Abstract
Central nervous system (CNS) cryptococcosis is a common opportunistic fungal infection in immunocompromised patients, and the imaging findings differ from those in immunocompetent patients. Here, we present the imaging findings in an immunocompetent woman of a rare case of central nervous system cryptococcal choroid plexitis with trapped temporal horns, enlarged enhancing bilateral choroid plexuses and multiple intraventricular choroid plexus cysts.
Central nervous system (CNS) cryptococcal infection usually manifests as meningitis, meningoencephalitis, encephalitis or ventriculitis, depending on the patient's immunity and inflammatory response. Choroid plexus involvement, with trapping of the temporal horns, is a rare manifestation of this infection in immunocompetent hosts.
Case report
A 65-year-old woman presented with insidious onset of fever and abnormal behaviour of 20 days' duration. She had a two-month history of intermittent holocranial headache of moderate to severe intensity, for which she took no definitive treatment. She also had memory loss, visual hallucinations and demonstrated behavioural change. She failed to recognise family members and needed help in all daily activities. She also demonstrated social disinhibition and had urinary incontinence. She had a history of pulmonary tuberculosis 10 years previously, for which treatment details were not available.
On admission, the patient was drowsy and unresponsive to oral commands but responded to painful stimuli. She had neck stiffness, was moving all four limbs, and had brisk deep tendon reflexes and bilateral equivocal plantars. Ocular movements and fundi were normal. Her cerebrospinal fluid (CSF) was subjected to cytological, biochemical and microbiological evaluation. Cytology of the CSF showed a total cell count of 230 cells per mm3 with a predominance of lymphocytes (87%). CSF protein and glucose were 1000 mg dl−1 and 48 mg dl−1, respectively.
A lumbar puncture was performed; the CSF showed the typical encapsulated yeast cells on Indian ink preparation, and a latex agglutination test was strongly positive. Culture tests for pyogenic fungal organisms and Mycobacterium tuberculosis were negative. Serum tests for HIV were non-reactive.
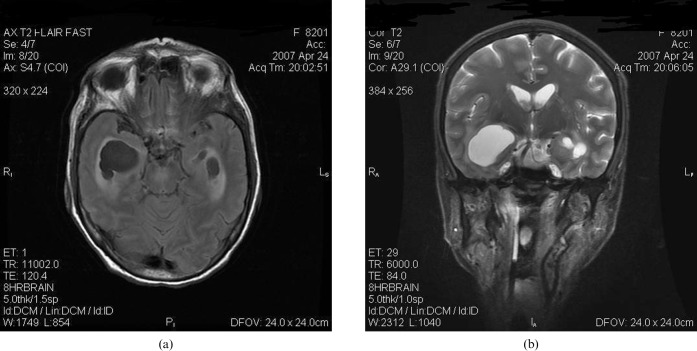
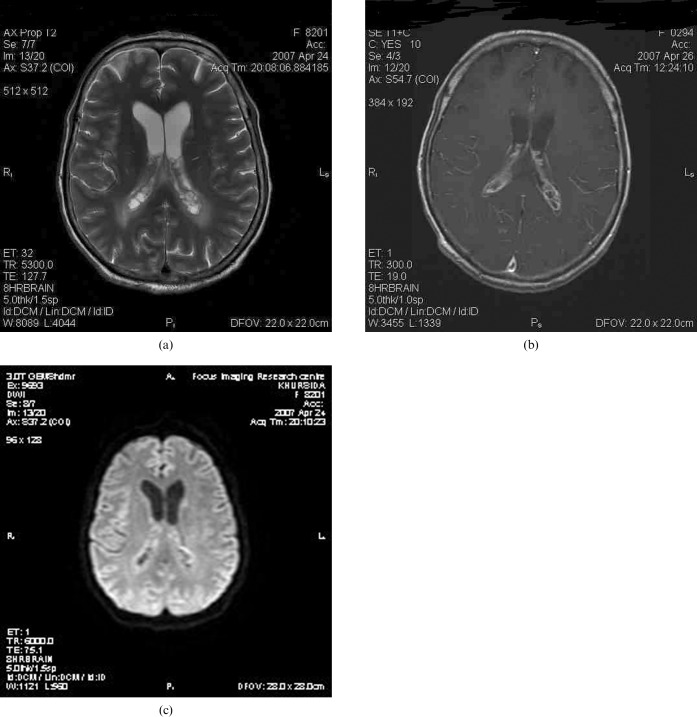
On MRI, there was marked asymmetric dilatation and trapping of both temporal horns (right > left), with internal septations and periventricular extravasation of CSF (Figure 1a,b). Both choroid plexuses within the atria and occipital horns were enlarged, with a multiloculated cystic appearance and abnormal hyperintense signal on T2 weighted fluid-attenuated inversion-recovery (FLAIR) images. Heterogeneous rim enhancement was observed on post-contrast scans (Figure 2a,b). In addition, dilated hyperintense Virchow Robin (VR) spaces were noted in the left basal ganglia (Figure 1b). Diffusion-weighted images revealed no significant restriction of diffusion within the choroid plexus cysts and trapped temporal horns (Figure 2c).
Figure 1.
(a) Axial T2 fluid-attenuated inversion-recovery and (b) coronal T2 weighted images show asymmetrically dilated and trapped temporal horns with periventricular extravasation of cerebrospinal fluid. Prominent perivascular spaces with adjacent oedema can be noted in the left basal ganglia.
Figure 2.
(a) Axial T2 weighted image at the level of the lateral ventricular trigones reveals multiple bilateral intraventricular choroid plexus cysts and adjacent periventricular white matter oedema. (c) Contrast-enhanced axial T1 weighted image at the same level shows multilocular rim enhancement in both choroid plexuses. (c) Diffusion-weighted image (b-value _ 1000) shows no significant restriction of diffusion.
The clinical, laboratory and imaging findings established a diagnosis of cryptococcal meningitis with choroid plexitis and the patient was started on intravenous antifungal antibiotics. Her sensorium improved over the next four weeks but she developed electrolyte imbalances (hyponatraemia and hypokalaemia). Her improvement with antifungal treatment was monitored through repeated CSF examinations, which showed a reduction in total protein and cell counts.
Discussion
Cryptococcus neoformans is a fungal pathogen that causes invasive infection of the CNS (and is particularly important in the era of the HIV/AIDS epidemic). The encapsulated yeast-like fungus was first isolated from fruit juice by San Felice in 1894 and was shown to be present in natural sources such as milk. Various types of soil contaminated with pigeon excretia provide an environmental source of this widely prevalent organism [1].
Infection occurs through inhalation of a small diameter (<10 μm) yeast-like organism, which enters the respiratory passage but then remains dormant depending on the host reaction [2]. The mode of spread to the CNS is through haematogeneous dissemination from the lungs. Subsequently, the fungus spreads to the CSF to cause meningitis, encephalitis and ependymitis; it is this leptomeningeal spread that is responsible for the clinical manifestations, rather than choroid plexitis alone, which is often asymptomatic [3]. The CNS is the preferred site for crpytococcal infection, as the soluble anticryptococcal factors present in serum are absent from CSF. In addition, the inflammatory response evoked is minimal, as the polysaccharide capsule of the fungus hinders phagocytosis and impairs leukocyte migration [4].
The spectrum of radiological findings includes dilated perivascular spaces, gelatinous pseudocysts, intraparenchymal cryptococcomas, miliary nodules, meningeal involvement, intraventricular/choroid plexus masses and hydrocephalus [3]. Dilated VR spaces in the basal ganglia are reported to be the most common imaging finding. As the infection spreads, mucoid gelatinous material produced by the capsule of the fungus gets enmeshed with budding cryptococci, distending and dilating the VR spaces and resulting in the formation of cysts, called gelatinous pseudocysts. These are thought to be an “unreactive” form of meningoencephalitis, preferentially located in the basal ganglia, thalamus, midbrain and dentate nuclei. Gelatinous peusdocysts are non-enhancing lesions and appear similar to CSF on all imaging sequences. Some cases appear as intermediate signal on T1 weighted images owing to the presence of mucin/mucoid material that shortens T1 relaxation time [5, 6].
The lesions extend occasionally to the parenchyma adjacent to the perivascular space, with formation of intraparenchymal cryptococcomas and miliary lesions. These lesions are microabscesses containing amorphous debris and cryptococcal organisms encased in an outer fibrous capsule, and are observed in the brain parenchyma, leptomeningeal and cisternal spaces. They are of low intensity on T1 weighted images and high intensity on T2 weighted images. Immunocompetent patients are more likely to present with cryptococcomas and, because gelatinous peusdocysts represent lesions associated with a disruption of the blood–brain barrier, they may enhance on contrast [7, 8].
Kuwahara et al [9] suggested that cerebral sulcal hyperintensity on FLAIR images is an early sign of an indolent process/recurrent inflammation, even when the CSF findings following lumbar puncture are normal. This finding implies a high protein concentration in the localised subarachnoid space.
Gelatinous pseudocysts and cryptococcomas in the choroid plexus are relatively specific for CNS cryptococcosis. A unilateral or bilateral enlargement of the choroid plexus that enhances on contrast is a relatively rare manifestation of cryptococcal infection. Choroid inflammation can progress to ependymitis, intraventricular synechiae, loculation or enlargement, and entrapment of the temporal horn owing to the obstruction of flow by cryptococci, as seen in our case. Contrast enhancement may not occur in the presence of impaired immunity [3, 8]. The choroid plexus, which lies at the interface between the CSF and the systemic circulation, forms an important portal for entry. The papillary fronds of the choroid plexus which protrude into the ventricle have an external epithelial lining that is continuous with the ependyma and encloses a vascularised mesenchymal core [10]. It is thus an important site for initial dissemination for many infections such as tuberculosis, cytomegalovirus, cryprococcosis, bacteria and parasites, as well as non-infectious processes such as sarcoidosis, xanthogranulomas and rheumatoid nodules. Similarly purulent ventriculitis and neoplastic lesions, such as lymphomas and germinomas, can cause ventricular enhancement by virtue of their subependymal location.
The serotype of the organism, size of inoculum and immune status of the patient are considered to be important factors in determining the pathogenesis, and thereby the radiological spectrum, of the disease. Two varieties of different virulence that cause disease in normal host are noted: (i) C. neoformans. var. neoformans, consisting of serotypes A and D, which cause disease in immune suppression, and (ii) C. neoformans. var. gatti, consisting of serotypes B and C. Virulence is caused by the production of oxidase and protease enzymes, and the antiphagocytic properties of the carbohydrate capsule [11].
The imaging findings differ between immunocompetent and immunodeficient individuals. MR findings of intraparenchymal crypotococcomas and enhancement are noted in the former whereas, in the latter, MR can be normal or show mildly dilated VR spaces, cortical atrophy and rarely meningeal enhancement. Meningeal involvement is often inferred from progressive ventriculomegaly in sequential images, but neither is specific for cryptococcosis [3, 8]. Therefore, diagnosis frequently depends on the identification of cryptococci in CSF through the Indian ink preparation (Figure 3) or on the detection of cryptococcal antigen in CSF to complement the imaging findings. The former is useful when >10 colony forming units (CFU) ml−1 of yeast are present. Other than Indian ink, Alcian blue and mucicarmine are the stains used to detect the polysaccharide capsule of yeast in tissue.
Figure 3.
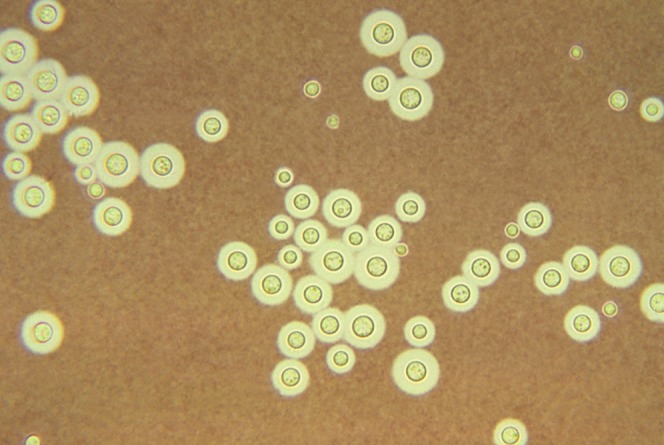
A photomicrograph of light Indian ink preparation of cerebrospinal fluid shows the typical encapsulated yeast cells.
The choice of antifungal treatment depends on the site of infection and the immune status of the patient; options include polyene-amphotericin B (Amp B), azoles (fluconazole, itraconazole) and flucytocine. Serially preformed lumbar punctures may serve to reduce headache in a patient with raised intracranial pressure. The surgical option is reserved for patients with hydroceohalus and raised intracranial tension [11].
In summary, imaging findings of a strongly enhancing choroid plexus, cystic lesions in the basal ganglia, meningeal enhancement and an intraventricular lesion in a patient with headache, vomiting and personality changes should suggest a diagnosis of cryptococcal infection, as a delay in antifungal therapy and use of steroids could result in a fatal outcome.
References
- 1.Kuroki M, Phichaichumpon C, Yasuoka A, Chiranairadul P, Chosa T, Sirinirund P, et al. Enviornmental isolation of Cryptococcus neoformans from an epidemic region of HIV associated cryptococcus in Thailand. Yeast 2004;21:809–12 [DOI] [PubMed] [Google Scholar]
- 2.Neilson JB, Fromtling RA, Bulmer GS. Cryptococcus neoformans: size range of infectious particles from aerosilised soil. Infect Immun 1977;17:634–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovoor JME, Mahadevan A, Narayan JP, Govindappa SS, Satishchandra P, Taly AV, et al. Cryptococcal choroid plexitis as a mass lesion: MR imaging and histopathologic correlation. Am J Neuroradiol 2002;23:273–6 [PMC free article] [PubMed] [Google Scholar]
- 4.Igel HJ, Bolande RP. Humoral defense mechanisms in cryptococcosis; substances in normal human serum, saliva and CSF affecting growth of Cryptococcus neoformans. J Infect Dis 1966:116–75 [DOI] [PubMed] [Google Scholar]
- 5.Andreula CF, Burdi N, Carella A. CNS cryptococosis in AIDS. Spectrum of MR findings. J Comput Tomogr 1993;17:438–41 [DOI] [PubMed] [Google Scholar]
- 6.Wehn SM, Heinz ER, Burger PC, Boyko OB. Dilated Virchow-Robin spaces in cryptococcal meningitis associated with AIDS; CT and MR findings. J Comput Tomogr 1989;13:756–62 [DOI] [PubMed] [Google Scholar]
- 7.Nicholas JP, Erini VM. MR of choroid plexus: involvement in intracranial cryptococosis. J. Comput Tomogr 1993;17:547–50 [DOI] [PubMed] [Google Scholar]
- 8.Berkefeld J, Enzenberger W, Lanfermann H. Cryptococcal meningoencephalitis in AIDS: parenchymal and meningeal forms. Neuroradiology 1999;41:129–33 [DOI] [PubMed] [Google Scholar]
- 9.Kuwahara S, Kawada M, Uga S. Cryptococcal meningoencephalitis presenting with an unusual magnetic resonance imaging appearance—case report. Neurol Med Chir (Tokyo) 2001;41:517–21 [DOI] [PubMed] [Google Scholar]
- 10.Hanly A, Petito CK. HLA-DR reactive dendritic cells of human choroids plexus: a potential reservoir of HIV in central nervous system. Human Pathol 1998;29:88–93 [DOI] [PubMed] [Google Scholar]
- 11.Subramanian S, Mathai D. Clinical manifestations and management of cryptococcal infection. J Postgrad Med 2005;51:521–6 [PubMed] [Google Scholar]