Abstract
Background:
Azadirachta indica has been reported to correct altered glycaemia in diabetes.
Objective:
The aqueous extract of A. indica leaf and bark has been evaluated for its effect on antioxidant status of alloxan diabetic rats and compared with insulin treatment.
Materials and Methods:
The oral effective dose of A. indica leaf (500 mg/kg body weight) and A. indica bark (100 mg/kg body weight) were given once daily for 21 days to separate groups of diabetic rats. At the end of the experimental period blood glucose level and activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), glucose-6-phosphate dehydrogenase (G-6-PD), and membrane lipid peroxidation were determined in different fractions of liver and kidney tissues.
Results:
Diabetic rats showed high blood glucose (P<0.01), increased level of malondialdehyde (P<0.05) and a significant decrease in the activity of antioxidant enzymes. Treatment with insulin, A. indica leaf extract (AILE), and A. indica bark extract (AIBE) restored the above altered parameters close to the control ones.
Conclusions:
Both AILE and AIBE were found significantly effective in reducing hyperglycemia-induced oxidative stress. The findings suggest further investigations for the possible use of A. indica as alternative medicine to prevent long-term complications of diabetes.
Keywords: Azadirachta indica, glutathione peroxidase, oxidative stress, superoxide dismutase
INTRODUCTION
Diabetes mellitus is a common disorder associated with increased morbidity, mortality rate, and can be characterized by hyperglycemia due to defects in insulin secretion, insulin action, or both, causing metabolic and physiological changes in various organs.[1] Hyperglycemia is the important factor in the onset and progress of diabetic complications mainly by producing oxidative stress.[2] Oxidative stress may result from over production of precursors to oxygen free radicals and/or decreased efficiency of antioxidant system.[3] Superoxide dismutase, catalase, and glutathione peroxidase (GPx) are the biological antioxidant enzymes that directly scavenge free radicals and prevent their conversion to toxic products.[4] Diabetes is associated with altered levels of these enzymes that result in increased oxidative stress, which may be caused due to several mechanisms, including glucose autoxidation and nonenzymatic glycation.[5–8]
The discovery of insulin was a boon for the treatment of diabetes mellitus but it fails to prevent the long-term complications. Various hypoglycemic drugs are also being used for the treatment but their use is restricted by their limited action and accompanying side effects. During the last five decades, considerable progress has been achieved regarding the biological activity and medicinal application of Azadiracta indica A Juss (Neem). Each part of Neem tree has some medicinal property and thus commercially exploitable.[9] A. indica has been commonly used to treat diabetes in Indian system of medicine from time immemorial.[10] There are several reports that suggest the hypoglycemic potential of A. indica.[11–13] The present study explores the possibility of using A. indica leaf and bark extracts, and evaluates their effect on altered level of antioxidant enzymes in different tissues of alloxan-induced diabetic rat model.
MATERIALS AND METHODS
A. indica leaf extract (aqueous)
One kilogram of freshly collected, shade dried, powdered leaves of A. indica were ground in 4 L of distilled water and allowed to soak overnight at room temperature. The suspension was then centrifuged at ×5000g for 20 min and filtered through Whatman No. 1 filter paper. The filtrate was lyophilized to yield 12.9 g of dry powder and stored at –20°C. A measured amount of the extract was dissolved in distilled water at a suitable concentration prior to experiment.
A. indica bark extract (aqueous)
Air dried bark from a full grown Neem tree, devoid of external hard wood was cut into small pieces and 100 g was soaked overnight in 1 L distilled water at room temperature with occasional shaking. After filtration, the brown red extract was lyophilized to yield 3.7 g of dry powder and stored at –20°C. A required concentration of powder was prepared in distilled water prior to experiment.
Animals treatment
Healthy female rats of Wistar strain (2–3 months old) weighing between 180 and 210 g were selected for the study. All animals were kept at 25°C–30°C and 45%–55% relative humidity, acclimatized with standard chow and water ad libitum throughout the study under 12:12 h light:dark cycle. Diabetes was induced by following the method of Sochor et al.[14] All animals were carefully monitored and experimental protocols were in accordance with the recommendations of the institutional Animal Ethical Committee. Alloxan monohydrate, insulin, and glutathione reductase (GR) were purchased from Sigma Aldrich, USA and other chemicals and reagents used were of analytical grade.
The rats with fasting glucose level above 360 mg/dL were considered diabetic and randomly divided into the following groups (n=6):
Group 1: Normal Control (NC) treated with vehicle alone (healthy rats with normal blood glucose level).
Group 2: Diabetic Control (DC) untreated.
Group 3: Insulin-treated diabetic group (D+I) (received intraperitoneal injection of 2 units of protamine–zinc insulin once daily).
Group 4: A. indica Leaf Extract–treated diabetic group (D+AILE) (500 mg/kg body weight).
Group 5: A. indica Bark Extract–treated diabetic group (D+AIBE) (100 mg/kg body weight).
The experimental groups received orally aqueous solution of extract by intragastric tube. For each dose, the required amount of the lyophilized powder calculated from the body weight of the animal was weighed, dissolved in distilled water at a suitable concentration so that the desired amount for each dose can be administered in 0.5 mL to each animal. To ensure the stability of the crude extracts and thus the efficacy, the treatment was given for 21 days. Effective dosages were selected after doing pilot studies.[15]
Estimation of enzyme activities
At the end of the experiment, rats were sacrificed by cervical dislocation. Liver and kidney were rapidly excised and washed with chilled normal saline. The tissues were then blotted dry and weighed. Tissue homogenates (10% w/v) were prepared in 0.25 M Sucrose, 0.02 M Triethanolamine hydrochloride buffer of pH 7.4 containing 0.12 M Dithiothreitol (DTT). Homogenates were then centrifuged at ×1000g for 10 min to remove nuclei and cell debris. The supernatant was again centrifuged at ×12,000g for 40 min to obtain cytosolic fraction. All the procedures were carried out at 4°C. The supernatants were then used for determination of enzyme activity.
The activity of superoxide dismutase (SOD) was measured by the method of Marklund and Marklund.[16] The change in absorbance was monitored at 420 nm for 3 min at 25°C against blank. One unit of enzyme activity is defined as the amount of enzyme that causes 50% maximal inhibition of pyrogallol autoxidation. The assay of catalase (CAT) was performed by following the method of Aebi.[17] Change in absorbance was monitored at 240 nm at 25°C. One unit of enzyme activity is defined as the amount of enzyme required to decompose 1 μmol of H2O2. The activity of GPx was measured using a coupled enzyme assay as described by Lawrence and Burk.[18] The decrease in absorbance was monitored at 25°C at 340 nm. One unit of enzyme activity is defined as 1 μmol of nicotinamide adenine dinucleotide phosphate oxidase (NADPH) oxidized/min/mg protein. The GR activity was measured in the soluble tissue extracts by the method of Erden et al.[19] The decrease in absorbance was monitored at 25°C at 340 nm. One unit of enzyme activity is defined as 1 μmol of NADPH oxidized/min/mg protein. The glucose-6-phosphate dehydrogenase (G-6-PD) activity was assayed by following the method described by Cohen and Rosemeyer.[20] The change in absorbance was monitored at 340 nm for 3 min at 25°C against blank. One unit of enzyme activity is defined as 1 nmol NADP reduced/min/mg protein.
Lipid peroxidation
The level of lipid peroxidation was assessed by measuring the formed malondialdehyde (MDA), an end product of fatty acid peroxidation, by using thiobarbituric acid reactive substance (TBARS) method.[1] The concentration of MDA–TBA complex was determined spectrophotometrically at 532 nm against blank and results are expressed as nmol MDA formed/mg protein.
Glycemic index
Blood glucose was determined by using Glucose-kit from Span Diagnostics, India, which quantitatively estimates D-glucose, the form that is present in blood plasma. Glycosylated hemoglobin (GHbA1c) was estimated by Ion Exchange Resin method using kit purchased from Aristha Pharmaceuticals, India. Soluble protein was determined by method of Bradford using bovine serum albumin as standard.[21]
Statistical analysis
All values were calculated as mean±SEM. The ANOVA test followed by Dunnett's multiple comparison test was employed for statistical comparison between control and various groups. Significance was considered at P<0.05.
RESULTS
General parameters
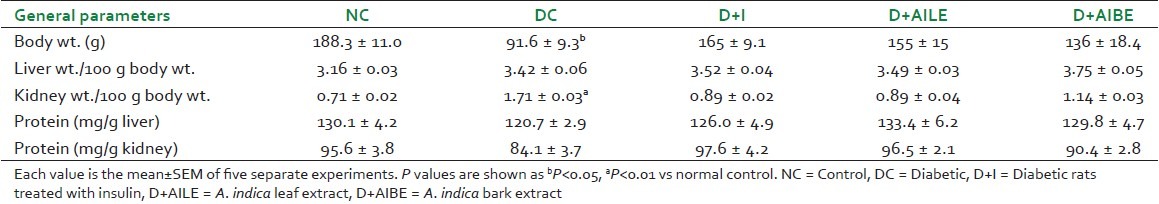
All diabetic animals showed the symptoms of glycosuria, polydipsia, polyphagia, and reduced rate of growth. Physiological parameters, such as body weight, tissue weight, and tissue protein, are summarized in Table 1. After 21 days of diabetes induction, body weight was significantly reduced in the diabetic group. Both AILE and AIBE were able to restore back the body weight of the diabetic rats to control levels. Liver weight of the diabetic rats decreased when compared with that of the controls, although the same being compared on a functional basis as liver/100 g body weight did not show any significant difference between the control, diabetic, and the treatment groups. There was an increase in kidney weight of diabetics as compared with the controls and the difference in the weights was even more significant when expressed as per 100 g body weight.
Table 1.
General parameters of all the groups
Alloxan-induced diabetic rats showed marked hyperglycemia with almost 3-fold higher blood glucose concentration when compared with the control values [Table 2]. Treatment with AILE and AIBE, however, resulted in a partial revival of euglycemia with improved body weight. As evident from Table 2, AILE was more effective in lowering hyperglycemia in diabetic rats as compared with AIBE. Glycosylated hemoglobin, measured as % of HbA1c, was significantly increased (P<0.05) in untreated diabetic animals when compared with healthy controls [Table 2]. Although, the decrease of HbA1c was observed in all the diabetic-treated groups, treatment was more effective in AILE-treated group.
Table 2.
Changes in glycemic index in all the groups

There was no significant change in the tissue protein content of diabetic and diabetic-treated groups when compared with controls. All the enzyme activities are expressed as per milligram protein and therefore, represent true changes under these conditions.
Effect on antioxidant enzymes
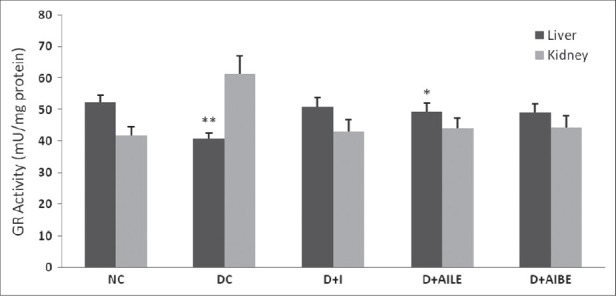
As is evident from Figure 1, SOD activity showed a significant reduction in diabetic rat liver (P<0.01), which agrees well with earlier published data.[5] Similarly, kidney showed a lower activity of SOD in the diabetic state (P<0.05). Figure 2 shows CAT activity decreasing significantly in liver and kidney of diabetic rats. In diabetic state, GPx activity decreased significantly in liver (P<0.05) and increased in kidney [Figure 3]. GR activity decreased significantly in liver and shows a small increase in the kidney of diabetic rat [Figure 4]. Treatment of diabetic animals with insulin, AILE, and AIBE reversed the changes in these enzymes to control values.
Figure 1.
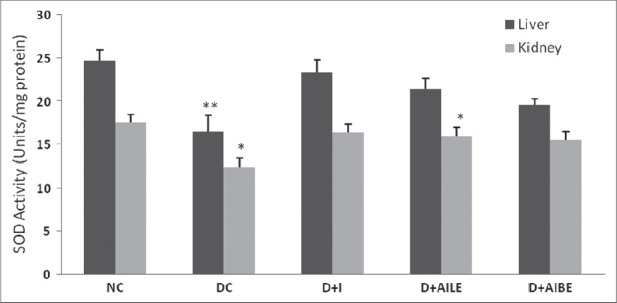
Change in the activity of superoxide dismutase in liver and kidney of control, diabetes-induced, and diabetic rats after 21 days of treatment. Results are expressed as mean±SEM of five separate experiments. *P<0.05, **P<0.01 vs normal control
Figure 2.
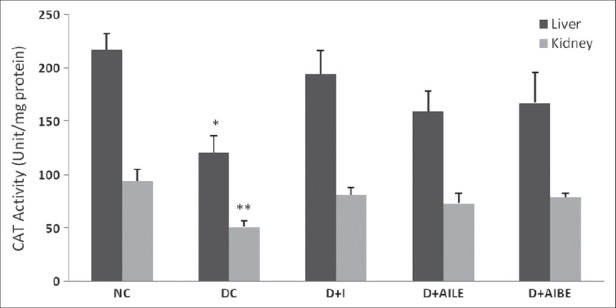
Change in the activity of catalase in liver and kidney of control, diabetes-induced, and diabetic rats after 21 days of treatment. Results are expressed as mean±SEM of five separate experiments. *P<0.05, **P<0.01 vs normal control
Figure 3.
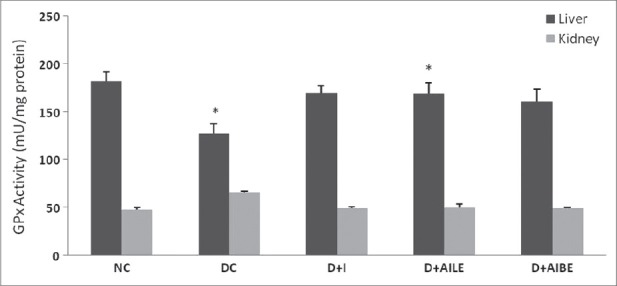
Change in the activity of glutathione peroxidase in liver and kidney of control, diabetes-induced, and diabetic rats after 21 days of treatment. Results are expressed as mean±SEM of five separate experiments. *P<0.05 vs normal control
Figure 4.
Change in the activity of glutathione reductase in liver and kidney of control, diabetes-induced, and diabetic rats after 21 days of treatment. Results are expressed as mean±SEM of five separateexperiments. *P<0.05, **P<0.01 vs normal control
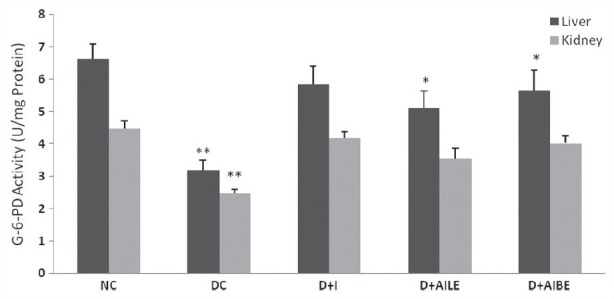
In hyperglycemic rats G-6-PD activity reduced significantly (P<0.01) in liver and kidney tissues [Figure 5]. Treatment with insulin, AILE, and AIBE restored the altered activity close to control levels. AIBE was more effective in reversing the change in G-6-PD activity.
Figure 5.
Change in the activity of glucose-6-phosphate dehydrogenase in liver and kidney of control, diabetes-induced, and diabetic rats after 21 days of treatment. Results are expressed as mean±SEM of five separate experiments. *P<0.05, **P<0.01 vs normal control
Effect on lipid peroxidation
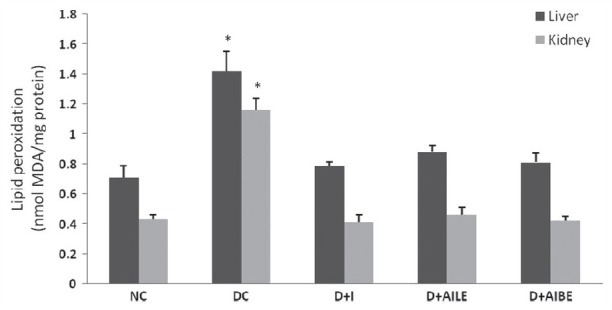
In the present study, the level of lipid peroxidation was determined in control, diabetes-induced, and diabetic rats treated with insulin and A. indica extracts, and results are summarized in Figure 6. Lipid peroxidation was found to increase in both liver and kidney of diabetic rats as results showed significant rise (P<0.05) in MDA level. Treatment with AILE and AIBE reversed the above-altered parameter to normal values. AIBE was found to be more effective in controlling diabetes induced changes in lipid peroxidation.
Figure 6.
Change in the malondialdehyde levels in liver and kidney of control, diabetes-induced, and diabetic rats after 21 days of treatment. Results are expressed as mean±SEM of five separate experiments. *P<0.05 vs normal control
DISCUSSION
The present study showed that 21 days of treatment with insulin, AILE, and AIBE resulted in a marked reduction in hyperglycemia in diabetic animals and also improved the bodyweight. Therefore, after showing the improvement in glucose homeostasis in the treated animals, the metabolic consequences of this treatment in liver and kidney was further examined.
In diabetes, the persistence of hyperglycemia has been reported to cause increased production of oxygen free radicals through auto-oxidation and nonenzymatic glycation. If the diabetic state is associated with a generalized increase in tissue oxidative stress it might be reflected in the changes in tissue antioxidant system. Therefore, the activities of some major antioxidant enzymes were measured in control and experimental rats. Diabetic rats showed altered levels of the antioxidant enzymes studied in liver and kidney, thus generating oxidative stress in these tissues. Under normal physiological condition there is a critical balance in the generation of oxygen free radicals and its antioxidant defense systems used by organism to deactivate and protect themselves against free radical toxicity.[22] Impairment in the oxidant/antioxidant equilibrium in favor of the former provokes a situation of oxidative stress, which is known to be a component of molecular and cellular tissue damage mechanisms in a wide spectrum of human diseases and contributes substantially to the pathogenesis of diabetic complications.[23] In vitro and in vivo studies have reported that in a variety of tissues, hyperglycemia and possibly elevated free fatty acid levels result in the generation of oxygen free radicals and considerably increased oxidative stress.[24–26]
Various tissues are more prone to oxidative damage and could result in various complications in long-term diabetes, implying that, the restoration of antioxidant status is an important parameter to evaluate the effect of antidiabetic compound. In the present study, major antioxidant enzymes, such as SOD, CAT, GPx, and GR, are studied to evaluate the antioxidant status in liver and kidney of A. indica-treated diabetic rats. Treatment with insulin, AILE, and AIBE restored the altered levels of SOD, CAT, GPx, and GR close to their normal values in liver and kidney of diabetic rats. The efficacy of AILE and AIBE treatment was found comparable to the insulin treatment. The mechanism responsible for the decreased activity of these enzymes in diabetic state is not fully known, it is suggested that phosphorylation and decreased expression may play a role.
NADPH is the principle intracellular reductant and the entire antioxidant system relies on an adequate supply of NADPH. G-6-PD is the principle source of NADPH,[27,28] which is of central importance to cellular redox regulation and any changes in G-6-PD will alter NADPH levels and thus impact the entire antioxidant system and makes tissues very vulnerable to oxidative damage.[29] Also, G-6-PD is the rate limiting enzyme of pentose phosphate pathway, which helps in maintaining the normal blood glucose level.[30] Any imbalance in glucose homeostasis will result in altered activity of the enzyme. Significantly, reduced activity of G-6-PD was observed in hyperglycemia-induced diabetic rats. The treatment with AILE and AIBE resulted in euglycemia and significant restoration of the G-6-PD activity in liver and kidney.
Hyperglycemia has been shown to generate free radicals from auto-oxidation of glucose, formation of advanced glycated end products, and increased polyol pathway with concomitant increase in cellular lipid peroxidation and damage of cellular membranes.[31] In the present study the formation of TBARS, a product of lipid peroxidation, was significantly increased in diabetic liver and kidney tissues. The results are in agreement with earlier published data of Kakkar et al.,[32] and Mohamad et al.[33] This increased lipid peroxides formation during diabetes disturbs the anatomical integrity of the membrane leading to the inhibition of several membrane bound enzymes.[34] The results revealed a sharp decreased level of TBARS in diabetic rats treated with insulin, AILE and AIBE when compared to untreated diabetic rats indicating the efficacy of treatment.
A. indica may exhibit its therapeutic effects through modulation of insulin secretion. The present study showed the hypoglycemic and antioxidant properties of A. indica. A reduction in the production of free radicals and lipid peroxides formation by restoring the antioxidant enzymes was observed in the present study, which can beneficially prevent the diabetes-associated tissue damage. As concluded from the studied parameters, AILE and AIBE were found effective in controlling the hyperglycemia-induced oxidative stress and stabilizing the physiological parameters and antioxidant defense system. Further studies are in progress to ascertain which photochemical fraction is most efficacious in the treatment of diabetes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Genet S, Kale RK, Baquer NZ. Alteration in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: Effect of vanadate and fenugreek (Trigonella foenum graecum) Mol Cell Biochem. 2002;236:7–12. doi: 10.1023/a:1016103131408. [DOI] [PubMed] [Google Scholar]
- 2.Giugliana D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–67. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 3.Baynes JW. Role of oxidative stress in development of complications of diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 4.Freeman BA, Crapo JD. Biology of diseases free radicals and injury. Lab Invest. 1982;47:412–26. [PubMed] [Google Scholar]
- 5.Wohaieb SA, Godin DV. Alterations in free radicals tissue defence mechanisms in streptozotocin-induced diabetes in rats: Effects of insulin treatment. Diabetes. 1987;36:1014–8. doi: 10.2337/diab.36.9.1014. [DOI] [PubMed] [Google Scholar]
- 6.Hunt JV, Smith CT, Wolff SP. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990;39:1420–4. doi: 10.2337/diab.39.11.1420. [DOI] [PubMed] [Google Scholar]
- 7.Ceriello A, Quatraro A, Giugliano D. New insights on non-enzymatic glycosylation may lead to therapeutic approaches for the prevention of diabetic complications. Diabet Med. 1992;9:297–9. doi: 10.1111/j.1464-5491.1992.tb01783.x. [DOI] [PubMed] [Google Scholar]
- 8.Packer L. The role of antioxidative treatment in diabetes mellitus. Diabetologia. 1993;36:1212–3. doi: 10.1007/BF00401070. [DOI] [PubMed] [Google Scholar]
- 9.Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay V. Biological and medicinal properties of Neem (Azadirachta indica) Curr Sci. 2002;82:1136–45. [Google Scholar]
- 10.Shukla R, Sharma SB, Prabhu KM, Murthy PS. Medicinal plant for treatment of diabetes mellitus. Ind J Clin Biochem. 2000;15:169–77. doi: 10.1007/BF02867556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehdi AA, Chandra A, Singh K, Shukla S, Mishra LC, Ahmed S. Effect of herbal hypoglycemic agents on oxidative stress and antioxidant status in diabetic rats. Indian J Clin Biochem. 2003;18:8–15. doi: 10.1007/BF02867361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Hawary ZM, Kholeif TS. Biochemical studies on hypoglycemic agents (1) Effect of Azadirachta indica leaf extract. Arch Phytother Res. 1990;13:108–12. [Google Scholar]
- 13.Chattopadhyaya RR. Possible mechanism of antihyperglycemic effect of Azadirachta Indica leaf extract. J Ethnopharmacol. 1990;67:373–6. doi: 10.1016/s0378-8741(99)00094-x. [DOI] [PubMed] [Google Scholar]
- 14.Sochor M, Baquer NZ, Mclean P. Glucose over and under utilization in diabetes: Comparative studies on changes in activities of enzymes of glucose metabolism in rat kidney and liver. Mol Physiol. 1985;7:51–68. [Google Scholar]
- 15.Shailey S, Basir SF. Long term effect of Azadirachta indica on hyperglycemia, associated hyperlipidemia and tissue glycogen content in alloxan induced diabetic rats. J Pharm Res. 2011;4:3. [Google Scholar]
- 16.Maklund S, Maklund G. Involvement of superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Bichem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 17.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods in enzymatic analysis. New York: Academic Press; 1974. pp. 673–84. [Google Scholar]
- 18.Lawrence RA, Burk RF. Gutathione peroxidase activity in selenium deficient rat liver. Biochem Biophys Res Commun. 1976;71:1161–5. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 19.Erden M, Bor NM. Changes in reduced glutathione, glutathione reductase and glutathione peroxidase after radiation in guinea pigs. Biochem Med. 1984;31:217–27. doi: 10.1016/0006-2944(84)90026-7. [DOI] [PubMed] [Google Scholar]
- 20.Cohen P, Rosemeyer MA. Glucose-6-phosphate dehydrogenase from human erythrocytes. In: Colowick SP, Kaplan NO, editors. Methods in Enzymology. Vol. 41. New York: Academic Press; 1975. pp. 208–16. [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 22.Sies H. Oxidative stress: Oxidants and antioxidants. New York: Academic Press; 1991. pp. 2–8. [DOI] [PubMed] [Google Scholar]
- 23.Halliwel B. Free radicals, antioxidants and human diseases: Cause or consequence? Lancet. 1994;344:721–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 24.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress mediated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 25.West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–80. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 26.Mak DH, Ip SP, Li PC, Poon MK, Ko KM. Alterations in tissue glutathione antioxidant system in streptozotocin-induced diabetic rats. Mol Cell Biochem. 1996;162:153–8. doi: 10.1007/BF00227543. [DOI] [PubMed] [Google Scholar]
- 27.Hashida K, Sakakura Y, Makino N. Kinetic studies on the hydrogen peroxide elimination by cultured PC12 cells: Rate limitation by glucose-6-phosphate dehydrogenase. Biochim Biophys Acta. 2002;1572:85–90. doi: 10.1016/s0304-4165(02)00282-9. [DOI] [PubMed] [Google Scholar]
- 28.Leopold JA, Zhang YY, Scribner AW, Stanton RC, Loscalzo J. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arteriosceler Thromb Vasc Biol. 2003;23:411–7. doi: 10.1161/01.ATV.0000056744.26901.BA. [DOI] [PubMed] [Google Scholar]
- 29.Felix K, Rockwood CD, Pretsch W, Bornkamm GW, Janz S. Redox imbalance and mutagenesis in spleens of mice harboring a hypomorphic allele of Gpdx(a) encoding glucose-6-phosphate dehydrogenase. Free Radic Biol Med. 2003;34:226–32. doi: 10.1016/s0891-5849(02)01243-1. [DOI] [PubMed] [Google Scholar]
- 30.Mayes PA. The pentose phosphate pathway and other pathway of hexose metabolism. In: Murray RK, Granner DK, Mayes VW, editors. Herper's Biochemistry. USA: McGraw-Hill; 2000. pp. 219–37. [Google Scholar]
- 31.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free fatty acids stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–45. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 32.Kakkar R, Kalra J, Mantha SV, Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem. 1995;151:113–9. doi: 10.1007/BF01322333. [DOI] [PubMed] [Google Scholar]
- 33.Mohamad S, Taha A, Bamezai RN, Basir SF, Baquer NZ. Lower doses of vanadate in combination with Trigonella restore altered carbohydrate metabolism and antioxidant status in alloxan-diabetic rats. Clin Chim Acta. 2004;342:105–14. doi: 10.1016/j.cccn.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Jamme I, Petit E, Divoux D, Gerbi A, Maixent JM, Nouvelot A. Modulation of mouse cerebral Na+/K+ATPase activity by oxygen free radicals. Neuroreport. 1995;29:333–7. [PubMed] [Google Scholar]


