Abstract
The aim of this study was to investigate the effects and mechanisms of mesenchymal stem cells (MSCs) on haematopoietic reconstitution in reducing bone marrow cell apoptosis effects in irradiated mice, and to research the safe and effective dosage of MSCs in mice with total body irradiation (TBI). After BALB/c mice were irradiated with 5.5 Gy cobalt-60 γ-rays, the following were observed: peripheral blood cell count, apoptosis rate, cell cycle, colony-forming unit-granulocyte macrophage (CFU-GM) and colony-forming unit-fibroblast (CFU-F) counts of bone marrow cells and pathological changes in the medulla. The survival of mice infused with three doses of MSCs after 8.0 Gy or 10 Gy TBI was examined. The blood cells recovered rapidly in the MSC groups. The apoptotic ratio of bone marrow cells in the control group was higher at 24 h after radiation. A lower ratio of G0/G1 cell cycle phases and a higher ratio of G2/M and S phases, as well as a greater number of haematopoietic islands and megalokaryocytes in the bone marrow, were observed in the MSC-treated groups. MSCs induced recovery of CFU-GM and CFU-GM and improved the survival of mice after 8 Gy TBI, but 1.5 × 108 kg−1 of MSCs increased mortality. These results indicate that MSCs protected and treated irradiated mice by inducing haematopoiesis and reducing apoptosis. MSCs may be a succedaneous or intensive method of haematopoietic stem cell transplantation under certain radiation dosages, and could provide a valuable strategy for acute radiation syndrome.
Currently, substantial progress has been made in the treatment of acute radiation syndrome (ARS) [1–6]. The study of haematopoietic growth factors and haematopoietic stem cell transplantation (SCT) has already progressed to the molecular and genetic level [4, 7, 8]. However, there are still some problems associated with SCT for ARS, such as donor deficiency, a high mortality for conditioning and complications resulting from transplantation. Thus, some experts continue to question the effect of transplantation on ARS. It is known that the microenvironment in bone marrow plays a key role in haematopoietic recovery after irradiation injury. The haematopoietic stem cells (HSCs) and the microenvironment, in a pathological state, often damage and aggravate mutually [9]. Therefore, the treatment of both is indispensable in ARS. Mesenchymal stem cells (MSCs) are adherent cells capable of self renewal and multilineage differentiation [10]. In addition, MSCs can produce several essential haematopoietic growth factors, including interleukin (IL)-6, IL-11, leukemia inhibitor factor (LIF), stem cell factor (SCF), Flt3 ligand and stromal derived factor (SDF) [11]. The microenvironment in bone marrow consists of stroma cells derived from MSCs. Cytokine and extracellular matrices are important factors in HSC growth and multiplication [12]. Transplantation of MSCs is used for both autologous and allogeneic transplant [13, 14], but there are fewer reports on the radiation protection and therapy effects of MSCs on mice with acute radiation injury [15, 16]. The present study was undertaken to examine the effects of three dosages of MSCs on the survival of mice exposed to lethal doses of total body irradiation (TBI), and to explore the mechanisms by which MSCs significantly improve haematopoietic recovery. This was accomplished by observing peripheral blood counts, apoptosis, p53 expression, CFU-GM and CFU-F counts, the cell cycle of bone marrow cells, and pathological changes in variety of the medulla.
Methods and materials
Mice
MSC donor mice (male C57BL/6) and bone marrow cell (BMC) donor mice and recipients (female BALB/C) 6–7 weeks of age were purchased from the Experimental Animal Facility of the Academy of Military Medicine Sciences, China. Mice were housed five to a cage and received commercial rodent chow and autoclaved acidified water. All studies were performed in accordance with protocols approved by the Institutional Care Committee.
Isolation and culture expansion of MSCs
Femurs were taken from donor mice killed by cervical dislocation under CO2 anaesthesia. Mononuclear cells were separated by centrifugation at 1000×g for 10 min at room temperature on a percoll gradient (density, 1.082 g ml−1). The interface was collected and resuspended in phosphate buffered saline (PBS). The cells were suspended in Dulbecco's Modified Eagle Medium-low glucose (DMEM-LG), supplemented with 10% foetal bovine serum (FBS) and 10 ng ml−1 epidermal growth factor (EGF); 1 × 106 cells cm−2 were plated in culture flasks (Hyclone, Logan, UT). The culture was maintained at 37°C in a humidified environment containing 5% CO2. After 48 h, the non-adherent cells were removed and the medium replaced. When 80–90% confluence was reached, adherent cells were trypsinised with 0.02% trypsin (Hyclone, Logan, UT) at 37°C for 5 min.
Phenotype of MSCs
The harvested cells were washed and resuspended in PBS in aliquots of 1 × 106 cells. The cells were stained with fluorescein-conjugated antibody against CD34, CD45, CD105, CD29 and Sca-1 (PharMingen, San Diego, CA) [12, 17] and incubated for 30 min in the dark at room temperature (1 μg antibody per 106 cells). After being washed and resuspended, the cells were analysed in a flow cytometer using cell Quest software (Becton-Dickinson, San Jose, CA).
Differentiative ability of MSCs
For osteogenic differentiation, MSCs were plated in induction media (DMEM, 10% FBS, 10 mM β-glycerophosphoric acid, 50 μM vitamin C, 0.25 μM dexamethasone) in a density of 5000 cells cm−2, cultured for 3 weeks and then stained for the presence of mineral with the von Kossa method. The cells were cultured in adipogenic induction media (DMEM, 10% FBS, 0.1 μM dexamethasone, 50 μM indomethacin and 0.5 mM 1-methyl-3-isobutylxanthine) with a density of 104 cells cm−2. The culture medium was replaced every 2 days and stained with oil red O solution to show lipids in adipose cells.
Radiation
Recipient mice were irradiated using a cobalt-60 source at a dose rate of 3.99 × 10−2 C kg−1 min−1. For haematopoietic studies, TBI was performed as a single exposure of 5.5 Gy [18]. 2.5 × 107 kg−1 of MSCs were infused via the tail vein 4 h after irradiation. Control mice were injected with a comparable volume of PBS.
For survival studies, recipient BALB/c mice were transplanted with 2.5 × 107 kg−1, 5 × 107 kg−1 or 1.5 × 108 kg−1 of MSCs after 8 Gy TBI, while being cotransplanted with 1 × 109 kg−1 of BMCs of donor mice. Control mice were only infused with 1 × 109 kg−1 of BMCs.
MSCs – engraftment detection
The sry gene of the Y chromosome in the BMCs of recipient female mice were detected by polymerase chain reaction (PCR) on days 7 and 30. The primer sequence of the Y chromosome forward primer was 5′-ATTTATGGTGTGGTCCCG-3′, and the reverse primer 5′-GCTGTAAAATGCCACTCC-3′ (Bioasia, SangHai, China). The PCR conditions were: 36 cycles at 95°C for 50 s, 55°C for 60 s, 72°C for 60 s, followed by 1 cycle at 72°C for 10 min [19, 20].
Peripheral blood counts
Each mouse was bled by retro-orbital puncture for blood cell counts every 3–4 days. Blood (20 μl) was collected in a vial containing 1 μl of 0.5 M ethylenediaminetetraacetic acid. Blood cell counts were performed automatically on an F-820 vet haematology analyser (Sysmex, Japan).
CFU-GM and CFU-F assay
At days 4, 12 and 25, cells were brushed out from the femur. 2 × 105 mononuclear cells were plated per well using six-well tissue culture plates. The media was composed of α-MEM (minimum essential medium) supplemented with 20% horse serum, 100 ng ml−1 granulocyte monocyte colony stimulating factor (GM-CSF) and 3% L-glutamine. CFU-GM were counted at day 7 of the culture at 37°C in a humidified environment containing 5% CO2, with colonies consisting of at least 50 cells. Bone marrow cells were plated at 1 × 106 in RPMI 1640 media including 10% FBS and 10−6 mol l−1 hydrocortisone. The fibroblast colonies were counted using an inverted microscope at ×25 on day 14. Cell clusters consisting of at least 40 fibroblasts were scored as a CFU-F colony.
Cell-cycle and apoptosis ratio and pathological changes in the bone marrow
After irradiation, the myelocyte count was taken and adjusted to a density of 5 × 105 cells. The cells were washed twice with PBS, resuspended with 5% calf blood serum PBS (0.3 ml), added to 0.7 ml anhydrous alcohol and mixed at −20°C for 24 h, before being added to 200 μl (1 mg ml−1) of RNA enzyme at 37°C for 30 min and then 100 μg ml−1 propidium iodide (PI) dyes (300 μl) for 20 min. Finally, the sample was measured with flow cytometry. The mice sternum section was observed using haematoxylin and eosin stain at days 12 and 23.
Statistical analyses
Results of replicate experiments were represented as the mean ± standard deviation (SD). Each data point represents 5–10 mice. Data were analysed by a t-test for haematological data and by a Kaplan–Meier analysis for survival experiments with SAS 9.0 software.
Results
Identification and engraftment of MSCs
MSCs have a spindle-shaped fibroblastic appearance and are positive for CD29, CD44, CD105 and Sca-1. No positive cells for the haematopoietic antigens CD34 or CD45 could be detected by flow cytometry. Adipogenic differentiation was shown by the accumulation of lipid vacuoles, which were stained by oil red O. Osteogenic differentiation was indicated by calcium deposition seen with the von Kossa method. The positive expression of Sry genes of MSCs was detected in the bone marrow of recipient female mice on days 7 and 30 (Figure 1).
Figure 1.
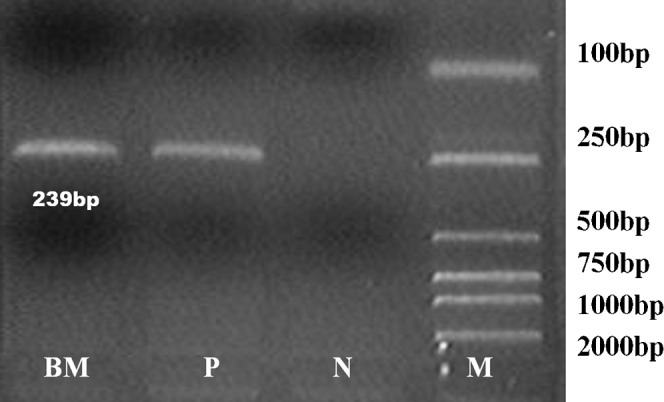
The sry gene of mesenchymal stem cells (MSCs) of mice was detected on day 30 after irradiation and MSC infusion. BM, bone marrow cells; P, positive control; N, negative control; M, DL-2000 marker; sry, 239 bp.
Peripheral blood assay
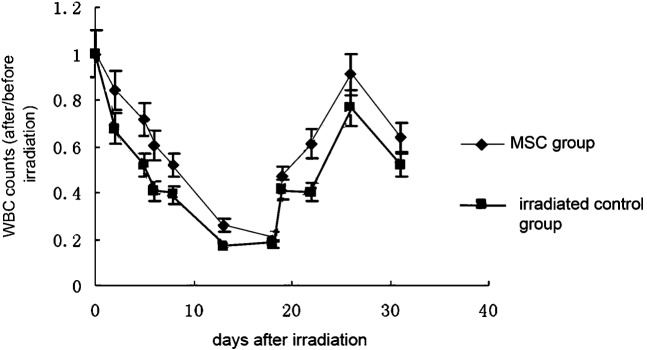
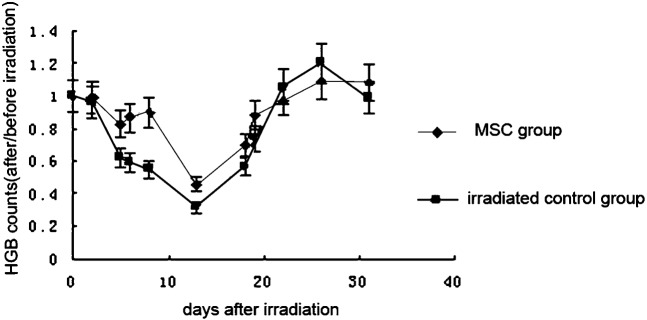
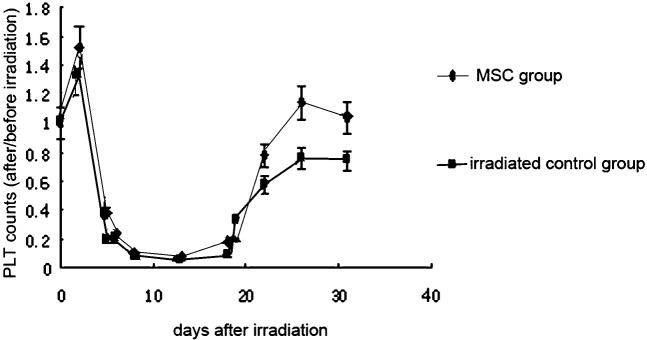
The number of white blood cells (WBCs) was always significantly higher in the initial stage, nadir stage and recovery stage of ARS in the MSC-treated groups than in the control group (p < 0.05). The WBC count in the MSC groups dropped to a nadir that was 20% of the pre-irradiation count on day 17. WBC recovery accelerated after 17 days and recovered to 90% of the pre-irradiation level on day 26. By contrast, WBC counts in the control group dropped until the nadir was about 17% of its original level. After day 17, counts slowly increased and only recovered to 80% of the original amount on day 26 (Figure 2). The haemoglobin count in the two groups showed no statistically significant difference. It dropped to the nadir and began to recover almost on the same day. Normal levels were reached at day 22 (Figure 3). The platelet count was slightly higher in the MSC-treated groups than in the control group in the initial and nadir stages. In the recovery stage, the counts were significantly increased and recovered to the pre-irradiation level at day 23 in the MSC group. The count in the control group only reached 60% at day 23. The recovery still lingered at 80% and recovery was not complete at day 30 (Figure 4).
Figure 2.
Effect of mesenchymal stem cells (MSCs) on the leukocyte count in sublethally irradiated mice. Each data point represents the mean ± standard deviation obtained from eight mice. Statistical significance was determined by comparison between the irradiated control group and the MSC group (p < 0.05) (every group, n _ 20). WBC, white blood cell.
Figure 3.
Effect of mesenchymal stem cells (MSCs) on the haemoglobin (HGB) count in sublethally irradiated mice. Each data point represents the mean ± standard deviation obtained from eight mice. Statistical significance was determined by comparison between the irradiated control group and the MSC group on days 5, 6, 8, 13, 18 and 19 (p < 0.05).
Figure 4.
Effect of mesenchymal stem cells (MSCs) on the platelet (PLT) count in sublethally irradiated mice. Each data point represents the mean ± standard deviation obtained from eight mice. Statistical significance was determined by comparison between the irradiated control group and the MSC group on days 20, 26 and 31 (p < 0.05), (every group, n _ 20).
CFU-GM and CFU-F assay
The percentage of CFU-GM was 12 ± 4.8 in the MSC group on day 4, 24 ± 3.6 on day 12 and 102 ± 4.8 on day 25. That in the control group was 0.36 ± 0.12 on day 4, 9.6 ± 2.4 on day 12 and 70.8 ± 3.6 on day 25. The amount of CFU-GM in MSC-treated groups was significantly different compared with the control level at each observed time point (p < 0.01). The percentage of CFU-F in the MSC-treated mice was 45 ± 3.6 on day 4, 36 ± 4.5 on day 12 and 55.8 ± 6.3 on day 25. That in the control group was 16.2 ± 3.6 on day 4, 30.6 ± 5.4 on day 12 and 37.8 ± 3.6 on day 25. The amount of CFU-F in MSC-treated groups was significantly different compared with the control level at each observed time point (p < 0.01).
Pathological changes in bone marrow
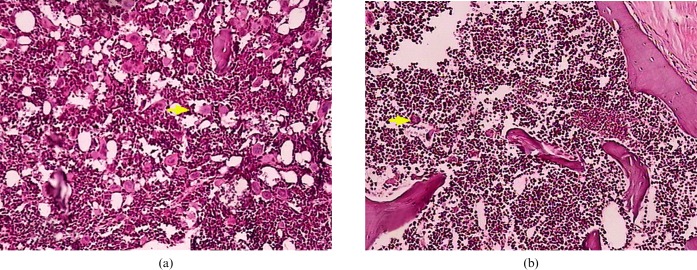
The bone marrow showed active proliferation in the two groups of mice on day 12 after radiation. The counts of megakaryocites were from 0 to 1 per high powerfield in the control group, and 4–5 in the MSC group. On day 23, all haematopoietic lineages had recovered to the normal ratio. 8–10 megakaryocytes were observed in the control group, whereas 50–60 megakaryocytes were observed in the MSC group (Figure 5).
Figure 5.The.
The pathological section of the sternum medulla in the (a) mesenchymal stem cell and (b) control groups (haematoxylin and eosin, ×200).The arrows indicate megacaryocytes (every group, n _ 5).
Cell-cycle and apoptosis
The apoptosis ratio of BMCs was lower in the MSC group than in the control group 24 h after irradiation (p < 0.05) (Table 1). The ratio of G0/G1 had decreased to a greater level in the MSC group at day 2 (p < 0.01). The ratio of G2/M and S phases was higher in the MSC group (p < 0.05) (Table 1).
Table 1. The cell-cycle and apoptosis of bone marrow cells in two irradiated groups (%).
| Time post irradiation | G0–G1 | S | G2–M | Apoptosis | |
| Normal | 70.81 ± 3.44 | 23.6 ± 3.76 | 5.45 ± 0.82 | 0.68 ± 0.22 | |
| 6 | Control | 76.77 ± 4.21 | 7.81 ± 1.56 | 14.42 ± 3.14 | 2.94 ± 0.43 |
| MSCs | 77.75 ± 3.32 | 10.37 ± 2.45 | 11.88 ± 3.42 | 2.36 ± 0.16 | |
| 12 | Control | 79.84 ± 3.28 | 3.81 ± 3.73 | 16.35 ± 2.17 | 5.24 ± 0.24 |
| MSCs | 75.33 ± 4.12 | 3.07 ± 1.93 | 21.6 ± 1.98 | 6.36 ± 0.34 | |
| 24 | control | 82.32 ± 3.54 | 4.27 ± 3.72 | 13.41 ± 2.78 | 4.48 ± 0.33 |
| MSCs | 86.14 ± 4.45 | 4.9 ± 1.11 | 8.96 ± 1.56 | 2.59 ± 0.45a | |
| 72 | Control | 64.83 ± 3.98 | 32.68 ± 3.45 | 2.49 ± 1.23 | 1.4 ± 0.45 |
| MSCs | 57.69 ± 2.05a | 38.31 ± 4.22a | 4.23 ± 0.87 | 0.8 ± 0.32 |
ap < 0.05 compared with the control in each time point (every group, n _ 20). MSCs, mesenchymal stem cells.
Survival experiments
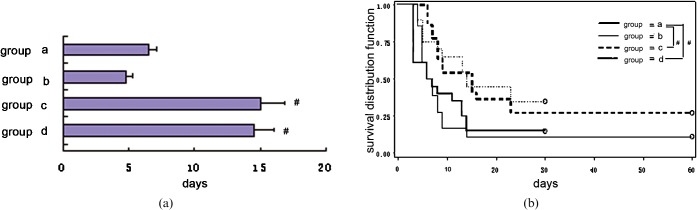
Mortality to a level of 100% was induced by 8 Gy TBI within 30 days without transplant of BMCs. The survival of MSCs in the 2.5 × 107 kg−1 plus BMCs group (Group D) was 30% after 8 Gy TBI. Survival in the 5 × 107 kg−1 plus BMCs group (Group C) was 43%, and in the 1.5 × 108 kg−1 plus BMCs group (Group B) was 12%. Finally, the survival of the BMCs alone group (Group A) was only 20% within 30 days. The survival of Groups C and D was significantly different compared with that of Group A. These results are shown in the Kaplan–Meier plots in Figure 6.
Figure 6.
(a) Mean survival time and (b) survival curve for each group. The mean survival time was 8.55 ± 0.94 days in Group A (BALB/c mice treated with 1 × 109 kg−1 bone marrow cells (BMCs) after 8 Gy of total body irradiation (TBI)). The mean survival time in Group B (BALB/c mice treated with BMC+1.5 × 108 kg−1 MSCs after 8.0 Gy TBI) was 7 days. The mean survival time in Group C (BMCs+5 × 107 kg−1 MSCs) was 14.5 ± 1.65 days. The mean survival time in Group D (BMCs+2.5 × 107 kg−1 MSCs) was 15.1 ± 1.55 days. # indicates p < 0.05 when compared with group A (every group, n _ 20).
Discussion
In our study, we cultured MSCs from the bone marrow; the MSCs and their capacity for differentiation were identified with monoclonal antibodies. The sry gene of MSCs from male donor mice was detected in the bone marrow of recipient mice. The peripheral blood counts showed that the three blood lineages in the MSC groups were higher than those in the control group after 5.5 Gy of irradiation. This knowledge could be beneficial for mice in order to reduce infection and blood complications and, moreover, allow patients to safely overcome the severe stage of ARS. The rapid recovery of thrombocytes in the MSC groups implied that MSCs dramatically enhance the recovery of haematopoiesis because platelets increase very slowly after irradiation injury.
Counts of the CFU-GM showed that the granulocyte macrophage line had greater radiosensitivity and faster depression and recovery. The CFU-GM counts were higher in the MSC groups than in the control group. MSCs may alleviate the progenitor's injuries by ameliorating the microenvironment and secreted cytokines; they may also improve proliferation. The stromal cells are the primary cells damaged by radiation in the microenvironment [21]. The CFU-F counts in the control group decreased slowly and lingered at a low level for a long time. They also recovered slowly, with levels still <50% of the normal counts at day 25. The counts in the MSC groups had recovered to >60% of the normal counts at day 25, showing that the MSCs protected and improved the microenvironment.
The cell-cycle is a complicated process co-regulated by a series of proteins, kinases and cytokines. Irradiation can cause cell-cycle disorder in the bone marrow, including G1 and G2/M blocking. DNA synthesis was depressed after irradiation >2 Gy [22, 23]. Our data showed that MSCs enhanced progression of cells from the resting phase to the cell-cycle-accelerated DNA synthesis and mitosis processes, thus progressing the proliferation of haemocytes and taking advantage of haematopoietic recovery. Apoptosis is the most important cause of bone marrow injuries by irradiation in medium- or severe haematopoietic radiation injury [24]. The occurrence of apoptosis is intimately related to oncogene and anti-oncogene regulation. p53 is the important checkpoint protein that controls the switch from G1 to S in the cell cycle. p53 determines whether cells go into the S phase or undergo apoptosis [25, 26]. The DNA damaged by irradiation activates two kinases: ATM (ataxia telangiectasia mutated) and ATR (ATM-Rad3-related) [27]. These are crucial molecular signals in supervising radiation injury, by “sensing” the injury and activating a downstream response component. ATM and ATR phosphorylate the serine of p53 and counteract the depression of Mdm2 to p53. Increased p53 activates the downstream activity of p21, which restricts the dependence of cell-cycle kinases, blocks the S phase and induces apoptosis. Apoptosis of cells in the MSC groups was reduced when compared with that in the control group at 24 h. The data indicate that the cytokines from the MSCs reduced the expression of p53 in irradiated cells. They also caused cells to leave the G0/G1 block, accelerated DNA synthesis and mitosis, and decreased apoptosis [28–30].
There are three major mechanisms of MSC-induced haematopoiesis. First, despite a transient increase in cytokines after irradiation, cytokines are comparatively scarce because cell factor receptors are damaged and the tolerance of cytokine receptors is advanced [31, 32]. Therefore, the cytokines from the MSCs can make up for the cytokine shortage. It is known that MSCs secrete a large number of cytokines in vitro, including GM-CSF, stem cell factor (SCF), thrombopoietin (TPO), IL-6, IL-11, Flt3 ligand and stromal cell-derived factor-1 (SDF). SCF is the stem cell factor that induced the most primitive of stem cells — granulocytes [33, 34], whereas TPO can stimulate colony-forming megakaryocytes [35, 36]. IL-6 is the multiple-function factor that activates the immune and haematopoietic system, which can activate B cells and improve the number of thrombocytes and granulocytes [37]. IL-11 can enhance the proliferation of all the blood cell lineages [38]. SDF induces transendothelial migration of haematopoietic stem cells to the bone marrow. SDF is classified as a cxc-chemokine and is also a chemoattractant for monocytes and lymphocytes [39]. Second, complete recovery of the haematopoietic microenvironment destroyed by irradiation is complex and occurs slowly [40]. MSCs can differentiate to stroma cells, fibroblast cells and adipose cells, which reconstitute the microenvironment and improve the number of haematopoietic islands. The islands induce stem cell proliferation and differentiation. Third, MSCs protect the haematopoietic cells from apoptosis and enhance the cell-cycle transition after irradiation. One explanation for this may be that MSCs have radioprotective effects by secreting IL-11, SCF, Flt-3L and IL-7 [41].
It is generally believed that the damage induced by high-level irradiation to the haematopoietic system results in the death of mice within a period of 30 days, and mortality can be directly attributed to infections and haemorrhagic complications resulting from neutropenia and thrombopenia. In our study, the high survival rate induced by MSCs was related to accelerated platelets and neutrophilic recovery. It is noteworthy that the survival of the 5 × 107 kg−1 and 2.5 × 107 kg−1 MSC groups showed no statistically significant difference, and that of the 1.5 × 108 kg−1MSC group was less than that of the BMC group. This finding occurred because MSCs are strongly sticky and favour conglobation. For these reasons, the over dosage of MSCs may be embolise in mice organisation to cause mice death.
MSCs reduced apoptosis, enhanced cell-cycle transition, improved the microenvironment, and accelerated haematopoietic stem cell proliferation and differentiation, and may be an effective treatment strategy for ARS.
References
- 1.Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. Int J Radiat Biol 2004;80:3–10 [DOI] [PubMed] [Google Scholar]
- 2.Fliedner TM, Graessle D, Paulsen C, Reimers K. Structure and function of bone marrow hemopoiesis: mechanisms of response to ionizing radiation exposure. Cancer Biother Radiopharm 2002;17:405–26 [DOI] [PubMed] [Google Scholar]
- 3.Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ, Farese AM. The hematologist and radiation casualties. Hematology 2003;2003:473–96 [DOI] [PubMed] [Google Scholar]
- 4.Hirama T, Tanosaki S, Kandatsu S, Kuroiwa N, Kamada T, Tsuji H, et al. Initial medical management of patients severely irradiated in the Tokai-mura criticality accident. Br J Radiol 2003;76:246–53 [DOI] [PubMed] [Google Scholar]
- 5.Meineke V, van Beuningen D, Sohns T, Fliedner TM. Medical management principles for radiation accidents. Mil Med 2003;168:219–22 [PubMed] [Google Scholar]
- 6.Waselenko JK, MacVittie TJ, Blakely WF. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med 2004;140:1037–51 [DOI] [PubMed] [Google Scholar]
- 7.Vávrová J, Vokurková D, Mareková M, Bláha M, Jebavý L, Filip S. Antiapoptotic cytokine IL-3 + SCF + FLT3L influence on proliferation of gamma-irradiated AC133+/CD34+ progenitor cells. Folia Biol 2002;48:51–7 [DOI] [PubMed] [Google Scholar]
- 8.Audet J, Miller CL, Eaves CJ, Piret JM. Common and distinct features of cytokine effects on hematopoietic stem and progenitor cells revealed by dose-response surface analysis. Biotechnol Bioeng 2002;80:393–404 [DOI] [PubMed] [Google Scholar]
- 9.Greenberger JS, Anderson J, Berry LA, Epperly M, Cronkite EP, Boggs SS. Effects of irradiation of CBA/CA mice on hematopoietic stem cells and stromal cells in longterm bone marrow cultures. Leukemia 1996;10:514–27 [PubMed] [Google Scholar]
- 10.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesemchymal stem cells. Science 1999;284:143–7 [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary LR, Spelsberg TC, Riggs BL. Production of various cytokines by normal human osteoblast-like cells in response to IL1 and tumor necrosis factora. Endocrinology 1992;130:2528–34 [DOI] [PubMed] [Google Scholar]
- 12.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells and stromal cells. J Cell Physiol 1998;176:186–92 [DOI] [PubMed] [Google Scholar]
- 13.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 2007;110:2764–7 [DOI] [PubMed] [Google Scholar]
- 14.Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia 2007;21:1733–8 [DOI] [PubMed] [Google Scholar]
- 15.Bensidhoum M, Chapel A, Francois S, Demarquay C, Mazurier C, Fouillard L, et al. Homing of in vitro expanded Stro-1- or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood 2004;103:3313–9 [DOI] [PubMed] [Google Scholar]
- 16.Fouillard L, Chapel A, Bories D, Bouchet S, Costa JM, Rouard H, et al. Infusion of allogeneic-related HLA mismatched mesenchymal stem cells for the treatment of incomplete engraftment following autologous haematopoietic stem cell transplantation. Leukemia 2007;21:568–70 [DOI] [PubMed] [Google Scholar]
- 17.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone 1992;13:69–80 [DOI] [PubMed] [Google Scholar]
- 18.Cui YF, Gao YB, Yang H, Xiong CQ, Xia GW, Wang DW. Apoptosis of circulating lymphocytes induced by whole body gamma-irradiation and its mechanism. J Environ Pathol Toxicol Oncol 1999;18:185–9 [PubMed] [Google Scholar]
- 19.O'Donoghue K, Choolani M, Chan J, de laFuente J, Kumar S, Campagnoli C, et al. Identification of fetal mesenchymal stem cells in maternal blood: implications for non-invasive prenatal diagnosis. Mol Hum Reprod 2003;9:497–502 [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Ma L, Ma GJ, Jiang XY, Zhao CH. Transplantation of mesenchymal derived stem cells followed by G-CSF injection can reconstitute hematopoiesis of lethally irradiated BALB/c mice. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2002;24:20–4 [PubMed] [Google Scholar]
- 21.FitzGerald TJ, McKenna M, Rothstein L, Daugherty C, Kase K, Greenberger JS. Radiosensitivity of human bone marrow granulocyte-macrophage progenitor cells and stromal colony-forming cells: effect of dose rate. Radiat Res 1986;107:205–10 [PubMed] [Google Scholar]
- 22.Goto S, Watanabe M, Yatagai F. Delayed cell cycle progression in human lymphoblastoid cells after exposure to high2LET radiation correlates with extremely localized DNA damage. Radiat Res 2002;1586:678–86 [DOI] [PubMed] [Google Scholar]
- 23.Parmar J, Marshall ES, Charters GA, Holdaway KM, Shelling AN, Baguley BC. Radiation induced cell cycle delays and p53 status of early passage melanoma cell lines. Oncol Res 2000;12:149–55 [DOI] [PubMed] [Google Scholar]
- 24.Peng R, Wang D, Wang B, Xia G, Li Y, Xiong C, et al. Apoptosis of hemopoietic cells in irradiated mouse bone marrow. J Environ Pathol Toxicol Oncol 1999;18:305–8 [PubMed] [Google Scholar]
- 25.DeSimone JN, Bengtsson U, Wang X, Lao XY, Redpath JL, Stanbridge EJ. Conplexity of the mechanisms of initiation and maintenance of DNA damage-induced G2-phase arrest and subsequent G1-phase arrest: TP53-dependent and TP53-independent roles. Radiat Res 2003;159:72–85 [DOI] [PubMed] [Google Scholar]
- 26.Wieler S, Gagné JP, Vaziri H, Poirier GG, Benchimol S. Poly (ADP-ribose) poly-merast-1 is appositive requlator of the p53-mediated G1 arrest response following ionizing radiation. J Biol Chem 2003;278:18914–21 [DOI] [PubMed] [Google Scholar]
- 27.Foray N, Marot D, Gabriel A, Randrianarison V, Carr AM, Perricaudet M, et al. A subset of ATM- and ATR-dependent phosphorylation events requires the BRCA protein. EMBO J 2003;22:2860–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lataillade JJ, Clay D, Bourin P, Hérodin F, Dupuy C, Jasmin C, et al. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G0/G1 transition in cells: evidence for an autocrine/paracrine mechanism. Blood 2002;99:1117–29 [DOI] [PubMed] [Google Scholar]
- 29.Drouet M, Mathieu J, Grenier N, Multon E, Sotto JJ, Herodin F. The reduction of in vitro radiation-induced fas-related apoptosis in CD34 progenitor cells by SCF, Flt-3 ligand, TPO, and IL-3 in combination resulted in CD34 proliferation and differentiation. Stem Cells 1999;17:273–85 [DOI] [PubMed] [Google Scholar]
- 30.Herodin F, Bourin P, Mayol J, Lataillade J, Drouet M. Short-term injection of antiapoptotic cytokine combinations soon after lethal irradiation promotes survival. Blood 2003;101:2609–16 [DOI] [PubMed] [Google Scholar]
- 31.MacVittie TJ. Therapy of radiation injury. Stem Cells 1997;15:263–8 [DOI] [PubMed] [Google Scholar]
- 32.Quelle FW, Wang J, Feng J, Wang D, Cleveland JL, Ihle JN, et al. Cytokine rescue of p53-dependent apoptosis and cell cycle arrest is mediated by distinct Jak kinase signaling pathways. Genes Dev 1998;12:1099–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broudy VC. Stem cell factor and hematopoiessis. Blood 1997;90:1345–64 [PubMed] [Google Scholar]
- 34.Drouet M, Mourcin F, Grenier N, Leroux V, Denis J, Mayol JF, et al. Single administration of stem cell factor, FLT-3 ligand, megakaryocyte growth and development factor, and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: long-term follow-up of hematopoiesis. Blood 2004;103:878–85 [DOI] [PubMed] [Google Scholar]
- 35.Geddis AE, Linden HM, Kaushanshy K. Thromobopoietin: a pan-hematopoietic cytokine. Cytokine Growth Factor Rev 2002;13:61–73 [DOI] [PubMed] [Google Scholar]
- 36.Mouthon MA, Gaugler MH, Van derMeeren A, Vandamme M, Gourmelon P, Wagemaker G. Single administration of thrombopoietin to lethally irradiated mice prevents infectious and thrombotic events leading to mortality. Exp Hematol 2001;29:30–40 [DOI] [PubMed] [Google Scholar]
- 37.Lazzari L, Lucchi S, Rebulla P, Porretti L, Puglisi G, Lecchi L, et al. Long-term expansion and maintenance of cord blood haematopoietic stem cells using thrombopoietin Flt3-ligand interleukin IL-6 and IL-11 in aserum-free and stroma-free culture system. Br J Haematol 2001;112:397–404 [DOI] [PubMed] [Google Scholar]
- 38.Van derMeeren A, Mouthon MA, Gaugler MH, Vandamme M, Gourmelon P. Administration of recombinant human IL11 after supralethal radiation exposure promotes survival in mice: interactive effect with thrombopoietin. Radiat Res 2002;157:642–9 [DOI] [PubMed] [Google Scholar]
- 39.Perez LE, Alpdogan O, Shieh JH, Wong D, Merzouk A, Salari H, et al. Increased plasma levels of stromal-derived factor-1(SDF-1/CXCL12) emhance human thrombopiesis and mobililize human colony-forming cells(CFC) in NOD/SCID mice. Exp Hematol 2004;32:300–7 [DOI] [PubMed] [Google Scholar]
- 40.Galotto M, Berisso G, Delfino L, Podesta M, Ottaggio L, Dallorso S, et al. Stromal damage as a consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol 1999;27:1460–6 [DOI] [PubMed] [Google Scholar]
- 41.Yaãez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 2006;24:2582–91 [DOI] [PubMed] [Google Scholar]