Abstract
Objectives:
Zornia diphylla (L.) Pers is an ethnomedical herb. The aim of the study is to scientifically verify the traditional use of Z. diphylla as an anticancer medicine.
Materials and Methods:
Different extracts, fractions, and chemical isolates of the whole plant were screened for cytotoxicity to Dalton's lymphoma ascites (DLA) cells by the Trypan blue exclusion method and MTT assay. Column chromatographic and preparative TLC techniques were used for the isolation of active fraction (AF) and active principle. Cytotoxicity of AF to different cell types was tested. The apoptotic activity of AF was evaluated by morphological observations, nuclear condensation, and comet assay. In vivo antitumor activity of AF was determined in DLA-challenged mice. Short-term (29 days) preliminary toxicity evaluation of AF was done in mice.
Results:
n-Hexane extract (but not water and ethanol extracts) showed significant cytotoxicity. AF, isolated from n-hexane extract, induced apoptotic cell death (in vitro) to DLA cells, but not to normal thymocytes and macrophages. A steroid positive active principle was isolated which showed 100% cytotoxicity at 5 μg/mL level. Interestingly, AF (50 mg/kg) protected all the mice challenged with one million DLA cells/mouse. AF (up to 10 times higher than the therapeutic dose) did not exhibit any conspicuous adverse toxic symptoms in the toxicity evaluation.
Conclusion:
Z. diphylla (AF) showed promising in vitro and in vivo anticancer activity against DLA cells, and it was devoid of any toxicity to mice in short-term toxicity evaluation. The herb is promising for the development of a valuable anticancer medicine.
Keywords: Anti-cancer, apoptosis, comet assay, Dalton's lymphoma ascites, Zornia diphylla (L.) Pers
INTRODUCTION
Cancer remains as an unconquered disease to a large extent. Although there are many chemotherapeutic agents available to treat cancer, these are not satisfactory to cure most of the cancers. Plants were used in the treatment of cancer from time immemorial.[1] Many plant-derived compounds are used in conventional medicine as chemotherapeutic agents with some level of success. These include taxol, etoposide, camptothecin, vincristine, and vinblastine. Even today, natural products remain as a rich source for novel anticancer drugs. Many medicinal plants used in ethnomedical practices to treat cancer in remote villages and tribal pockets in India and elsewhere are not known to the main stream population. In this context, an ethnobotanical survey conducted by the authors in remote villages in the Western Ghats area of Thiruvananthapuram District of Kerala State, India, revealed the folklore use of Zornia diphylla (L.) Pers in the treatment of cancer and fungal diseases. Therefore, detailed studies are being carried out in this laboratory to determine the likely utility of this plant to develop antifungal and/or anticancer drugs.
Z. diphylla is a prostrate herbaceous flowering plant, coming under the family Fabaceae. This plant grows as a weed in many parts of Kerala State, India. The ethnomedical literature shows that Z. diphylla is used to treat dysentery, venereal diseases and to induce sleep in children.[2] In spite of its ethnomedical use, the plant was not subjected to pharmacological and phytochemical studies. This study was carried out to determine the utility of this plant for developing a safe and effective anticancer agent.
MATERIALS AND METHODS
Plant materials
Z. diphylla was collected as a whole plant from Neyyar Dam area, Thiruvananthapuram District, Kerala State and was identified by the Taxonomists of Tropical Botanic Garden and Research Institute (TBGRI). A voucher specimen, No. 50976, was deposited in the Herbarium of TBGRI.
Chemicals and reagents
RPMI-1640 culture media, phosphate buffered saline (PBS), Trypan blue, penicillin, streptomycin (Himedia, Mumbai, India), 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT), agarose, acridine orange, ethidium bromide, vincristine, curcumin (Sigma Aldrich, St. Louis, USA), and fetal bovine serum (GibcoBRL, USA) were used. All other chemicals and reagents used were analytical grade and purchased from E. Merck India Ltd.
Equipments used
A carbon dioxide incubator (Shel lab, NC, USA), an electrical balance (Sartorius GMBH, Germany), an ELISA plate reader (Qualisystems, GSK, India), a membrane filter unit (Millipore, USA), a thermostatically controlled water bath (Julabo Labortec Hink GMBH, Germany), a rotavapor and a vacuum pump (Buchi, Switzerland), microcentrifuge (minispin, Eppendorf, USA), an inverted fluorescent-phase contrast microscope (Olympus, USA), and a high performance thin layer chromatography (HPTLC) system equipped with sample applicator LINOMAT- V, Scanner III, and integration software CATS 4.04 (CAMAG, Switzerland) were used.
Animals
Swiss albino mice (25–30 g body weight) were used for the experiments which were reared in TBGRI animal house and fed with standard pellet diet and water ad libitum. Animal experiments were approved by the Institute Animal Ethics Committee (IAEC), and the animals were maintained under standard laboratory conditions as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
DLA cells
Dalton's Lymphoma ascites (DLA) cells, obtained from Amala Cancer Research Centre, Trissur, India, were maintained as transplantable tumors in the peritoneal cavity of mice.
Collection of thymocytes and macrophages
Mice were sacrificed by cervical dislocation and thymus glands were carefully separated without adjoining lymph nodes. The separated thymus glands were transferred to RPMI-1640 medium, and single cell suspension of thymocytes was prepared. Viability was assessed by the Trypan blue exclusion method using a Neubauer counting chamber. For the collection of macrophages, mice were killed by cervical dislocation and immediately 5 mL chilled RPMI-1640 medium was injected into the peritoneal cavity and peritoneal exudates cells (PEC) were collected. The glass adherent cell population (macrophages) was separated, and viability was assessed by the Trypan blue exclusion method.[3]
Preparation of plant extracts
The whole plants were collected, cleaned, dried under shade in the laboratory, and powdered. The powder was extracted sequentially with n-hexane, ethanol, and water. In order to get complete extraction, each extraction was repeated three times with fresh solvent each time. (Since the heat sensitivity of the extracts with reference to bio-activity is not known, the extraction was carried out at low temperature without using rigorous extraction procedures). Alcohol and n-hexane extracts obtained were dried using a rotary evaporator at 40°C. The water extract was freeze-dried in a lyophilizer.[4]
Fractionation of n-hexane extract
The n-hexane extract (active extract) was fractionated by column chromatography using n-hexane, chloroform, and ethyl acetate. Precisely 4 g of the extract was loaded in a column packed with silica gel of mesh size 60–120, and seven fractions each of 500 mL were eluted with n-hexane (100%), n-hexane: chloroform (9:1), n-hexane: chloroform (3:1), n-hexane: chloroform (1:1), n-hexane: chloroform (1:3), chloroform (100%), and ethyl acetate (100%). All fractions were dried of solvent-free using a rotary evaporator at 40°C under reduced pressure.
Separation of active compound
The active fraction (AF) was analyzed by HPTLC. Briefly 10 μg of active AF was spotted in a readymade silica gel 60 F254 (Merck) plate and developed with n-hexane–chloroform (3:2) solvent system and individual component bands were derivatized with an anisaldehyde–sulfuric acid spray reagent. The HPTLC profile of the AF was performed using a CAMAG HPTLC system. Preparative thin layer chromatography (TLC) was carried out in a silica gel-G plate (20 × 20 cm2). The AF was subjected to preparative silica gel-G TLC using an n-hexane–chloroform (3:2) solvent system. After separation, individual bands in the TLC plate were visualized by spraying with an anisaldehyde–sulfuric acid spray reagent masking the major portion of the preparative TLC plate followed by heating at 80°C. The silica gel corresponded to individual bands were carefully separated and eluted with chloroform, filtered, and dried solvent-free using a rotary evaporator. The individual compounds or components thus obtained were tested for their cytotoxicity to DLA cells.[3] The active compound was subjected to chemical analysis.[5]
Cytotoxicity assays
Trypan blue exclusion method
Effect of various extracts on short-term cell viability was assessed by incubating 1 × 106 DLA cells in 1 mL of PBS containing vehicle (0.1% DMSO) and different concentrations of extracts (25–400 μg/mL) for 3 h in a CO2 incubator at 37°C, 5% CO2, 95% air, and 95% relative humidity. The cell viability was assessed by the Trypan blue exclusion method.[3] Cytotoxic effects to DLA cells of various fractions of the active n-hexane extract (10–50 μg/mL), components of AF (5 μg/mL), active component (1–5 μg/mL), and curcumin (10–400 μg/ mL) were also assessed. Effects of AF (25 and 50 μg/mL) and curcumin (50 μg/mL) on DLA cells, peritoneal macrophages, and thymocytes were also assessed.
MTT assay
MTT assay was performed essentially as described earlier.[6] Briefly 1 mL of 1 × 106 DLA cells was seeded in RPMI 1640 medium supplemented with 10% fetal bovine serum, streptomycin (100 μg/mL) and penicillin (100 units/mL) and incubated in a carbon dioxide incubator with 0.1% DMSO (vehicle) or various doses of AF (5–100 μg/mL) or curcumin (5–100 μg/ mL) for 48 h. After incubation, MTT solution (1.2 mg/mL) was added to each well, and the cells were incubated for an additional 4 h. The MTT formazan product was dissolved in DMSO, and the optical density was measured at 570 nm using an ELISA plate reader.
Apoptosis
Morphological observations
DLA cells (1 × 106/mL) were treated with 0.1% DMSO (vehicle) or curcumin (25 μg/mL) or the AF (25 μg/mL) in a carbon dioxide incubator for 48 h at 37°C, 5% carbon dioxide, 95% air, and 95% relative humidity and observed under a phase contrast microscope to assess nuclear condensation and membrane blebbing of the cells. Since the AF-treated cells showed membrane blebbing and nuclear condensation, detailed studies were carried out to observe morphological changes including formation of apoptotic bodies. Briefly, the treated cells were washed with PBS and mixed with acridine orange-ethidium bromide stain (100 μg/mL). After staining, the cells were observed under an inverted fluorescent microscope using a blue filter and photographed with a digital camera.[3]
Comet assay
Comet assay was carried out essentially as described elsewhere.[7] Briefly, DLA cells (1 × 106 cells/mL) were treated with 0.1% DMSO (vehicle) or AF (25 μg/mL) or curcumin (25 μg/mL) in a 24 well plate for 24 h in RPMI-1640 medium supplemented with 10% fetal bovine serum, streptomycin (100 μg/mL), and penicillin (100 units/mL) in a carbon dioxide incubator with 5% carbon dioxide at 37°C and cells were harvested, washed, and suspended in PBS. Ten microliters of the cell suspension (10,000 cells) were added to 75 μL of low melting point agar, mixed thoroughly and spread uniformly over the normal melting agar in a frosted slide. Over the cell paved layer, a third-layer of low melting point agar was added and a cover slip was placed over it after solidification. These prepared slides were dipped in lyses buffer (pH 10.0) for 2 h, and kept in electrophoresis buffer (pH 13.0) for 20 min. The treated slides were electrophorezed in a horizontal electrophoresis unit. The slides were neutralized by drop wise addition of neutralization buffer (pH 7.5) and stained with ethidium bromide (20 μg/ mL). The stained slides were observed under a fluorescent microscope using green filter and photographed (Olympus digital camera).
In vivo antitumor activity assay in DLA challenged mice
In vivo antitumor activity of AF of the n-hexane extract was assessed as described previously.[3] Briefly, 48 male Swiss albino mice (20–25 g body weight) were challenged with an intraperitoneal injection of DLA cells (1 × 106 cells/0.5 mL) and divided into six groups of eight animals each. Group I was kept as control and received 1% Tween-80 in water. The groups II, III, and IV were administered the AF at 10, 25, and 50 mg/kg body weight, respectively. The groups V and VI received standard anticancer drug, vincristine 0.5 and 1mg/kg body weight, respectively. All animals received the drugs once daily (0.5 mL/animal) for 15 days. The mortality of animals was observed for 30 days.
Preliminary toxicity evaluation in mice
To study sub-acute (short-term) toxicity, four groups of mice each containing six male mice (25–30 g body weight) were used. The first group was kept as control and groups II, III, and IV received 100, 250 and 500 mg/kg of the AF, respectively. The drug was administered once daily (0.5 mL/animal) for 29 days (p.o.). The control group received the vehicle (1% Tween-80 in water) in an identical manner. The behavior of the animals was observed daily for 1 h for 14 days. Initial and final body weights, water and food intake and state of stool were observed. The animals were killed on the 30th day and serum biochemical and hematological parameters were determined. Important organs were dissected out, weighed, and observed for pathological changes. Serum glutamate pyruvate transaminase (GPT), glutamate oxaloacetate transaminase (GOT), alkaline phosphatase (ALP), glucose, urea, cholesterol, and total lipid were measured[8–10] using commercial assay kits (Autospan diagnostic India Pvt. Ltd.). Hemoglobin was measured using a hemoglobinometer with comparison standards. The peritoneal macrophages and total leucocytes were collected and counted as described elsewhere.[11]
Statistical analysis
Statistical comparison was done using one-way ANOVA followed by Dunnets’ post-hoc comparison when more than two groups were involved. P values <0.05 were considered statistically significant.
RESULTS
Cytotoxicity of Z. diphylla
Trypan blue exclusion method
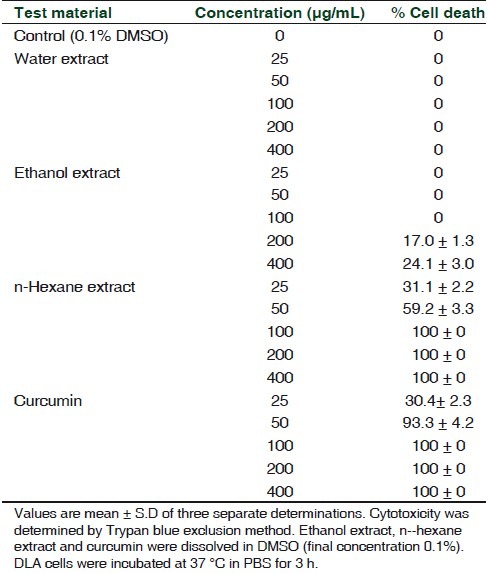
As shown in Table 1, among the various extracts of Z. diphylla incubated with DLA cells for 3 h, the n-hexane extract showed significant cytotoxicity. The extract showed 100% cytotoxicity at 100 μg/mL level. The cytotoxic effect of n-hexane extract to DLA cells was comparable to that of standard chemopreventive compound curcumin from turmeric. Curcumin is known to induce apoptosis.[12] The alcohol extract showed only marginal cytotoxicity (17% and 24% at 200 and 400 μg/mL, respectively) while the water extract was devoid of any cytotoxicity in all tested concentrations.
Table 1.
Cytotoxicity of different extracts of Z. diphylla to DLA cells
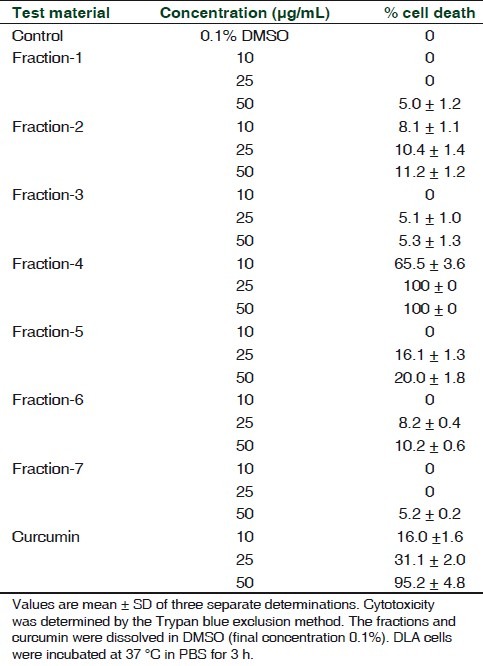
When various fractions of the n-hexane extract were tested for their cytotoxicity, the fourth fraction (eluted with n-hexane: chloroform, 1:1 ratio) showed significant cytotoxicity to DLA cells. This fraction of n-hexane extract (here after designated as AF) at 25 μg/mL level showed 100% cytotoxicity, while all other fractions exhibited marginal cytotoxicity only. The cytotoxicity of 25 μg/mL AF to DLA cells was better than that of 50 μg/mL curcumin [Table 2].
Table 2.
Cytotoxicity of different fractions of the n-hexane extract of Z. diphylla to DLA cells
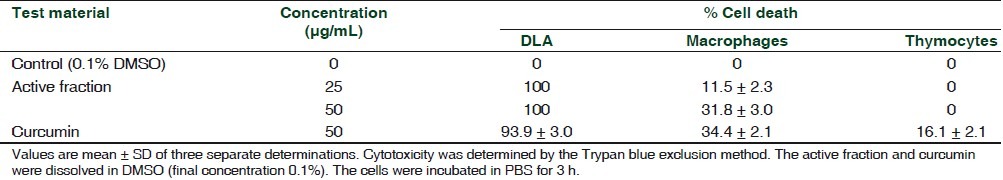
When different cell types were compared, AF showed significant cytotoxicity to DLA cells, but it was devoid of any cytotoxicity to thymocytes in the two concentrations (25 and 50 μg/mL) tested. A dose-dependent marginal cytotoxicity was observed in the case of macrophages [Table 3].
Table 3.
In vitro cytotoxicity of the active fraction of the n-hexane extract of Z. diphylla to different cell types
AF was resolved into five individual compounds in HPTLC [Figure 1]. Among the five compounds obtained from silica gel-G preparative TLC of AF, the fast moving compound with an Rf value of 0.83 (component number five) showed 100% cytotoxicity to DLA cells at 5 μg/mL level and all other compounds did not show any significant cytotoxicity at this concentration [Table 4]. The active compound showed positive reaction to steroid (Liberman–Buchart test).
Figure 1.
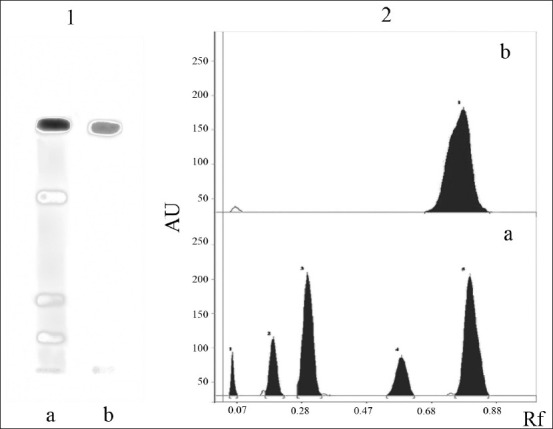
High performance thin layer chromatogram (HPTLC) of the active fraction (AF) and active principle isolated from Z. diphylla. (1) Silica gel 60F254 HPTLC using n-hexane: chloroform (1:1) as a solvent system. Individual components were derivatized with anisaldehyde-sulfuric acid spray reagent. (a) HPTLC of active fraction (AF) showing five components (bands). The fifth band with an Rf value of 0.83 is the active compound (principle). (b) HPTLC of active principle (Rf 0.83) isolated from AF. (2) Silica gel 60F254 HPTLC scanned densitometrically at 580 nm after derivatization. (a) HPTLC of AF showing five components. (b) HPTLC of active principle (Rf 0.83) isolated from AF
Table 4.
Cytotoxicity to DLA cells of different components (separated by TLC) of the active fraction obtained from the n-hexane extract of Z. diphylla
MTT assay
In 48 h culture of DLA cells, AF showed dose-dependent cytotoxicity in MTT assay, and 100% cytotoxicity was observed at 25 μg/mL level. When compared with the standard compound curcumin, AF showed 100% cell death at 25 μg/mL whereas curcumin showed only 76% cell death in the same concentration [Figure 2].
Figure 2.
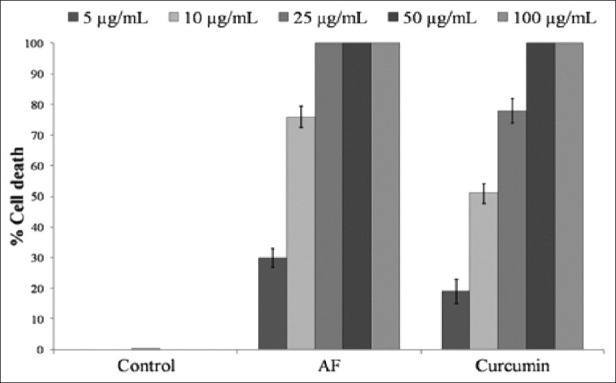
Cytotoxicity to DLA cells of the active fraction of the n-hexane extract of Z. diphylla. AF, Active fraction; cytotoxicity was determined by MTT assay in 48 h culture. values are mean ± SD of three separate determinations. Compared to control all values are statistically significant, P ≤ 0.5
Apoptosis
AF- or curcumin-treated DLA cells, when stained with AO-Et Br, showed membrane blebbing in a fluorescent microscope. The cells treated with 25 μg/mL of AF appeared orange-red in color (dead cells), while DMSO control cells appeared yellowish-green (live cells) [Figure 3]. In phase contract microscopy both the curcumin- and AF- (25 μg/mL) treated DLA cells showed nuclear condensation whereas vehicle- (DMSO) treated control cells appeared in normal conditions [Figure 4].
Figure 3.
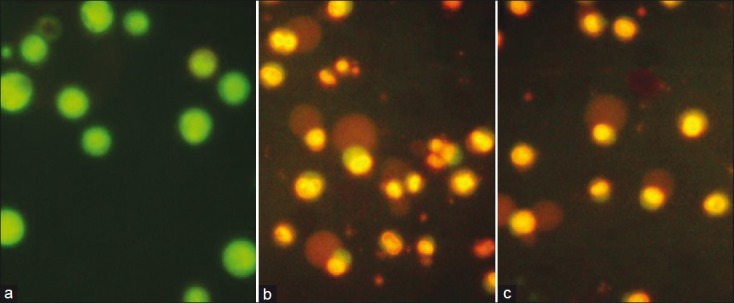
DLA cells stained with acridine orange–ethidium bromide (Ao-Et Br) under a fluorescent microscope. (a) DMSO control DLA cells appeared in yellowish green color (live). (b) The active fraction (25 μg/mL) of Z. diphylla-treated DLA cells showing cell death (orange-red) and apoptotic bodies. (c) Curcumin (25 μg/mL)-treated DLA cells showing cell death (orange-red) and apoptotic bodies
Figure 4.
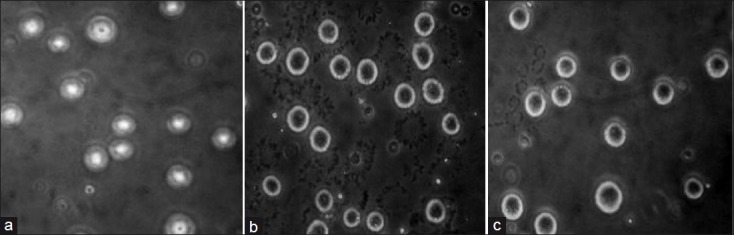
Nuclear condensation induced by Z. diphylla (active fraction) in a phase contrast microscope. (a) Control DLA cells (without nuclear condensation), (b) active fraction (25 μg/mL) treated DLA cells showing nuclear condensation, and (c) Curcumin (25 μg/mL) treated DLA cells showing nuclear condensation
In comet assay DMSO-treated control cells did not show any comet formation but AF- or curcumin-treated cells showed significant comet formation in fluorescent microscopy due to DNA degradation. The tailing of comet was longer in AF-treated cells than that of curcumin-treated cells [Figure 5].
Figure 5.
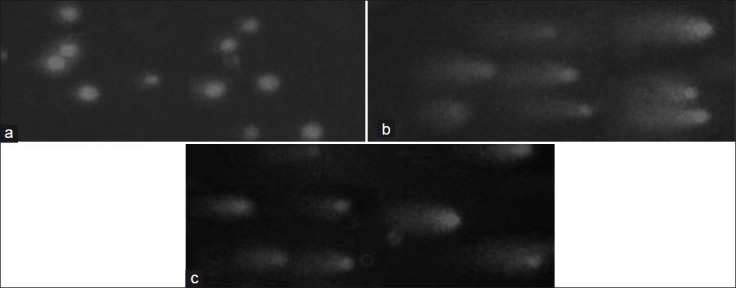
Comet assay. (a) DMSO control DLA cells with intact DNA without DNA tailing. (b) The active fraction (25 μg/mL) of Z. diphylla-treated DLA cells with nuclear DNA degradation and tailing typical to comet formation. (c) Curcumin (25 μg/mL) treated DLA cells with nuclear DNA degradation and tailing typical to comet formation
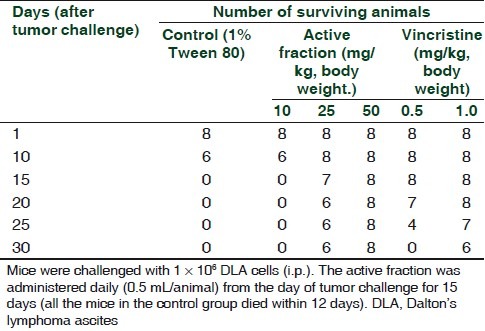
In vivo antitumor activity
Administration of AF daily for 15 days to DLA challenged mice at a dose of 50 mg/kg body weight resulted in protection of all the challenged mice, while 25 mg/kg protected only 75% of the animals. All the untreated DLA challenged animals (vehicle control group) died on or before twelth day after the challenge. The standard anticancer drug, vincristine (1mg/kg), showed 75% protection to DLA challenged mice and the effect was comparable to that of 25 mg/kg AF [Table 5].
Table 5.
Antitumor activities of the active fraction isolated from the n-hexane extract of Z. diphylla in DLA challenged mice
Preliminary toxicity evaluation
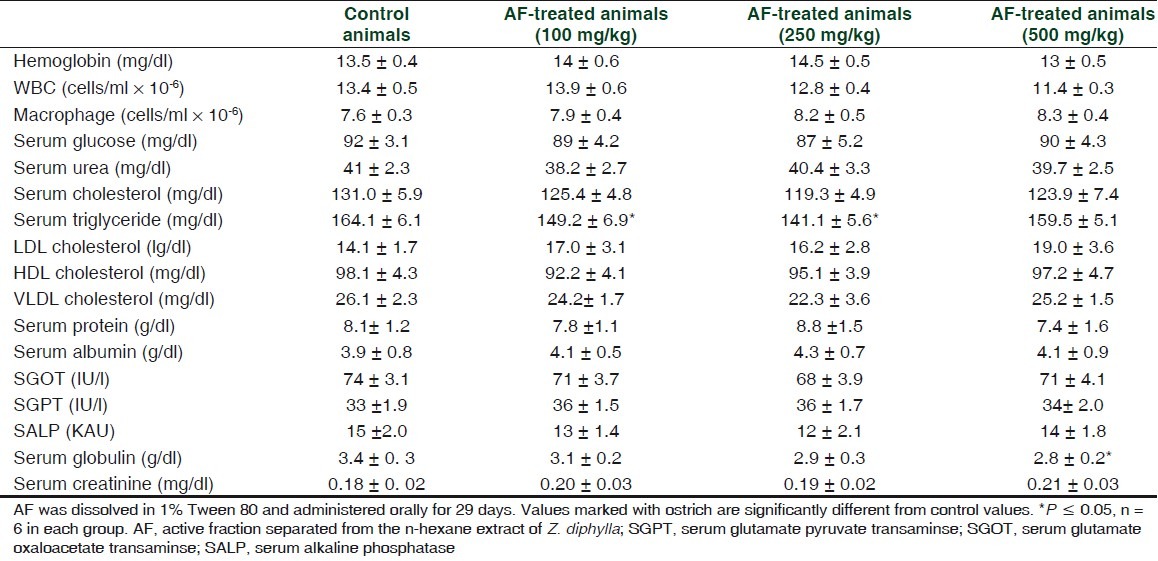
In short-term (29 days), toxicity studies AF at a dose of 5 or 10 times higher than that of therapeutic dose did not exhibit any conspicuous toxic symptoms in mice. Administration of AF did not change food and water intake significantly (data not given). However, at the highest dose studied (500 mg/kg), there was a significant (P ≥ 0.05) decrease in the weight gain of the animals. 2.40 ± 0.18, 2.33 ± 0.15, 2.05 ± 0.13, and 1.78 ± 0.10 g were the body weight gains during the experimental period for 0 (control), 100, 250, and 500 mg/kg AF-treated groups, respectively. AF administration did not result in any gross behavioral changes. Serum biochemical and hematological parameters of the test groups did not show any significant deviations from the values of normal control animals up to a dose of 250 mg/kg. However, a marginal decline in serum globulin levels was observed at the highest dose studied [Table 6]. The serum triglyceride (TG) level decreased in 100 and 250 mg/kg treated groups, but not in the 500 mg/kg group.
Table 6.
Effect of short-term (29 days) administration of the active fraction (isolated from n-hexane extract of Z. diphylla) to mice on hematological and serum biochemical parameters
DISCUSSION
A highly active anticancer fraction (AF) and an active principle, which showed positive reaction to steroid, have been isolated for the first time from Z. diphylla. The structure of the steroid positive, active principle is not known; it remains to be elucidated. Elucidation of the structure of the active steroid may pave the way for the production of the active principle by chemical synthesis.
Some of the potent anticancer plants are highly toxic.[13] Although studies in the recent past have revealed anticancer activity in many medicinal plants, none of them was completely free of toxicity at doses 10 times higher than therapeutic dose. In this context, it should be noted that this herbal drug was devoid of toxicity even at 500 mg/kg. The therapeutic dose was 50 mg/kg. Thus, it appears that AF is a very safe herbal drug. Above 1mg/kg, vincristine exhibits toxicity. The doses of vincristine selected for this study were based on prior studies.[3]
In the toxicity evaluation, serum triglyceride levels decreased at 100 and 250 mg/kg levels, but not at 500 mg/ kg. The reason for the same is not known. At the highest dose, the body would have developed some adaptive response. However, the decrease in TG may be considered as a beneficial effect.
At least one of the mechanisms of anticancer activity of Z. diphylla could be the induction of cell-specific apoptosis. There are nutraceuticals and herbal drugs known to induce apoptosis influencing expression of specific genes and thereby act as chemo-preventive/protective agents against carcinogenesis.[14–16] Examples of these include curcumin from turmeric,[9,17] sulforaphane from vegetables such as cauliflower and broccoli,[18,19] rice bran polyphenol[20] and triterpene from Emelia sancifolia.[3] However, in the present case, the steroid from Z. diphylla appears to be highly specific to cancer cells in inducing apoptotic cell death. As shown in Table 3, compared to curcumin, the well-known chemo-preventive phytochemical from Curcuma longa, AF was more cell specific in inducing cytotoxicity. However, this study has to be extended including many cancer as well as normal cell types to establish its cancer cell-specific apoptosis inducing property.
In the in vivo studies, 50 mg/kg AF completely protected all the tumor challenged mice. Although this dose is relatively high, AF is devoid of toxicity even at 500 mg/kg. Further, in the case of AF, the concentration required for 100% DLA cell death in vitro was 25 μg/mL whereas that for active principle isolated from AF was 5 μg/mL only. Therefore, the active principle may protect all DLA challenged mice in vivo at 10 mg/kg level.
Z. diphylla is a locally available weed which can be cultivated easily to obtain the desired quantity of plant material. Thus, this plant can be cultivated for large-scale production of raw material for drug development. Seasonal and ecotype variations in the medicinal quality of the plant, if any, remain to be studied.
At any rate, this study opened up a new vista for the likely development of a successful drug for, at least, certain types of cancer. In this context, in should be noted that for full grown cancer the conventional medicine is devoid of a satisfactory treatment. Development of improved herbal drug with regard to safety and efficacy is the need of the hour. This study is a significant leap towards that direction.
ACKNOWLEDGMENTS
The authors express sincere thanks to Mr. G. Santhosh Kumar, Technical Assistant, TBGRI, for providing assistance in the animal experiments. The authors are thankful to Dr. B. Sabulal, Head, Phytochemistry and Phytopharmacology Division, for his encouragement and useful suggestions, to Prof. N. Ravi and Dr. T. Sabu for their help in the correct taxonomical identification of the plant, and to Ms. Rajani Kurup for her help in the phytochemical analysis of the active fraction.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hartwell JL. Plants Used Against Cancer. Massachusetts: Quarterman Lawrence; 1982. [Google Scholar]
- 2.Khare CP. Indian Medicinal Plants: An Illustrated Dictionary. Heidelberg: Springer-Verlag; 2004. [Google Scholar]
- 3.Shylesh BS, Ajikumaran NS, Subramoniam A. Induction of cell-specific apoptosis and protection from Dalton's lymphoma challenge in mice by an active fraction from Emilia sonchifolia. Indian J Pharmacol. 2005;37:232–7. [Google Scholar]
- 4.Subhisha S, Subramoniam A. Antifungal activities of a steroid from Pallavicinia lyellii, a liverwort. Indian J Pharmacol. 2005;37:304–8. [Google Scholar]
- 5.Wagner H, Bladt S, Zgainski EM. Plant Drug Analysis. Berlin, Heidelberg, New York, Tokyo: Springer-Verlag; 1984. [Google Scholar]
- 6.Bernas T, Dobrucki J. Mitochondrial and nonmitochondrial reduction of MTT: Interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry. 2002;47:236–42. doi: 10.1002/cyto.10080. [DOI] [PubMed] [Google Scholar]
- 7.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 8.Subramoniam A, Evans DA, Rajasekharan S, Pushpangadan P. Hepatoprotective activity of Trichopus zeylanicus extract against paracetamol induced hepatic damage in rats. Indian J Exp Biol. 1998;36:385–9. [PubMed] [Google Scholar]
- 9.Tathagata C, Suman P, Tanya D, Gaurisankar SA. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280:20059–68. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 10.Reitman S, Frankel S. Calorimetric method for the determination of serum glutamate oxalo-acetate and glutamate pyruvate transaminase. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 11.Kind PR, King EJ. Estimation of plasma phosphatases by determination of hydrolysed phenol with antipyrine. J Clin Pathol. 1954;70:322–30. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jam NC. Schalm's Veterinary Haematology. 4th ed. Philadelphia: Lea and Fabiger; 1986. [Google Scholar]
- 13.Jose Thomas T, Panikker B, Subramoniam A, Krishnan Nair M, Panickkar KR. Anti-tumor property and toxicity of Barringtonia recemosa Roxb seed extract in mice. J Ethnopharmacol. 2002;82:223–7. doi: 10.1016/s0378-8741(02)00074-0. [DOI] [PubMed] [Google Scholar]
- 14.Szliszka E, Czuba ZP, Sedek L, Paradysz A, Krol W. Enhanced TRAIL-mediated apoptosis in prostrate cancer cells by the bio-active compounds neobavaisoflavone and psoralidin isolated from Psoralia corylifolia. Pharmacol Rep. 2011;63:139–48. doi: 10.1016/s1734-1140(11)70408-x. [DOI] [PubMed] [Google Scholar]
- 15.Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni SJ, et al. Licochalone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 2011;302:69–75. doi: 10.1016/j.canlet.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Samadi AK, Tong X, Mukerji R, Zhang H, Timmerman BN, Cohen MS. Withaferin A, a cytotoxic steroid from Vassolia breviflora induces apoptosis in human head and neck squamous cell carcinoma. J Nat Prod. 2010;73:1476–81. doi: 10.1021/np100112p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumor and anti-oxidant activities of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 18.Yu R, Mandlekar S, Harvey KJ, Ucker DS, Kong AN. Chemopreventive isothiocynates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998;58:402–8. [PubMed] [Google Scholar]
- 19.Hecht SS, Kenny PM, Wang M, Upadhyaya P. Benzyl isothiocynate: An effective inhibitor of polycyclic aromatic hydrocarbon tumorigenesis in A/J mouse lung. Cancer Lett. 2002;187:87–94. doi: 10.1016/s0304-3835(02)00410-x. [DOI] [PubMed] [Google Scholar]
- 20.Kong CK, Lam WS, Chiu LC, Ooi VE, Sun SS, Wong YS. A rice bran polyphenol, cycloartenyl ferulate elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 to TRIL-induced apoptosis. Biochem Pharmacol. 2009;77:1487–96. doi: 10.1016/j.bcp.2009.02.008. [DOI] [PubMed] [Google Scholar]


