Abstract
Background:
Pulmonary tuberculosis (PTB) is an age old disease described in Vedic Medicine as ‘Yakshma’. Later on, in Ayurveda it earned a prefix and found way into mythology as ‘Rajayakshma’. After the discovery of streptomycin, the therapeutic management of PTB received a major breakthrough. The treatment module changed remarkably with the formulation of newer anti-tubercular drugs (ATD) with appreciable success. Recent resurgence of PTB in developed countries like United States posed a threat to the medical community due to resistant strains. Consequently, WHO looked toward traditional medicine. Literature reveals that Ayurvedic treatment of PTB was in vogue in India before the introduction of ATD with limited success. Records show that 2766 patients of PTB were treated with Ayurvedic drugs in a tertiary care hospital in Kolkata in the year 1933-1947.
Objectives:
To evaluate the toxicity reduction and early restoration by adjunct therapy of Ayurvedic drugs by increasing the bio-availability of ATDs.
Materials and Methods:
In the present study, treatment response of 99 patients treated with ATD as an adjunct with Aswagandha (Withania somnifera) and a multi-herbal formulation described in Chikitsa-sthana of Charaka samhita i.e. Chyawanprash were investigated. Hematological profile, sputum bacterial load count, immunoglobulin IgA and IgM, blood sugar, liver function test, serum creatinine were the assessed parameters besides blood isoniazid and pyrazinamide, repeated after 28 days of treatment.
Results:
The symptoms abated, body weight showed improvement, ESR values were normal, there was appreciable change in IgA and IgM patterns and significantly increased bioavailability of isoniazid and pyrazinamide were recorded.
Conclusion:
This innovative clinical study coupled with empowered research may turn out to be promising in finding a solution for the treatment of PTB.
Keywords: Adjunct therapy, anti-tubercular drugs, Ayurveda, tuberculosis
INTRODUCTION
Yakshma, a disease of ancient origin described in Vedic medicine, could be correlated well with Tuberculosis.[1] This belief-based concept seems to have been well ruminated upon, and eventually entered reason-based Ayurvedic treatises with a redefined name Rajayakshma. Rajayakshma (PTB) is considered to be a disease of grave prognosis along with Udara (ascites) and Balashosha (marasmus). Many religious, mythological and historical episodes are linked with these dreadful diseases. Many herbal and herbo-mineral drugs are being used for the treatment of Rajayakshma. However, the results are far from satisfaction.
In Rajayakshma, Dhatukshaya (tissue emaciation or loss) is universally accepted as one of the main reasons to initiate pathogenesis. And there is inevitable metabolic dysfunction (Dhatwagninasana), out of which rasa (tissue fluid), rakta (blood), mamsa (muscle), meda (adipose tissue) and sukra (generative tissue) are lost. Ultimately, deterioration of immunity (ojokshaya) is evident. In tuberculosis starting from Ojokshaya, sukra, meda dhatus to rasa dhatu is lost preceding each other, which is known as Pratilomakshaya and is an unusual metabolic change.[2] In Atharvaveda (6/13/1) involvement of ojas has been demonstrated and linked with Rajayakshma. Yakshma spreads from one person to another (Jayana) like the flight of birds (Atharvaveda 6.14.3).[1] Recent studies have revealed that Auyrvedic Rasayana like Aswagandha and Shilajeet therapy could modulate the immune profile in patients.[3]
J. B. Roy State Ayurvedic Medical College and Hospital, Kolkata has a glorious past. Established on 16th February 1916, systemic research started here as early as 1921 to develop newer Ayurvedic formulations for PTB. Pioneering initiative was taken by Kaviraj Gananath Sen (Ayurvedic Scientist) who prepared aerosol and medicated enema from indigenous plants and tried it on hospitalized patients.
Ayurvedic treatment of tuberculosis was initiated in 1933 by the establishment of Patipukur TB Hospital, Kolkata. Later on, a full-fledged research unit was commissioned with exclusive budget. Treatment guidelines were adopted on Ayurvedic principles for therapeutic management which was an unique effort of its kind in Pre-Independence India. This regimen was discontinued from 1st November 1947 on the introduction of synthetic ATDs. Drugs containing mercury, gold, calcium was prepared at the in-house pharmacy and was administered to the patients with fresh juice of herbs cultivated in the hospital garden. Formulations like Vasantamalati, Kanchanabhra rasa, Rajamriganka rasa were under use including Bhallataka (Semicarpus anacardium) rasayan, Mallasindura, Vasa (Adatoda vasica) etc. The statistics on the treatment of PTB using Ayurvedic medicine over a period of 13 years is of immense value.[4]
A few research outcomes relevant for public health from the Indian systems of medicine: Tuberculosis – The Patipukur TB Hospital was established in Calcutta in 1933 specifically for managing TB patients through Ayurveda drugs. The Ayurvedic management of PTB was discontinued in the year 1947 with the development and availability of modern anti-TB drugs. Modern TB-drugs were administered to about 2766 patients and the cure rate turned out to be 11.42% and the death rate 40.9%. When a group of patients on modern anti-TB drugs received supplementary Ayurvedic drugs, the cure rate was 41.3% and the death rate was 3.8%. Studies have also been conducted to assess the role of Ayurvedic treatment in resistant cases.[4]
In-house treatment was discontinued at the hospital and only outpatient treatment was monitored by the Department of Health and Family Welfare, Government of West Bengal. PTB being a global emergency is a threat to the world medical community. Increasing trend of multi drug resistance (MDR) among patients is alarming.[5] Keeping in view the possible toxic component of the anti-tubercular drug (ATD) therapy, the present study was started with two objectives at the initial stage:
Whether add-on therapy of Ayurvedic drugs along with ATD is in anyway beneficial to prevent or reduce toxicity?
Whether this module can increase the bio-availability of ATD and help in quick recovery?
In the present study Aswagandha, a well-known immuno-modulator with potential anti-stress activity and ancient Ayurvedic formulation Chyawanprash, which is used in Ayurveda for the treatment of cough, cold, tuberculosis and also for immune protection, were used as an adjunct to anti-tubercular drugs as an add-on therapy.[6–10]
MATERIALS AND METHODS
The clinical study is conducted in phases comprising 99 newly diagnosed pulmonary tuberculosis patients from both the sexes aged between 10 and 65 years and randomly divided into two groups irrespective of age, sex and religion.
Type of study conducted
Initial exploratory observational study (pilot study) and an open labeled trial with therapeutic control add on therapy.
Place of study
Patipukur T B Hospital annexed to J B Roy State Ayurvedic Medical College and Hospital, Department of Health and Family Welfare, Government of West Bengal, Kolkata and Southern Health Improvement Samity (Pioneer and Peer NGO, WHO affiliated, devoted for Tuberculosis Eradication, Implementing DOTs), Bhangar, 24 Parganas (South), Rural hospital in Sundarban area, 30 km away from Kolkata, West Bengal, India.
Enrollment and co-ordination procedure
Pulmonary tuberculosis patients judged eligible by the inclusion and exclusion criteria were formally informed about the study and those patients who agreed to participate followed by written informed consent were enrolled and randomly allotted to the different study groups. Inclusion criteria were:
Freshly diagnosed case of PTB
Age in between 12 years and 60 years
Both male and female subjects
No history of being administered any ATDs
Not suffering from any other chronic infections including HIV
No history of any concomitant serious illness related to brain, heart, kidney, liver etc. or hormonal disorders.
Patients who reported to the outpatient department of both the hospitals with complaint of cough for more than three weeks, sputum production, loss of body weight, respiratory symptoms like chest pain, breathlessness, constitutional symptoms like night sweating, anorexia, lassitude, debility, loss of body tissue, newly registered patients diagnosed of PTB with clinical investigations like sputum AFB positive, X-ray of chest (PA view) in compliance with the inclusion criteria and those patients who had not received ATD previously were included in the study.
Extra pulmonary tuberculosis patients who received ATD earlier, pregnant women, breast feeding mothers, those with other concomitant disorders, those who have received other drugs, those with complications like pleural effusion, fungal colonization of cavities, right ventricular failure, chronic renal failure, positive HIV with or without ARV therapy and those who are not ready to comply the trial protocol were excluded.
Assessment criteria
The confirmed patients of pulmonary tuberculosis fulfilling the inclusion and exclusion criteria were assessed with fixed subjective criteria like cough with or without fever for more than three weeks, sputum production, and debility, loss of body tissue, weakness and general condition of health. The objective criteria comprising of sputum AFB positive, X-ray of chest (PA view) and Monteux test and investigations including sputum bacterial load (negative patients after treatment consecutive three days on 24 h collected sputum), Hb%, ESR, absolute lymphocyte count, blood sugar, blood urea and serum creatinine), liver function test (serum bilirubin, SGOT, SGPT, alkaline phosphates), serum protein, Immunoglobulin like IgA, IgM and blood isoniazid and pyrazinamide, total body water, fat free mass, total body fat and BMI were estimated.[11–13]
Ethical consideration
The study was conducted with Good Clinical Practice and was confirmed by the approval of the Institutional Ethical committee, formed by the ICMR guidelines by the Department of Health and Family Welfare, Government of West Bengal. Provisions were made for withdrawal from the study for any reliable reason. Patients Information and IC in local language, i.e. Bengali was approved by IEC(HR) and in case of minors, the written consent was obtained from the minor as well as from legal guardian for compliance. All procedures in the study were carried out by maintaining strict confidentiality. Patient's identity, medical condition and data were not disclosed to or discussed with any third party. Though serious adverse effects were not expected, case record form and protocol had provisions for inclusion of serious adverse events just in case.
Medication and treatment plan
Anti-tubercular Drugs – Rifampicin, Pyrazinamide, Ethambutol, Isoniazid were supplied from the Hospital.
Ayurvedic drugs Aswagandha (Withania somnifera) and Chyawanprash, both the drugs as defined were gifted by Dabur Research Foundation, New Delhi.
Aswagandha (StresscomÒ capsule) 500 mg - 2 caps twice daily for 28 days
Chyawanprash (Indian Pharmacopeia) 1 kg bottle - 10 g thrice daily for 28 days
INVESTIGATIONS
Hematological and other biochemical parameters were determined according to standard method of investigation followed in the hospital laboratory.[12]
Immunoglobulin IgA, IgM, was estimated by agar gel prepared with human antibody IgA, IgM separately. The zone was calculated and matched with the standard scale provided by Hoechst, India.[14]
Sputum acid fast stain smear test was done microscopically. Presence of Acid fast bacilli was done. Further, the bacterial count of the smear field was counted microscopically.[15]
Plasma isonized and pyrazinamide were estimated in plasma following the method of Oslan and Deylon as described by J. Chattopadhyay, spectro-flurometrically at the Department of Chemistry, Bose Institute, Kolkata.[11,16]
Total body water (TBW), fat free mass (FFM), body mass index ( BMI) and total body fat (TBF) estimation on selected patients of pulmonary tuberculosis and of normal matching age volunteers were subjected to body composition (bioelectrical impedance study) employing the instrument Tanita (Germany). The results are calculated using programmed software. Bioelectrical impedance analysis (BIA) is a rapid, non-invasive and relatively inexpensive method for evaluation of body composition.[17]
Quality control and standardization
Quality control and standardization of Aswagandha (StresscomÒ Capsule) were done employing HPLC and HPTLC finger printing methods with withaferin A as standard. Quality control data of Chyawanprash was supplied from the Dabur Research Foundation [Figures 1, 2, 3].
Figure 1.

Chemical structure of withaferin A
Figure 2.

HPLC study of Aswagandha (StresscomÒ)
Figure 3.

HPTLC study of Aswagandha (StresscomÒ)
Chyawanprash is one of the oldest Ayurvedic formulations described in Charakasamhita in rasayanachikitsa, presently categorized as Awaleha-paka.[18] The pulp of main ingredient (Pradhan dravya) Amalaki (Phyllanthus emblica) is fried in ghee and sesame oil and Yamakadravyas.
After proper frying, decoction prepared with 41 herbs (Samsadhana dravya) is added with sugar (Bhavana dravya) and boiled up to semisolid form and taken out from the furnace but stirring continued. Thereafter, powder of seven herbs (Prakshepa dravya) along with honey is added and stirring is continued till a homogenous mixture is obtained. The finished product is being standardized with solvent extracts. The appearance of the semi-solid mass was brownish black, predominantly having sweet and pungent aroma, sweet and astringent after taste with smell of prakshepa dravyas.[19]
Water solubility is 7.51%, saline solubility is 7.72% and saturation is 60%. It contains sterol, reducing sugar, glycosides, alkaloids besides Na, K, Ca, Fe, silica, oxalate and phosphate etc. On HPTLC finger printing numerous spots are detected with different solvent extracts e.g. hexane, chloroform, butane and alcohol. In mobile phase toluene, ethyl acetate and methanol 80:20:2 was used. But standardization is done for quality control with alcoholic extract of Chyawanprash. Four prominent spots are detected at Rf value 0.097, 0.23, 0.487 and 0.575 under UV 254 and 366 nm.[18,20]
RESULTS
In total 110 patients were registered in the pilot and therapeutic control study. In pilot study, 41 patients registered and among them 2 dropped out. While in therapeutic control 69 patients were registered, out of them 9 dropped out. Out of 99 patients, 98 pertaining to both males and females are within the age group 16 to 60 years of age (one patient aged 11 yrs). The patients under this study include, Hindu 46 and Muslim 74; Hindu: Muslim ratio was 1:1.64, while female (41): male (69) ratio was 1:1.68.
Initial exploratory observational study (Pilot study)
Initial exploratory observational clinical study was conducted with 39 patients in two groups in two phases. In the first phase, 24 patients were divided into two groups and in second phase 15 patients in three groups through permuted block randomization system. In first phase, one group was treated with ATD and other group with ATD + Aswagandha 12 each. As the patients were enrolled with AFB positive and freshly detected, the test was repeated after 28 days. Surprisingly it was seen that in ATD group of patients, 4 patients’ AFB was found positive even after 28 days. Hence, it was planned to observe bacterial load of individuals. 15 patients were again freshly enrolled in three equal groups adding new group of ATD + Chyawanprash for piloting.
Among the three groups of patients in ATD group, presence of AFB was confirmed in two patients. Subsequently, the study design was modified and systematic therapeutic study was planned to determine the bacterial load count sequentially to detect the negativity time and bacterial count. Moreover, blood isoniazid and pyrazinamide, along with immunoglobulin and body composition study were included.
Open trial with therapeutic control
60 patients participated in the entire study with full compliance. Patients who reported after 29th day were not included. The reasons were ignorance, negligence and callousness about the importance of the study in spite of our best efforts. Changes in clinical symptoms scores on 15th and 29th day post medication on the basis of selected symptomatology was made whereas severe (03), moderate (02), mild (01) and normal (00) are scored in Table 1.
Table 1.
Change in clinical symptoms scores on 15th and 29th day post medication

The hemopoetic parameter was expressed as percent change from the baseline pre-treated data. There were remarkable changes on ESR, body weight and immunoglobulin [Table 2]. Sputum AFB positivity and bacterial load counts are represented in percentage in Table 3. The bacterial load in number declined in all the groups. The difference in group on add-on therapy is appreciable in comparison to ATD group. In Chyawanprash add-on group the bacterial load negativity started from 17th day against Aswagandha group from 20th day and completed on 26th day. Even on 29th day 50 percent of ATD group patients had bacterial load positive [Table 3 and Figure 4]. Bacterial load count at different days is summarized in Table 4.
Table 2.
Improvement on hemopoetic parameters of two adjunct groups

Table 3.
Treatment response on AFB positivity and bacterial load count in sputum test
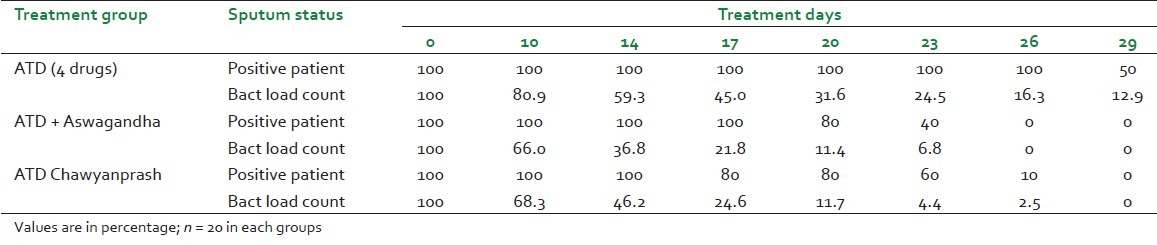
Figure 4.

Bacterial load percent change
Table 4.
Bacterial load count on different days

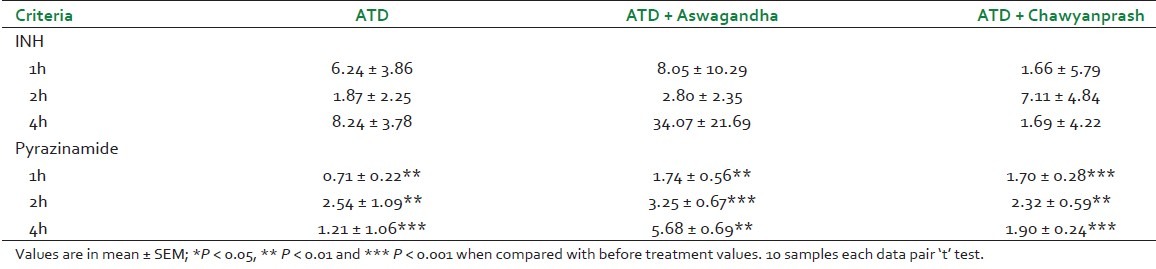
Blood INH and pyrazinamide were estimated after first dose of drugs and repeated on day 29. 0 hour data of 29th day was considered as 24 h after drug administration. Absolute bioavailability could not be calculated for limitation. To determine bioavailability two routes of administration were required. In our study, drug available in blood after absorption in oral route increased. The change was significant with pyrazinamide. The results are summarized in Table 5.
Table 5.
Estimation of blood INH and pyrazinamide on 0 day and 29th day
Post interventional different biochemical parameters are summarized in Table 6. In ATD group, two patients’ blood sugar and serum bilirubin increased from pre drug administration level. While on add-on therapy group, three patients had blood sugar on the higher normal side came down to normal level (mean 96.2 to 83.5 mg/dl). In four patients the serum bilirubin higher than normal (mean 1.32 to 0.91 mg %) came down [Table 6].
Table 6.
Post interventional biochemical study in three different treatment groups

Bioelectrical impedance analysis
Dhatukshaya was recorded on total body water (TBW), body mass index (BMI), fat free mass (FFM) and total body fat (TBF).[17] It was observed that rasa, rakta, mamsa and meda dhatu are lost in patients of PTB. The results are summarized in Figures 5–8.
Figure 5.

Body mass index of tuberculosis patients
Figure 8.

Total body fat of tuberculosis patients
Figure 6.

Fat free mass of tuberculosis patients
Figure 7.

Total body water of tuberculosis patients
DISCUSSION
The present study based on adjunct therapy of Ayurvedic medicine with anti tubercular drugs on the therapeutic management of pulmonary tuberculosis has opened up new dimensions. Considering the re-emergence of TB, WHO looked to intricate traditional medicine in this context e.g Ayurveda. In National Health Policy 1981 amended from time to time brought to light the role of ISM and H drugs specially Ayurveda in primary, secondary and tertiary healthcare. In the domain of primary healthcare, preventive aspects of common diseases were enlisted. Ayurvedic treatment may be considered as adjunct to standard treatment guidelines.[21] Among them, cancer, diabetes and pulmonary tuberculosis may be amenable to Ayurveda. In the domain of primary healthcare, initiatives have been taken on preventive care by the Government of India through AYUSH in National Rural Health Mission (NRHM). India has got its rich heritage of primary healthcare delivery system and advantage of those provisions may be explored with scientific evidences which will best suit for Indian population (Health policy, ISM, 2002).[22] In the present millennium Rajayakshma nomenclature as PTB appears as great threat in healthcare delivery especially at the primary care level. In the present scenario, Ayurveda must not remain silent to rationalize the therapeutic approach. At this juncture, incorporation of Ayurvedic regimen in the treatment may be a hope-finding solution (WHO 2000) and requires appreciation with scientific reasoning.[5] Rasayana Chikitsa of Ayurveda is in existence since Vedic period in different forms. Single drug Aswagandha and ancient formulation Chyawanprash were included for they were in use through the ages and were well known to Indian community and is widely available. The specific actions of those drugs in the poshaka rasa level (nutrient supplement) agni level (metabolic appreciation) and srotas level (tissue nourishment) are well accepted.[23] Good number of rasayana drugs had shown immunomodulatory activity to boost up or restore functional immunity in response to defense mechanism.[24] The rasayana drugs also show tissue and disease specific immunomodulatory activity.[25] The rasayana drugs not only play a role in immunity but also due to its anti-stress, inotropic and antioxidant activity help in the quality of life improvement, observed in patients as improvement of symptoms.
In this project rasayana drugs as adjunct to ATD regimen is reported after 28 days of treatment. Further, ATD was continued for 8 more months according to WHO norms. On initial exploratory observational study, out of 3 groups comprising 39 patients AFB was positive in 6 patients after 28 days that too on ATD group only. Usually sputum smear test is not done frequently. In WHO clinical manual monitoring of PTB with sputum positive clearly mentioned that sputum examination at diagnosis and to monitor it at the completion of initial phase i.e after 8 weeks of ATD treatment. Our observation was that out of 17 PTB patients under ATD treatment, 6 (35.2%) patients were AFB positive after twenty days of treatment with HRZE. In other groups that were on adjunct therapy, all the 23 patients were found to be AFB negative. In our study design we did lay emphasis on these aspects. Our objective focus was on the bioavailability study of ATD along with ESR estimations and other hematological and biochemical parameters. Looking toward the prognostic value of ESR, it was considered as an important marker. On ATD 18.67% ESR reduced while on Aswagandha 64.55% and with Chaywanprash added group 66.46%. On biochemical investigation no appreciable change was noticed. In two patients in add on therapy group, blood sugar which was higher than normal came down to normal level. Serum isoniazid estimation 2h after 1st dose and on 29th day after 2h blood samples was studied. The results were inconclusive.
Later on, with gained experience and on evaluation of the results we had changed our study protocol to get comprehensive information. Sequential bacterial load studies were incorporated. To assess the bacterial load, consecutive 3 days samples were collected for 24 h. In total 60 patients’ results are projected as ‘protocol complete’, out of which 50% showed AFB positive and total bacterial load was thinner in ATD group. This information directed to comprehend that add-on therapy of Ayurvedic rasayana adjunct to ATD could reduce the bacterial load early. This information is of immense importance on public health issues. Dhanukar et al. (1988) in experimental model had clearly shown that Tinospora cardifolia (Guduchi) could protect E. coli infection and acts as an immunomodulator.[26] Thattee and Dhanukar (1998) had indicated that Ayurvedic rasayana are immune stimulants in nature.[27,28] Jivanti (Desmotricum fimbriatum Bl) an orchid plant had shown anti stress, antioxidant, and immune stimulant activity with quick shift of bacterial load in PTB patients.[29,30] The water extract of those drugs are used in Chyawanprash preparation.
Bioavailability of isoniazid and pyrazinamide showed 7-10% increased value after 28-days treatment. The present study indicates increased drug absorption status but not absolute bioavailability. In true sense estimation of bioavailability is done on the administration of drugs in two routes. In experimental situation, Ayurvedic plant product of Piper nigrum (Piperine) increases bioavailability of rifampicin[31] Recently, Indian Institute of Integrative Medicine (IIIM, Jammu) had transferred its technology to pharmaceutical industry for further evaluation in this regard. In a major breakthrough, the pharmaceutical industry has since marketed pipererine with a reduced dosage of rifampicin viz. 10 + 200 mg in capsule form. Immunoglobulin IgA and IgM estimations are other prognostic markers to determine the activity of rasayana drugs.[32]
Tuberculosis (Yakshma) nomenclature as Balasa in the Vedic literature was confirmed scientifically by employing Bioelectrical Impedance analysis. In PTB patients, total body water (TBW) was found to be abnormal. Only 2 female (out of 15) and one male patients (out of 35) recorded normal TBW. In all other patients the values were less than normal. Fat free mass, total body fat and BMI were found to be low in PTB patients. After 28 days’ treatment, body weight proportionally increased. But post treatment evaluation of TBW, TBF, FFM and BMI was not possible. This study emphasizes on the need for consumption of more water and exposure to sun rays in the treatment of PTB. Biochemical studies to determine the toxicological component include liver function test, blood urea and serum creatinine. In all the patients treatment with Ayurvedic rasayana drugs reduced the values pertaining to liver function test while in ATD patients it was on increasing trend. In this series only two patients on ATD developed jaundice and appropriate measures were taken and were subsequently considered as drop outs. On the other hand, two patients on the adjunct group whose serum bilirubin was on the higher side (1.8 and 1.7 mg %) at the time of inclusion came down to near normal.
Similar add on therapy were conducted with Ayurvedic drugs adjunct to anti-diabetic drugs like chromium complex, glipizide, metformin and pioglitazone. The effects were assessed on fasting and post prandial blood sugar, HbA1c, HsCRP, LDL and micro albumin after 60 days of treatment. Further reduction was observed compared to anti diabetic drugs used alone.
The practice of Ayurveda has survived through the centuries and is a living tradition in India even today. The rich heritage of Ayurveda should be rationalized and revamped for the use of the suffering millions the world over. The present finding points toward appreciable increase in body weight and IgM and decreased ESR and IgA. Decreased TBW in PTB requires replenishment of Rasa dhatu. The toxic components are protected along with increased availability of ATD in blood.
CONCLUSION
This innovative research provides plethora of information which is empowered by research on synergism. Nurturing these innovative ideas and utilizing core competence will, in the long run, hopefully bring up solutions in public health initiatives to boost health care delivery system in India in pulmonary tuberculosis care.
ACKNOWLEDGEMENT
The authors thankfully acknowledge the gift of Stresscom Capsule and Chywanprash from Dabur Research Foundation, New Delhi. The need of such studies of national importance emphasized and encouraged by Prof. Ranjit Roychaudhury and Dr. S.K. Sharma, Advisor Ayurveda, Government of India, we are thankful to them, their academic discussions enriched the study design. Mr. M.A Wohab and Miss Sabitri Paul of Southern Health Improvement Samity, Bhangar Hospital for allowing us to work in their hospital.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Samhita A. In: Panduranga PS, editor. Bombay: Government Central Book Depot; 1973. [Google Scholar]
- 2.Samhita C. Vidyatini Hindi Commentary. In: Shastri K, Chaturvedi GN, editors. Part I and II. Varanasi: Chaukhamba Sanskrit Series; 1969. [Google Scholar]
- 3.Debnath PK, Chattopadhyay J, Ghosal D, Bhattacharya P. Immunomodulatory Role of Ayurvedic Rasayan for Quality of Life. International Nat Conf. 1998:38. [Google Scholar]
- 4.Sailaja C. Journal of Health & Population in Developing Countries. Vol. 3. WHO publication; 2000. A few research outcomes relevant for Public Health from Indian System of Medicine; p. 109. [Google Scholar]
- 5.General Guidelines for methodologies on research and evaluation of traditional medicine. Geneva (WHO, QOL): World Health Organization; 2000. p. 59. [Google Scholar]
- 6.Bhattacharya SK, Muruganandam A. Adaptogenic activity of Withania somnefera: An experimental model using a rat model of chronic stress. Pharmacol Biochem Behav. 2003;75:547–55. doi: 10.1016/s0091-3057(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 7.Katiyar CK, Brindabanam NB, Tewari P, Narayana DB. Immunomodulation products from Ayurveda: Current status and future perspectives. In: Upadhaya SN, editor. Immunomodulation. New Delhi: Norosa Publishing House; 1997. [Google Scholar]
- 8.Brahma SK, Debnath PK. Therapeutic importance of rasayana drugs with special reference to their multi dimensional actions. Aryavaidyam. 2003;16:160–3. [Google Scholar]
- 9.Gulati K, Roy A, Debnath PK, Bhattacharyya SK. Immunomodulatory Indian medicinal plants. J Nat Remedies. 2002;2:121–31. [Google Scholar]
- 10.Ojha LK, Bajpai HS, Sharma PV, Khanna MN, Shukla PK, Sharma TN. Chyawanprash as anabolic agent Experimental Study (preliminary work) J Res Indian Med. 1973;8:11–4. [Google Scholar]
- 11.Oslan WA, Dayton PG, Israili ZH, Pruti AW. Spectroflurometric assay of isoniazid and acetyl sioniazid in plasma adopted to paediatric studies. Clin Chem. 2007;23:745–8. [PubMed] [Google Scholar]
- 12.Henry RJ, Winkelman JW. Clinical Chemistry Principles and Techniques. 2nd ed. Harper & Row; 1974. [Google Scholar]
- 13.Drobniewski F, Caws M, Gibson A, Young D. Modern laboratory diagnosis of tuberculosis. Lancet Infect Dis. 2003;3:141–7. doi: 10.1016/s1473-3099(03)00544-9. [DOI] [PubMed] [Google Scholar]
- 14.Taylor RN, Fulford KM, Huong AY. Comparison of kinetic and end-point diffusion methods for quantitating human serum immunoglobulins. J Clin Microbiol. 1978;8:23–7. doi: 10.1128/jcm.8.1.23-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldfogel GA, Sewell DL. Preparation of Sputum smears for Acid fast Microscopy. J Clin Microbiol. 1981;14:460–1. doi: 10.1128/jcm.14.4.460-461.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chottopadhyay J. Study on the effect of Ayurvedic medicine on the bioavailability of Anti-tubercular drugs, Ph.D. (Ay.) thesis. Kolkata: University of Calcutta; 2006. [Google Scholar]
- 17.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis part I: Review of principles and methods. Clin Nutr. 2004;23:1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health & F W. 2nd ed. New Delhi: Government of India; 2003. Anonymous. The Ayurvedic Formulary of India. Part I; p. 37. [Google Scholar]
- 19.Ojha JK, editor. Chyawanprash from Vedic and Genomic era. Delhi: Chaukhamba Sanskrit Pratishtha; 2003. [Google Scholar]
- 20.Sur TK, Pandit S, Mukherjee R, Debnath PK, Bandopadhyay SK, Bhattacharya D. Effect of Sonachandi Chyawanprash and Chyawanprash Plus, two Herbal Formulations on Immunomodulation. Nepal Med Coll J. 2004;6:126–8. [PubMed] [Google Scholar]
- 21.National Health Policy, Ministry of Health and Family Welfare, Government of India. 1981 [Google Scholar]
- 22.National Health Policy, Indian System of Medicine and Homoeopathy. Ministry of Health and Family Welfare, Government of India. 2002 [Google Scholar]
- 23.Datta Goutam K, Debnath PK. Stress Adaptation in Ayurveda by Immunomodulatory Rasayana in National Seminar on Rasayana Proceedings published by CCRAS, New Delhi. 2001:60–75. [Google Scholar]
- 24.Bhattacharya SK, Goel RK, Kaur R, Ghosal S. Anti-stress activity of sitoindoles VII, VIII, new steryl glycosides from Withania somnefera. Phytother Res. 1987;1:32–3. [Google Scholar]
- 25.Debnath PK, Mitra A, Hazra J, Pandit S, Biswas TK, Jana U, et al. In: Evidence based medicine – A clinical experience on Ayurveda Medicine in Recent Advances in Herbal Drug Research and Therapy. Roy A, Gulati K, editors. New Delhi: I K International Publishing House Pvt. Ltd; 2010. pp. 49–73. [Google Scholar]
- 26.Rege NN, Abraham P, Bapat RD, Ray V, Thatte UM, Dahanukar SA, et al. Immunomodulation. New Delhi: Narosa Publishing House; 1999. p. 105. [Google Scholar]
- 27.Dhanukar SA, Thatte UM, Mere PM, Karandikar SM. Immunotherapeutic modification by Tinospora Cordifolia of abdominal sepsis induced by caecal ligation rats. Indian J Gastroenterol. 1988;7:21–3. [PubMed] [Google Scholar]
- 28.Dhanukar SN, Thatte UM, Rege NN. Immunostimulant from Ayurvedic Medicinal Plants. In: Wager, editor. Immunomodulatory agents from plants. Basel: 1999. [Google Scholar]
- 29.Chakrabarty M, Datta G, Ghosh S, Debnath PK. Induction of antioxidative Enzyme by the Ayurvedic Herb Desmotrichum Fimbratum Bl. Indian J Exp Biol. 2001;39:485–6. [PubMed] [Google Scholar]
- 30.Datta GK, Charaborty M, Ghosh S, Debnath PK. Hepatoprotective effect of Desmotricum fimbriatum BI. in mice with carbon tetrachloride induced liver damage. Biomed Res. 2002;13:81–4. [Google Scholar]
- 31.Zutshi RK, Singh R, Zutshi U, Johri RK, Atal CK. Influence of piperine on rifampicin blood levels in patients of pulmonary tuberculosis. J Assoc Physicians India. 1985;33:223–4. [PubMed] [Google Scholar]
- 32.Bansal P, Saand R, Srikanth N, Lavekar GS. Effect of traditionally designed nutraceutical on stress induced immunoglobulin changes at antartica. Afr J Biochem Res. 2009;39:84–8. [Google Scholar]


