for multiple independent tests. Plant characteristics of flower size and color were tested for correlations with positive or negative deviations from expected numbers of diseased specimens of perennial species using the nonparametric Spearman’s rank test in spss version 12 (SPSS Inc., Chicago, IL, USA).
The percentage of perennial species in the tribe Sileneae expected to be diseased in nature was estimated using two methods. First, the per cent diseased was determined among species where the binomial probability of not finding disease was < 0.05, < 0.10 and < 0.20 given the number of sheets examined and the expected overall frequency of disease in perennials (see Results section). Secondly, a simulation approach was used. For each species, a binomial distribution probability was calculated for finding at least one diseased specimen. In the simulation, a species was scored as being diseased if this calculated probability was greater than a uniform random value between zero and one. This simulation attempts to estimate the percentage of species that are diseased by including all perennial species surveyed rather than only the most intensively surveyed. The average probability of perennial species being diseased was then calculated across all species weighted by the number of specimens per species, and the simulation was averaged over 1000 iterations.
Phylogenetic basis of disease occurrence
To investigate whether there was a phylogenetic basis to high and low rates of disease incidence, a phylogeny was constructed using chloroplast ribosomal protein rps16 intron sequences of Silene available in GenBank National Centre for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/). Accession numbers are available in Table S2. The species were categorized into three groups: perennials with high disease (positive deviations from expected numbers of diseased specimens), perennials with low disease (negative deviations from expected numbers of diseased specimens), and annual species. DNA sequences were available for 14 perennial species with high disease, and this number of species was chosen from the categories of perennials with low disease, and from annual species. DNA sequences were aligned using ClustalW (http://www.ebi.ac.uk/clustalw/), and phylogenies were reconstructed with the mega 4.0 software (Kumar et al., 2004) by maximum parsimony analysis using the CNI heuristic search option, 100 random additions of sequences, and 1000 bootstrap pseudoreplicates. Bayesian posterior probabilities were determined using MrBayes version 3.1, and the model of molecular evolution that best fitted the data was determined using jModeltest (Posada, 2008). Under the Akaike information criterion (AIC), the data were best fitted by the TPM1uf + G model which assumes equals rates for three types of substitutions (see jModeltest manual for details) with a gamma distribution (G) of site-specific rates. The general time reversible (GTR) model with a gamma distribution (G) of site-specific rates was used in the Bayesian analysis because it is the most similar model to TPM1uf + G available in MrBayes 3.1, and it was ranked 8th among the 88 models compared with the AIC. Priors of state frequencies were left at default settings and Markov chains were initiated from a random tree and run until the average standard deviation of split frequencies remained below 0.01, that is, 600 000 iterations; posterior probabilities were derived from 9000 post-burn-in trees.
To test whether disease status was correlated with life-span (annual vs perennial) while controlling for the plant phylogeny, a continuous Markov model in a maximum likelihood framework was used, as described by Pagel (1994) and implemented in the program Mesquite 2.6 (Maddison & Maddison, 2009) with 1000 simulations. Evidence of phylogenetic signal for the discrete characters of annual vs perennial life-span and presence or absence of disease were tested in Mesquite 2.6 by comparing the observed state transition steps against a simulated distribution of state transition steps in which the character states were shuffled randomly among taxa (Maddison & Slatkin, 1991). In this analysis, a smaller number of observed state transition steps than expected from the randomized distribution (based upon 95% confidence intervals) would indicate that the discrete character is determined significantly by phylogenetic history; the simulated distribution of state transition steps was based on 1000 iterations. These analyses were intended to assess the distribution of plant life-span and disease status rather than to provide systematic revisions to the group, which are underway elsewhere.
Host and pathogen distributions
Because annual species were not found to be diseased (see Results section), we focused on the distributions of perennial species. Information on the geographical distributions of perennial Silene species in Europe was obtained from the Atlas Florae Europaeae Database (AFE) (http://www.fmnh.helsinki.fi/english/botany/afe/publishing/database.htm). These data were used to generate species richness maps. For comparison with the perennial species richness map, 10 European Silene species were chosen with the most significant binomial distribution probabilities for positive deviations from the expected number of diseased specimens, and 10 European Silene species were chosen with the largest numbers of examined specimens where no disease was found. This strategy was used to maximize confidence in assigning species to the two categories of plants with regard to observed rates of disease; the number of species to include in the heavily diseased category was arbitrarily set at a positive binomial distribution probability of 0.05, totaling 10 species in the AFE database, and an equal number of disease-free species was then also included. In addition, 10 annual Silene species were chosen with the largest numbers of examined specimens and used to generate a European distribution map. Among the European perennial species the correlation between the size of geographical ranges and the positive or negative deviation from expected numbers of diseased specimens was tested using the Spearman’s rank test in spss. The geographical range for each species was determined from the total number of 50 × 50 km grid points occupied by a species in the AFE database. Locality data recorded from herbarium labels of diseased specimens were used to generate a global distribution map of anther-smut disease found in the survey.
Pathogen DNA sampling and analysis
The ability to obtain Microbotryum from herbarium samples raises the question of whether it is still possible to isolate DNA and characterize relationships among these samples, especially as some of these specimens would be difficult to resample in the wild. We therefore tested whether DNA could be obtained from herbarium collected smut. We included anther-smut samples infecting the host genus Calandrinia, because recent phylogenetic studies (Le Gac et al., 2007) suggested that the anther smut from this genus (in the family Portulacaceae) falls within the clade containing anther smuts from the genus Silene. We therefore sought to confirm this in our sampling of anther smut from Calandrinia based on a larger number of herbarium specimens.
During the herbarium survey, samples of the infected anthers were collected, with permission, from some diseased specimens (see Fig. 4). DNA was extracted from infected anthers using the DNeasy Mini Kit (Qiagen), and PCR was performed using primers that anneal to the variable regions of the internal transcribed spacer region of the nuclear rRNA genes to amplify only Microbotryum DNA (intraITS forward: 5′-CTGTTTAACCAGGGCGTGAC; intraITS reverse: 5′-TGATCTCGAAGGTTAGGATGC). Accession numbers are available in Table S2. Field-collected material of Microbotryum from several hosts was also included as positive controls (see Fig. 4). DNA sequences were aligned using ClustalW, and phylogenies were reconstructed with the mega 4.0 software by maximum parsimony analysis using the CNI heuristic search option, 100 random additions of sequences, and 1000 bootstrap pseudoreplicates. Bayesian posterior probabilities for support of tree topology were determined as described in the section on Phylogenetic basis of disease occurrence.
Figure 4.
Phylogeny of Microbotryum (M.) based upon maximum parsimony analysis of DNA sequences for the internal transcribed spacer region of the nuclear rRNA genes. Support values for tree topology are shown when they had values of Bayesian posterior probabilities/maximum parsimony bootstraps at least equal to 0.9/60, respectively. The tree includes genotypes obtained from herbarium specimens (arrows) and from field collections that broadly represent the species diversity of Microbotryum species on the Caryophyllacaeae (see Le Gac et al., 2007). *The outgroup pathogen on Knautia arvensis (Dipsacaceae) was chosen according to Kemler et al. (2006) and Lutz et al. (2008) and the sequence was obtained from GenBank National Centre for Biotechnology Information (NCBI). Accession numbers are available in Table S2.
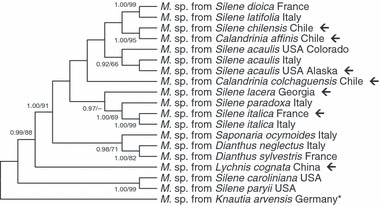
Analysis of DNA sequence data from herbarium samples confirms that the lineage of Microbotryum causing anther smut on the Caryophyllaceae has representatives infecting other plant families (Le Gac et al., 2007; but see Vánky, 1998, 2001). Samples collected from two species of South American Calandrinia (Fig. 2) group along with samples collected from species in the Caryophyllaceae rather than forming a sister clade (Fig. 4). Other species included in the phylogenetic analysis served as ‘controls’ and, as predicted, sequences of Microbotryum from herbarium specimens of Silene italica and Silene acaulis mapped adjacent to sequences of field-collected fungal specimens from the same host species. Moreover, sequences of anther smut on S. chilensis from South America fell within the clade containing Microbotryum from other Silene hosts.
Results
Rates of disease
The herbarium surveys included 42 707 specimens from 952 plant species, with the great majority belonging to tribe Sileneae and to other members of the Caryophylloideae subfamily (Table 1). Anther-smut disease was found on 391 herbarium specimens, which corresponded to disease on 114 plant species (Table 2). Anther-smut disease was present exclusively in perennial species and not in annuals (1.38% among 28 379 specimens of perennials, and no cases of disease among 14 101 specimens of annuals). Disease occurred on perennial species of all major taxonomic groups examined in this study (Table 2). The complete species list represents the most inclusive survey of this group to date (see Table S1 ).
The annual/perennial status of some species could not be determined from the literature (Table 1), but none of these was found to be diseased and most were represented by very few specimens (average = 1.9). The average number of specimens per species was 42 for perennials and 55 for annuals. Herbarium specimens labeled only with the genus name (e.g. ‘Silene indet.’) were examined but not included in further analyses; anther-smut disease was found on four of 178 Silene indet. specimens and four of 26 Lychnis indet. specimens.
In the tribe Sileneae, an estimated 84% of all perennial species are likely to be hosts to anther-smut disease in nature based on the data for species with a probability of not finding disease of < 0.05 (i.e. 26 out of 31 species with > 210 specimens having at least one that was diseased). Similar estimates were obtained by including Sileneae perennials with fewer herbarium specimens (i.e. where the probability of not finding disease if it were present was statistically less significant); the percentage of species found to be diseased was 82% for species with a P-value for not finding disease < 0.1 (n = 39 species, each with > 170 specimens), and 76% for a P-value < 0.2 (n = 54 species, each with > 120 specimens).
The simulation approach to estimate the proportion of perennial species with disease provided a comparable value of 81%. The percentage of examined Sileneae species that were classified as perennials vs annual was 79%. Therefore, with anther-smut disease on roughly 80% of perennials and an estimated 750–850 extant Sileneae species (e.g. Oxelman et al., 2001; Eggens et al., 2007), it is predicted that between 470 and 530 species might be diseased in nature for this tribe alone.
Among the perennial Sileneae species where fewer diseased specimens were found than expected, only Silene stellata, with no disease among 824 specimens, had a binomial distribution probability < 0.05 of having disease based on the overall average incidence of disease in perennials after correction for multiple independent tests (P-value corrected for 450 independent tests of perennials without disease = 0.008). Other species with large numbers of specimens but where no disease was found included Silene fortunei (411 specimens) and Silene involucrata (403 specimens), but for these to be significant would require fewer than 13 independent tests.
Species with statistically greater numbers of diseased specimens than expected based on binomial distribution probabilities included Silene saxifraga (32 diseased specimens among 491 specimens), Lychnis fulgens (13 among 116 specimens), and Silene parryi (21 among 373 specimens).
Analysis of the host phylogeny revealed that perennials with high disease frequencies were found together in multiple well-supported clades either with perennials having low or no disease, or with annual species (Fig. 1). There was no statistically significant evidence for a phylogenetic signal for either annual vs perennial life-span or presence or absence of disease; that is, the estimated number of state transition steps in the host phylogeny (n = 11) was not lower than expected by chance, indicating that these characters are highly labile during the evolution of the Sileneae. The association of life-span (annual vs perennial) and disease status was found to be statistically significant while controlling for the plant phylogeny (P-value from 1000 simulations = 0.001; independent log likelihood = 51.1985; correlated log likelihood = 43.5713).
Figure 1.
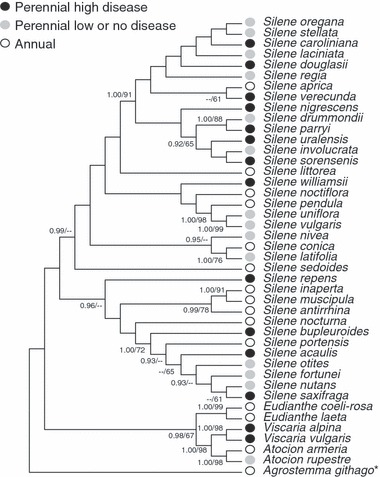
Phylogeny of plant species of the tribe Sileneae based upon maximum parsimony analysis of ribosomal protein rps16 DNA sequences. Support values for tree topology are shown when they had values of Bayesian posterior probabilities/maximum parsimony bootstraps at least equal to 0.9/60, respectively. DNA sequences were obtained from GenBank National Centre for Biotechnology Information (NCBI) for an equal number of plant species in the categories of annuals (open circles), perennials with high disease frequencies (closed circles), and perennials with low or no disease (gray circles). Perennial species were chosen as those with the most significant binomial distribution probabilities for positive or negative deviations from overall disease frequencies among perennials. Annuals were chosen as those with the largest numbers of specimens examined. *Agrostemma githago was chosen as the outgroup to the remainder of the Sileneae based on Oxelman et al. (2001). Accession numbers are available in Table S2.
No significant correlation of disease rates was found with flower size (correlation coefficient = −0.057, P = 0.476, n = 156) or with darkness of flower color (correlation coefficient = 0.083, P = 0.247, n = 195). Because these correlations were nonsignificant, they were not tested further by controlling for the plant phylogeny.
Host and pathogen distributions
The known geographical distribution of anther smuts (Fig. 2) was greatly expanded to include the presence of the pathogen in species of Sileneae from the Southern Hemisphere, both in South America (on Silene chilensis and Silene magellanica) and in southern regions of Africa (on Silene burchellii, Silene ornata, and Silene undulata). The difference between continents in the proportion of diseased perennial Sileneae specimens approached significance (χ2 = 8.41, df = 4, P = 0.078), and the trend was toward the least disease in the Southern Hemisphere and the most disease in Asia (Table 3).
Figure 2.
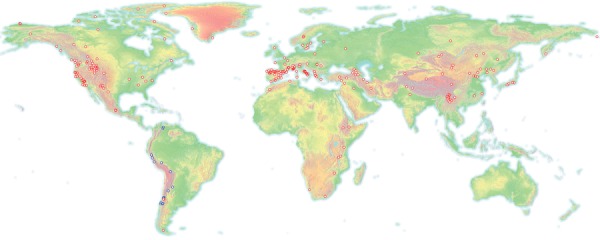
Distribution map of all diseased specimens found in this herbarium survey. Red markers, species in the Caryophyllaceae; blue markers, species of Calandrinia in the Portulacaceae. The map includes specimens with herbarium labels that were legible and contained locality data more specific than the country of origin. Continents are shaded according to land elevation.
Table 3.
Frequency of disease among herbarium specimens of Sileneae from different continents
| Continent | Specimens examined | Diseased specimens | Disease frequency |
|---|---|---|---|
| Africa | 1521 | 12 | 0.0079 |
| Asia | 5375 | 89 | 0.0166 |
| Europe | 9123 | 125 | 0.0137 |
| North America | 8709 | 111 | 0.0127 |
| South America | 263 | 2 | 0.0076 |
Within Europe, compiled distribution maps for perennial Silene species from the Atlas Florae Europaeae showed the highest species richness in southern mountain regions. Heavily diseased Silene species also appeared to be distributed in these regions (Fig. 3). By contrast, the most examined perennial species having no disease were more broadly distributed in regions of low Silene species richness. Annual Silene species exhibited a southern European distribution similar to that of perennial species, with each of the 10 annual species with the largest numbers of examined specimens overlapping in geographical distribution with the 10 most diseased perennial species (see Supporting Information Fig. S1). The geographical range size of perennial Silene in Europe was negatively correlated with the disease rates within species (Spearman’s rank correlation coefficient for all examined perennial species in the AFE database = −0.195, P = 0.048, n = 104; rank correlation coefficient including only diseased species = −0.456, P = 0.013, n = 29).
Figure 3.
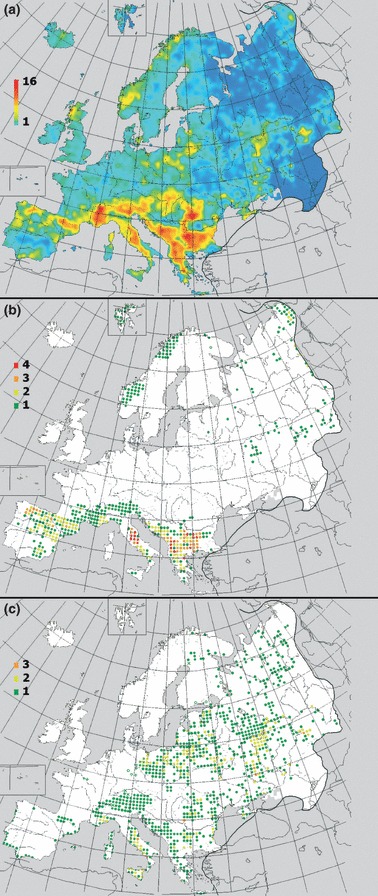
Species richness maps for perennial Silene species. (a) Distribution of all Silene species classified as perennial in the herbarium survey and included in the Atlas Florae Europaeae database (n = 104). (b) Distribution of the 10 most diseased Silene species, identified by the most significant positive deviations from expected number of diseased specimens based on binomial distribution probabilities. (c) Distribution of 10 Silene species with the largest number of examined species but where no disease was found. Color scales represent the numbers of overlapping species.
Discussion
Understanding the distribution of disease among related host species is a central challenge in pathogen ecology, especially in the context of disease emergence. Here we show that the hosts of anther-smut disease, caused by Microbotryum species, are exclusively perennial plants in the Caryophyllaceae. The occurrence of anther smut only on perennial plants is consistent with the disease lacking a free-living or environmentally resilient overwintering stage, but instead perennating inside the host and being spread directly from diseased flowers to healthy plants primarily by insect pollinators. This is in contrast to diverse fungal species in the Microbotryales (Kemler et al., 2006) and Ustilaginales (Fischer & Holton, 1957) affecting tissues other than the anthers, such as floral smuts and seed smuts, where transmission appears to be primarily via spores overwintering on the seed coat or in the soil.
Using a search of the literature, Thrall et al. (1993) also found that the proportion of species with anther-smut disease was far greater in perennials than in annuals. However, in their study disease was reported in a substantial proportion of the annuals (c. 11% of the records of disease were on annual species). Unfortunately, the original data set from that study is no longer available, but the literature reports may have included specimens from botanical gardens or experimental inoculations. For example, Goldschmidt (1928) records having obtained Silene noctiflora from a botanical garden, and he successfully inoculated Agrostemma githago experimentally. We have also found that annual species can become diseased following artificial inoculation (M. E. Hood et al., unpublished results). Similarly, infection of annual species in the field may result from a transient cross-species transmission involving disease from sympatric perennials, but the maintenance of disease on an annual species alone is not expected to occur in nature.
Previous reports of seed-destroying smuts related to Microbotryum on several annual Silene species suggest that a change in the pathogen’s transmission ecology may be required to persist on hosts with contrasting life histories (e.g. annual vs perennial). Based largely on the location of spores and larger spore size, the seed-smut pathogen on annual Silene species (i.e. Silene colorata, Silene crassipes and Silene apetala; see Vánky, 2005) was recently removed from Microbotryum and renamed Heradaea jehudana (Denchev et al., 2006). Development of smut spores in capsules, rather than anthers, suggests a nonvectored disease that is transmitted by environmental contamination, such that overlapping host generations are not required for disease persistence. Similar overwintering spores occur in Microbotryum species causing seed and ovary smuts on annuals from other plant families (Berner et al., 2006; Denchev et al., 2006). Whether the seed smut on annual Silene species is derived from an anther smut needs further study using molecular phylogenetics. However, Sloan et al. (2008) recently demonstrated that seed-smut disease symptoms can result simply from cross-species transmission of anther-smut-causing Microbotryum onto a novel Silene host. Moreover, the evolutionary transitions between anther and seed/ovary smuts appear to have occurred among Microbotryum lineages on the Caryophyllaceae (Kemler et al., 2006). Thus, persistence of Microbotryum on hosts with alternate life histories (i.e. from perennials to annuals) may be facilitated by a concomitant and fundamental change in the pathogen’s ecology (i.e. from vector-borne to environmental transmission).
That the disease is restricted to perennial species raises the question of whether anther smut imposes selection in Sileneae in favor of an annual life history. Whether a species exhibits an annual or perennial habits appear to be highly labile in the Sileneae, with multiple evolutionary transitions and no statistically significant evidence of a phylogenetic signal. Anther smut is widely distributed within this group of plants and has been shown to constrain host population growth and persistence (Antonovics, 2004). However, Thrall et al. (1993) stressed that the selective role of diseases, which may be transient within populations, needs to be considered in comparison to other evolutionary forces affecting host life history traits. Moreover, the evolution of the annual habit is unlikely to be caused by anther-smut disease because an annual variant would gain little immediate advantage in terms of escaping disease in a population consisting largely of perennials. However, the interactions with anther smut may still have evolutionary consequences when, for example, the disease is lost from a species evolving a more annual habit or from regions where populations have an environmentally imposed annual habit. A genetic basis for physiological resistance to anther smut has been demonstrated in multiple Silene species (Alexander et al., 1993; Cafuir et al., 2007) and can be associated with fitness costs in the absence of disease (Biere & Antonovics, 1996). Therefore, the loss of anther smut could contribute to the success of annuals by favoring the abandonment of costly resistance mechanisms even if the disease was not a causal factor in the evolution of host life-span. These possibilities should encourage more detailed phylogenetic studies on this host–pathogen system so that the emergence of annual life histories can be assessed for ancestral associations with disease.
Overall, the probability that anther smut is present on a perennial species is remarkably high, particularly in the tribe Sileneae. The question then arises as to why some perennials are disease-free. No infected plants were found for Silene stellata despite a large number of specimens examined. This species is broadly distributed in eastern North America and is common across much of its range. We have not observed infected plants in the field, even in areas where its close relatives, Silene caroliniana and Silene virginica, are frequently diseased (see Antonovics et al., 2003), and yet S. stellata can be readily infected by experimental inoculation (M. E. Hood et al., unpublished data). Other perennial species that so far appear healthy could also be targeted for more intensive herbarium surveys to establish disease-free status, which could then allow the assessment of shared traits that might discourage establishment of disease.
In the current study, we did not find any relationship between disease rates and flower size (as measured by petal limb length). While Thrall et al. (1993) initially speculated that larger flowers should attract larger pollinators that in turn should transmit disease at a higher rate and over larger distances, their results only approached significance at P < 0.1, and the analysis included both annual and perennial species. There was also not a significant correlation between disease rates and the darkness of flower color, which has previously been suggested as a potential confounding factor in how often disease is detected in the field or on herbarium specimens (Antonovics et al., 2003).
Disease rate among perennials was negatively associated with the host’s geographical range size. There are at least two possible explanations for this result. First, species with large geographical ranges may occupy many habitats less suitable for Microbotryum. For example, the broadly distributed host Silene vulgaris is frequently diseased above 2000 m, but many herbarium specimens come from lower elevations where this species is largely disease-free (M. E. Hood et al., unpublished data). Disease on the host Silene dioica may follow a similar elevational gradient (Bucheli et al., 2000). Secondly, very broadly distributed species may have much of their range in regions of low host species richness, and therefore experience a lower overall incidence of Microbotryum and fewer opportunities for host shifts, although the contribution of localized, transient host shifts to disease frequencies within host species is unknown. Both explanations are consistent with our observation that the distribution of species with low rates of disease appeared to overlap less with mountainous regions in southern Europe where we found the highest species richness of potential hosts. The current data are not sufficient to assess whether regions of high host diversity are ‘hotspots’ for disease emergence via host shifts, but this would be interesting to investigate in further studies. The importance of species richness has been suggested for emerging zoonotic diseases in humans (Jones et al., 2008), and recent phylogenetic and experimental studies have shown the significance of host shifts in the Microbotryum system (Refrégier et al., 2008).
The results demonstrate the broad distribution of anther-smut disease on the Caryophyllaceae. The full geographical distribution of anther-smut disease has not been widely recognized, particularly in the Southern Hemisphere. However, the herbarium survey was able to locate the pathogen on the Caryophyllaceae in the southernmost regions of South America (e.g. Punta Arenas, Chile) and Africa (e.g. near Port Elizabeth, South Africa). We have found one previous published report for the disease on Silene in South Africa, as a footnote (Reid & Hooper, 1957). The centers of diversity for Sileneae are in the Eastern Mediterranean and Central Asia (Eggens et al., 2007), and our results provide suggestive evidence that anther smut is less frequent among perennial species whose distribution is the result of dispersal to the Americas or to the Southern Hemisphere. This raises the possibility either of a broader scale relationship between disease incidence and host species richness or that escape from the pathogen for ecological or genetic reasons was associated with ancient migration patterns of hosts into the Americas or southern continents (Popp et al., 2005; Popp & Oxelman, 2007).
It should be emphasized that the data we present are not exhaustive for the distribution of anther smut. Many additional specific locations for the disease can be found in the literature, in mycological herbaria, and in previous field surveys of particular sets of host species or geographical regions (Antonovics et al., 2003; Hood & Antonovics, 2003). The close relationship between the pathogen from South American Calandrinia (Portulacaceae) and Silene (Le Gac et al., 2007) suggests the need to consider the distribution of Microbotryum outside the Caryophyllaceae. Moreover, the survey of specimens from nature does not fully reflect their actual geographical distributions because of a bias toward environments favored by botanists or toward countries where field studies have been more active. For example, the cluster of specimens in north-west Yunnan probably reflects the enormous collections by Père Jean Marie Delavay in the late 1800s and others who revisited that particular research area. Conclusions potentially influenced by unequal sampling effort should be confirmed by the collection of field data.
This study further demonstrates the utility of natural history collections for investigating large-scale disease distributions (Evans, 1987; Antonovics et al., 2003; Alexander et al., 2007; Malmstrom et al., 2007). As with previous studies on Microbotryum (Antonovics et al., 2003; Hood & Antonovics, 2003), there was little evidence from collectors’ notes or subsequent annotations that plant specimens were recognized as diseased. As further support that the data were not biased by the appearance of anther-smut symptoms, our analysis showed that disease rates were not correlated with either flower size or color, which could have affected how conspicuous the disease appears to collectors.
Acknowledgments
We thank the many herbaria mentioned in this paper, and the curators for allowing us to conduct these surveys. We thank Karen Searcy of the Massachusetts State Herbarium (MASS) for help in acquiring loaned materials, Katerina Byanova and Janice Djabatey for assistance with data compilation, and Tung Chao for translation of herbarium label information. We acknowledge grant support from the John Simon Guggenheim Memorial Foundation and the National Science Foundation (DEB-0747222) to MEH, the National Science Foundation Minority Postdoctoral Fellowship (DBI-0706721) to JIMA, University of Chile awards PFB-23 and ICM P05-002 to MTKA, and The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) support to BO, and Royal Society Incoming Fellowship and Center for Infection, Immunity, and Evolution Advanced Fellowship to ABP.
Supplementary Material
References
- Alexander HM, Antonovics J, Kelly A. Genotypic variation in plant disease resistance: physiological resistance in relation to field disease transmission. Journal of Ecology. 1993;81:325–334. [Google Scholar]
- Alexander HM, Price S, Houser R, Finch D, Tourtellotm M. Is there reduction in disease and predispersal seed predation at the border of a host plant’s range? – Field and herbarium studies of Carex blanda Journal of Ecology. 2007;95:446–457. [Google Scholar]
- Anderson RM, May RM. The population dynamics of microparasites and their invertebrate hosts. Philosophical Transactions of the Royal Society of London, Series B. 1981;291:451–524. [Google Scholar]
- Antonovics J. Long term study of a plant-pathogen metapopulation. In: Hanski I, Gaggiotti O, editors. Ecology, genetics, and evolution of metapopulations. Burlington, MA, USA: Academic Press; 2004. pp. 471–488. [Google Scholar]
- Antonovics J, Alexander HM. Epidemiology of anther-smut infection of Silene albaS. latifolia) caused by Ustilago violacea: patterns of spore deposition in experimental populations. Proceedings of the Royal Society of London B. 1992;250:157–163. [Google Scholar]
- Antonovics J, Hood ME, Partain J. The ecology and genetics of a host shift: Microbotryum as a model system. American Naturalist. 2002;160:S40–S53. doi: 10.1086/342143. [DOI] [PubMed] [Google Scholar]
- Antonovics J, Hood ME, Thrall P, Abrams JY, Duthie M. Herbarium studies on the distribution of anther-smut fungus (Microbotryum violaceum) and Silene species (Caryophyllaceae) in the eastern United States. American Journal of Botany. 2003;90:1522–1531. doi: 10.3732/ajb.90.10.1522. [DOI] [PubMed] [Google Scholar]
- Barrett LG, Thrall PH, Burdon JJ, Linde CC. Life history determines genetic structure and evolutionary potential of host-parasite interactions. Trends in Ecology and Evolution. 2008;23:678–685. doi: 10.1016/j.tree.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi G, Antonovics J, Biere A, Charlesworth D, Delph LF, Filatov D, Giraud T, Hood ME, Marais GAB, McCauley D, et al. Silene as a model system in ecology and evolution. Heredity. 2009;103:5–14. doi: 10.1038/hdy.2009.34. [DOI] [PubMed] [Google Scholar]
- Berner D, McMahon M, Kashefi J, Erbe E. First report of an ovary smut of Italian thistle caused by a Microbotryum sp. in Greece. Plant Disease. 2006;90:681. doi: 10.1094/PD-90-0681B. [DOI] [PubMed] [Google Scholar]
- Biere A, Antonovics J. Sex-specific costs of resistance to the fungal pathogen Ustilago violaceaMicrobotryum violaceum) in Silene alba. Evolution. 1996;50:1098–1110. doi: 10.1111/j.1558-5646.1996.tb02350.x. [DOI] [PubMed] [Google Scholar]
- Biere A, Honders SC. Anther smut transmission in Silene latifolia and Silene dioica: impact of host traits, disease frequency, and host density. International Journal of Plant Sciences. 1998;159:228–235. [Google Scholar]
- Bucheli E, Gautschi B, Shykoff JA. Host-specific differentiation in the anther smut fungus Microbotryum violaceum as revealed by microsatellites. Journal of Evolutionary Biology. 2000;13:188–198. [Google Scholar]
- Burleigh JG, Holtsford TP. Molecular systematics of the Eastern North American Silene (Caryophyllaceae): evidence from nuclear ITS and chloroplast trnL intron sequences. Rhodora. 2003;105:76–90. [Google Scholar]
- Cafuir L, Antonovics J, Hood ME. Tissue culture and quantification of individual-level resistance to anther-smut disease in Silene vulgaris. International Journal of Plant Sciences. 2007;168:415–419. [Google Scholar]
- Crawford RMM, Abbott RJ. Pre-adaptation of arctic plants to climate-change. Botanica Acta. 1994;107:271–278. [Google Scholar]
- Denchev CM, Giraud T, Hood ME. Three new species of Anthericolous smut fungi on Caryophyllaceae. Mycologia Balcanica. 2009;6:79–84. [Google Scholar]
- Denchev CM, Moore RT, Shin HD. A reappraisal of the genus Bauhinus (Microbotryaceae) Mycologia Balcanica. 2006;3:71–75. [Google Scholar]
- Desfeux C, Maurice S, Henry JP, Lejeune B, Gouyon PH. Evolution of reproductive systems in the genus Silene. Proceedings of the Royal Society of London B. 1996;263:409–414. doi: 10.1098/rspb.1996.0062. [DOI] [PubMed] [Google Scholar]
- Eggens F, Popp M, Nepokroeff M, Wagner WL, Oxelman B. The origin and number of introductions of the Hawaiian endemic Silene species (Caryophyllaceae) American Journal of Botany. 2007;94:210–218. doi: 10.3732/ajb.94.2.210. [DOI] [PubMed] [Google Scholar]
- Evans HC. Fungal pathogens of some subtropical and tropical weeds and the possibilities for biological control. Biocontrol News and Information. 1987;8:7–30. [Google Scholar]
- Fischer GW, Holton CS. Biology and control of the smut fungi. New York, NY, USA: Ronald Press; 1957. [Google Scholar]
- Forbis TA, Doak DF. Seedling establishment and life history trade-offs in alpine plants. American Journal of Botany. 2004;91:1147–1153. doi: 10.3732/ajb.91.7.1147. [DOI] [PubMed] [Google Scholar]
- Garber ED, Ruddat M. Transmission genetics of Microbotryum violaceumUstilago violacea): a case study. Advances in Applied Microbiology. 2002;51:107–127. doi: 10.1016/s0065-2164(02)51003-0. [DOI] [PubMed] [Google Scholar]
- Giles BE, Pettersson TM, Carlsson-Granér U, Ingvarsson PK. Natural selection on floral traits of female Silene dioica by a sexually transmitted disease. New Phytologist. 2006;169:729–739. doi: 10.1111/j.1469-8137.2005.01544.x. [DOI] [PubMed] [Google Scholar]
- Giraud T, Yockteng R, López-Villavicencio M, Refrégier G, Hood ME. Mating system of the anther smut fungus Microbotryum violaceum: selfing under heterothallism. Eukaryotic Cell. 2008;7:765–775. doi: 10.1128/EC.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt V. Vererbungsversuche mit den biologischen Arten des Antherenbrandes (Ustilago violacea Pers.) Zeitschrift fur Botanik. 1928;21:1–90. [Google Scholar]
- Heywood VH. Flowering plants of the world. New York, NY, USA: Mayflower Books, Inc; 1978. [Google Scholar]
- Holmgren PK, Holmgren NH. Index herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium; 1998. http://sweetgum.nybg.org/ih/ [Google Scholar]
- Hood ME, Antonovics J. Plant species descriptions show signs of disease. Proceedings of the Royal Society London Series B. 2003;270:S156–S158. doi: 10.1098/rsbl.2003.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Jalas J, Suominen J. Distribution of vascular plants in Europe. 3. Caryophyllaceae. In: Jalas J, Suominen J, editors. Atlas florae Europaeae. Cambridge, UK: Cambridge University Press; 1988. p. 416. [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens A. Comparative floral morphometrics in day-flowering, night-flowering and self-pollinated Caryophylloideae (Agrostemma, Dianthus, Saponaria, Silene and Vaccaria. Plant Systematics and Evolution. 2006;257:233–250. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. Reproduction and pollination in Central European populations of Silene and Saponaria species. Botanica Acta. 1996;109:316–324. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. Flower scent composition in night-flowering Silene species (Caryophyllaceae) Biochemical Systematics and Ecology. 2002a;30:383–397. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. Pollen grain numbers, ovule numbers and pollen-ovule ratios in Caryophylloideae: correlation with breeding system, pollination, life form, style number, and sexual system. Sexual Plant Reproduction. 2002b;14:279–289. [Google Scholar]
- Kemler M, Göker M, Oberwinkler F, Begerow D. Implications of molecular characters for the phylogeny of the Microbotryaceae (Basidiomycota: Urediniomycetes) BMC Evolutionary Biology. 2006;6:35. doi: 10.1186/1471-2148-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler M, Lutz L, Göker M, Oberwinkler F, Begerow D. Hidden diversity in the non-caryophyllaceous plant parasite members of Microbotryum (Pucciniomycotina: Microbotryales) Systematics and Biodiversity. 2009;7:297–306. [Google Scholar]
- Kephart S, Reynolds RJ, Rutter MT, Fenster CB, Dudash MR. Pollination and seed predation by moths on Silene and allied Caryophyllaceae: evaluating a model system to study the evolution of mutualisms. New Phytologist. 2006;169:667–680. doi: 10.1111/j.1469-8137.2005.01619.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Briefings in Bioinformatics. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Le Gac M, Hood ME, Fournier E, Giraud T. Phylogenetic evidence of host-specific cryptic species in the anther smut fungus. Evolution. 2007;61:15–26. doi: 10.1111/j.1558-5646.2007.00002.x. [DOI] [PubMed] [Google Scholar]
- Lutz M, Göker M, Piatek M, Kemler M, Begerow D, Oberwinkler F. Anther smuts of Caryophyllaceae: molecular characters indicate host-dependence species delimitation. Mycological Progress. 2005;4:225–238. [Google Scholar]
- Lutz M, Piatek M, Kemler M, Chlebicki A, Oberwinkler F. Anther smuts of Caryophyllaceae: molecular analyses reveal further new species. Mycological Research. 2008;112:1280–1296. doi: 10.1016/j.mycres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. 2009. Version 2.6 http://mesquiteproject.org. [Google Scholar]
- Maddison WP, Slatkin M. Null models for the number of evolutionary steps in a character on a phylogenetic tree. Evolution. 1991;45:1184–1197. doi: 10.1111/j.1558-5646.1991.tb04385.x. [DOI] [PubMed] [Google Scholar]
- Malmstrom CM, Shu R, Linton EW, Newton LA, Cook MA. Barley yellow dwarf viruses (BYDVs) preserved in herbarium specimens illuminate historical disease ecology of invasive and native grasses. Journal of Ecology. 2007;95:1153–1166. [Google Scholar]
- Marr DL, Delph LF. Spatial and temporal pattern of a pollinator-transmitted pathogen in a long-lived perennial, Silene acaulis. Evolutionary Ecology Research. 2005;7:335–352. [Google Scholar]
- Oxelman B, Lidén M, Rabeler RK, Popp M. A revised generic classification of the tribe Sileneae (Caryophyllaceae) Nordic Journal of Botany. 2001;20:743–748. [Google Scholar]
- Pagel M. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proceedings of the Royal Society London Series B. 1994;255:37–45. [Google Scholar]
- Piepenbring M. Smut fungi (Ustilaginomycetes and Microbotryales, Basidiomycota) in Panama. Revista de Biología Tropical. 2001;49:411–428. [PubMed] [Google Scholar]
- Popp M, Erixon P, Eggens F, Oxelman B. Origin and evolution of a circumpolar polyploid species complex in Silene (Caryophyllaceae) inferred from low copy nuclear RNA polymerase introns, rDNA, and chloroplast DNA. Systematic Botany. 2005;30:302–313. [Google Scholar]
- Popp M, Oxelman B. Origin and evolution of North American polyploid Silene (Caryophyllaceae) American Journal of Botany. 2007;94:330–349. doi: 10.3732/ajb.94.3.330. [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Rabeler RK, Hartman RL, Flora of North America Editorial Committee . Flora of North America North of Mexico 12 + vols. New York, NY, USA: Oxford University Press, New York; 2005. Caryophyllaceae; pp. 3–215. [Google Scholar]
- Refrégier G, Le Gac M, Jabbour F, Widmer A, Shykoff JA, Yockteng R, Hood ME, Giraud T. Cophylogeny of the anther smut fungi and their caryophyllaceous hosts: prevalence of host shifts and importance of delimiting parasite species for inferring cospeciation. BMC Evolutionary Biology. 2008;8:100. doi: 10.1186/1471-2148-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DA, Hooper SS. Ustilago violacea (Pers.) Fuckel on a hybrid Dianthus from South Africa. Kew Bulletin. 1956;11:163. [Google Scholar]
- Sloan DB, Giraud T, Hood ME. Maximized virulence in a sterilizing pathogen: the anther-smut fungus and its co-evolved hosts. Journal of Evolutionary Biology. 2008;21:1544–1554. doi: 10.1111/j.1420-9101.2008.01604.x. [DOI] [PubMed] [Google Scholar]
- Thrall PH, Biere A, Antonovics J. Plant life-history and disease susceptibility – the occurrence of Ustilago violacea on different species within the Caryophyllaceae. Journal of Ecology. 1993;81:489–498. [Google Scholar]
- Thrall PH, Burdon JJ. Host-pathogen life history interactions affect biological control success. Weed Technology. 2004;18:1269–1274. [Google Scholar]
- Vánky K. The genus Microbotryum (smut fungi) Mycotaxon. 1998;67:33–60. [Google Scholar]
- Vánky K. The new classification of the smut fungi, exemplified by Australasian taxa. Australian Systematic Botany. 2001;14:85–394. [Google Scholar]
- Vánky K. Three smut fungi new for Europe. Nova Hedwigia. 2005;80:387–396. [Google Scholar]
- Zhou L, Wu ZY, Lidén M, Oxelman B. Silene (110 spp.) In: Wu Z-Y, Raven PH, editors. Flora of China, Vol 6. Beijing: Missouri Botanical Garden; 2001. pp. 66–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


