Abstract
Objective:
To study the effect of metformin on amelioration of hepatotoxicity induced by methotrexate.
Materials and Methods:
After a 2-weeks of acclimatization period, the animals were randomly separated into three groups (seven rabbits each), all groups were maintained on standard chow diet throughout the experiment (8 weeks). Group 1 was treated with normal saline water (control), Group 2 with methotrexate (MTX, hepatotoxic), and Group 3 with MTX plus metformin. Induction of hepatotoxicity was carried out by administration of MTX to the rabbit in a dose of 0.25 mg/kg /day i.m. for 8 weeks.
Results:
The treatment with MTX to rabbits for 8 weeks resulted in significant changes in serum liver enzymes, as compared to the baseline group. SGOT, SGPT, ALP, and bilirubin were significantly increased (P < 0.001), while total serum protein was significantly decreased. Similarly, 8 weeks of MTX treatment produced significant (P < 0.001) prolongation in PT. PTT was not significantly changed. It was found that serum MDA levels and SOD activity were significantly increased (P < 0.001), while serum GSH levels were significantly decreased (P < 0.001). Adding metformin to MTX is found to be significantly (P < 0.001) reduced the liver function test and shortening of PT and a significant increase in TSP (P < 0.001).
Conclusion:
It can be concluded that administration of metformin restored the altered liver function parameters and produced significant improvement in liver histopathological findings. Therefore, this additive drug possesses hepatoprotection against MTX-induced hepatotoxicity.
Keywords: Hepatotoxicity, metformin, methotrexate
INTRODUCTION
Liver is the main organ responsible for the drug metabolism and appears to be the sensitive target site for substances modulating biotransformation.[1] Liver injury caused by drugs and other chemicals accounts for approximately 5% of all cases of jaundice and encompasses a wide spectrum of diseases ranging from acute and chronic hepatitis to bile duct abnormalities and neoplasm (oral contraceptive, clomiphene, and carbamazepine have been associated with hepatic adenomas).[2,3]
Methotrexate (MTX) is an effective treatment modality largely used in rheumatoid arthritis, psoriasis, leukemias, and some autoimmune disorders for more than 40 years.[4,5] Nowadays it is also used for sarcoidosis, inflammatory bowel diseases, and vasculitis and severe refractory asthma.[6] With this enlarged spectrum of clinical use for MTX, its toxicity on the liver has gained much more importance.[4] Oxidative stress has emerged as a key player in the pathogenesis of MTX-induced hepatotoxicity. The increased generation of reactive oxygen and nitrogen species, together with the decreased antioxidant defense, promotes the development and progression of hepatotoxicity.[7]
Metfomin is regarded as the first-line treatment in type 2 diabetes mellitus (DM). Recently, benefits in the macrovascular complications of diabetes have been attributed to it. Metformin offers many advantages over the currently available other oral antidiabetic drugs.[8,9]
Metformin can exert a direct vascular anti inflammatory effect by inhibiting NF-κB through blockade of the PI3K-Akt pathway, metformin dose-dependently inhibits interleukin (IL)-1β-induced release of the proinflammatory cytokines IL6 and 8 in the human vascular smooth muscle cells (SMCs), macrophages, and endothelial cells. It diminishes IL-1 β-induced activation and nuclear translocation of nuclear factor Kappa B (NF-κB) in SMCs.
Metformin also leads to overexpression of HO-1 that leads to protection against oxidative stress-induced apoptosis in primary hepatocyte in rats.[10–12]
In some studies, the effects of metformin on oxidative stress have been evaluated and conflicting data were obtained. It was demonstrated that metformin reduced oxidative stress while other study showed that it increases oxidative stress.[13,14] In other separate research work, the serum levels of total antioxidant status and MDA were not significantly changed with metformin treatment.[15,16]
This study was designed to test the effect of metformin in amelioration of hepatotoxicity induced by MTX.
MATERIALS AND METHODS
Animals
Twenty-one New Zealand white male rabbits, aged 3–4 months with weight of 1.5–2 kg, were used in the study. All experiments were approved by the Animal Care and Research Committee of the University of Kufa, and this investigation conforms with the Guide for the Care and Use of Laboratory Animals (National Research Council). The animals were placed in Kufa Medical College's animal house. The rabbits were housed in an isolated room, in a group caging system, at controlled temperature (25 ± 2 °C) and ambient humidity. Lights were maintained on a 12-h light/dark cycle. The animals had free access to water ad libitum.
Drugs
MTX (EBEWE Pharma, Austria) was used in a dose of 0.25 mg/kg/day i.m[17] and it was given to the rabbits according to body weight once daily while metformin was used in a dose of 200 mg/kg/day orally. A 500 mg tablet was dissolved in DW, and the drug was given to the rabbits according to body weight once daily through a stomach tube.[18,19]
Experimental protocol
After a 2-week acclimatization period, the animals were randomly separated into three groups (seven rabbits each), all groups were maintained on standard chow diet (4% fat, 18% protein, 60% carbohydrate, and 4% fibers) throughout the experiment (8 weeks). Group 1 was treated with normal saline water (control), Group 2 with methotrexate (MTX, hepatotoxic), and Group 3 with MTX plus metformin (Merck Sante s.a.s. 45400 SEMOY-FRANCE). Induction of hepatotoxicity was carried out by the administration of MTX to the rabbits in a dose of 0.25 mg/kg /day i.m. for 8 weeks.[17]
Biochemical procedures
From each rabbit, 15 ml of blood were collected from the heart directly using a disposable syringe. The blood sample was divided into two parts:
4.5 ml of the blood was placed in a tube that contained sodium citrate (Sun, Jordan), as anticoagulant. The plasma was prepared via centrifugation at 2500 rpm for 10 min for determination of prothrombin time and activated partial thromboplastin time.
10.5 ml of blood was placed in a serum tube and left for 30 min. The serum was prepared via centrifugation at 3000 rpm for 10 min and kept frozen at –20 °C (unless immediately analyzed) and then subdivided in two parts; one part was used for determination of SGPT, SGOT, ALP, total protein, and bilirubin and the other part was subsequently used for determination of oxidation parameters (SOD, GSH, and MDA).
Histological examination of the liver
All specimens were immediately fixed in 10% formaldehyde solution. After fixation they were processed in usual manner, and embedded in paraffin for subsequent histopathological examination for hepatotoxicity.
Statistics
All data are expressed as mean ± SEM. Groups of data were compared with an analysis of variance (ANOVA) followed by the Least Significant Difference test. The Mann–Whitney test was used to identify the difference between two groups. Turkey's multiple comparison tests, the statistical significance, strength, and direction of linear correlation between two quantitative variables were assessed by Spearman's rank linear correlation. Values of P < 0.001 were regarded as statistically significant.
RESULTS
Effect of MTX on liver function
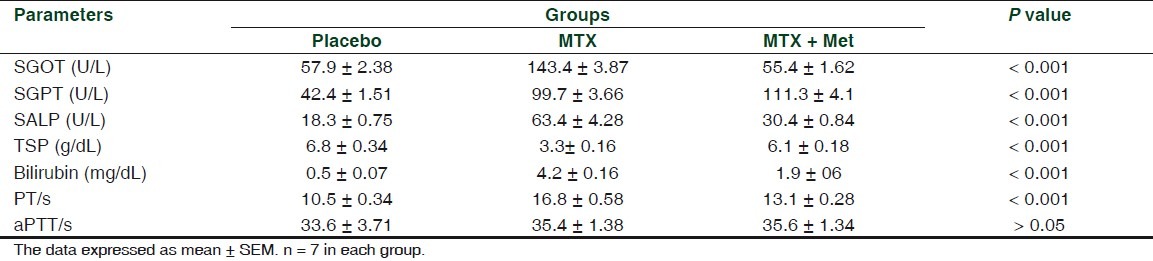
Treating rabbits with MTX for 8 weeks resulted in significant changes in serum liver enzymes, as compared to the placebo group. SGOT, SGPT, ALP, and bilirubin were significantly increased (P < 0.001), whereas total serum protein was significantly decreased. Similarly, 8 weeks of MTX treatment produced a significant (P < 0.001) prolongation in PT. PTT was not significantly changed [Table 1].
Table 1.
Changes in serum liver enzymes of rabbits treated with MTX and MTX + MET for 8 week
Effect of MTX on serum oxidation parameters
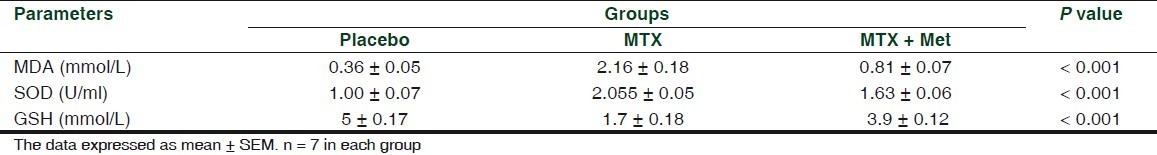
In comparison with the placebo group, MTX treatment for 8 weeks resulted in significant changes in oxidation parameters namely serum MDA level, SOD activity, and GSH level. It was found that serum MDA levels and SOD activity were significantly increased (P < 0.001) while serum GSH levels were significantly decreased (P < 0.001) [Table 2].
Table 2.
Changes in serum oxidation parameters of rabbits treated with MTX and MTX + Met for 8 weeks
Correlation between oxidation parameters and liver enzymes
The three oxidative stress parameters were tested for association with serum GOT, GPT, and ALP in the overall experiment, in a quest to explain the treatment effects observed through changes in oxidative stress parameters.
An increase in serum MDA was found to be associated with statistically significant mean increase in serum GOT (of 0.457 U/L), GPT (of 18.486 U/L), and ALP (of 11.873 U/L). MDA has strong positive correlation with serum GOT (r = 0.652), GPT (r = 0.91), and ALP (r = 0.845).
An increase in serum SOD was found to be associated with statistically significant mean increase in serum GOT (of 46.032 U/L), GPT (of 0.848 U/L). Serum SOD had the smallest and statistically insignificant effect on serum ALP. An increase in serum SOD of 1 U/L was found to be associated with a slight mean increase in serum ALP of 4.198 U/L. Serum SOD showed a strong positive correlation with serum GOT (r = 0.847), GPT (r = 0.68), and ALP (r = 0.691).
An increase in serum GSH was found to be associated with statistically significant mean decrease in serum GOT (of 8.567 U/L), GPT (of 7.615 U/L), and ALP (of 4.928 U/L). Serum GSH had a strong negative (indirect) correlation with serum GOT (r = –0.767), GPT (r = –0.879), and ALP (r = –0.82).
Correlation between oxidation parameters and TSP, bilirubin, and PT
An increase in serum MDA was found to be associated with statistically significant mean decrease in TSP (of 0.669 g/dL), increase in serum bilirubin (of 0.753 mg/dL), and PT (of 1.048 s). MDA had a strong negative correlation with serum TSP (r = –0.716), strong positive correlation with serum Bilirubin (r = 0.838), and PT (r = 0.772).
An increase in serum SOD was found to be associated with statistically significant mean decrease in TSP (of 1.815 g/dL), and increase in serum bilirubin (of 1.644 mg/dL). Serum SOD had statistically significant effect on serum PT. An increase in serum SOD of 1 U/L was found to be associated with mean increase in serum PT of 2.796 s. Serum SOD showed a strong negative correlation with TSP (r = –0.783) and a strong positive correlation with serum bilirubin (r = 0.875) and PT (r = 0.825).
An increase in serum GSH was found to be associated with statistically significant mean increase in TSP (of 0.075 g/dL), decrease in bilirubin (of 0.123 mg/dL), and PT (of 0.354 s). Serum GSH had a strong positive correlation with TSP (r = 0.648) and a strong negative correlation with bilirubin (r = –0.764) and PT (r = –0.747).
Effect of adding metformin on liver function
As compared to the hepatotoxic group, a combination drug treatment resulted in the following changes in liver function parameters:
Adding metformin to MTX was found to be significantly (P < 0.001) reduced GOT (by a mean of 32.143 U/L), GPT (by a mean of 44.286 U/L), and ALP (by a mean of 33 U/L). Furthermore, metformin caused a significant (P < 0.001) reduction in serum bilirubin levels and shortening of PT and significant rise in TSP (P < 0.001) [Table 1].
Effect of adding metformin on oxidation parameters
Adding metformin to MTX was found to be significantly (P < 0.001) reduced serum MDA level (by a mean of 1.354 mmol/L), SOD (by a mean of 0.428 U/ml), and increased GSH (by a mean of 2.194 mmol/L) [Table 2].
Effect of adding metformin on hepatic histopathology
The histopathological hepatic changes resulting from toxic insults were graded as mild, moderate, and severe changes [Figures 1 and 2]. These were tested in seven experimental animals in each treatment group as shown in Table 3. None had any abnormal hepatic changes in the placebo group .The median grade of hepatic changes was significantly different between all the three study groups. The median was the highest in the MTX-only group (severe) and the lowest in the placebo group (no abnormality). Adding metformin to MTX was associated with a median hepatic change that is significantly lower than the MTX-only group [Figures 3 and 4].
Figure 1.
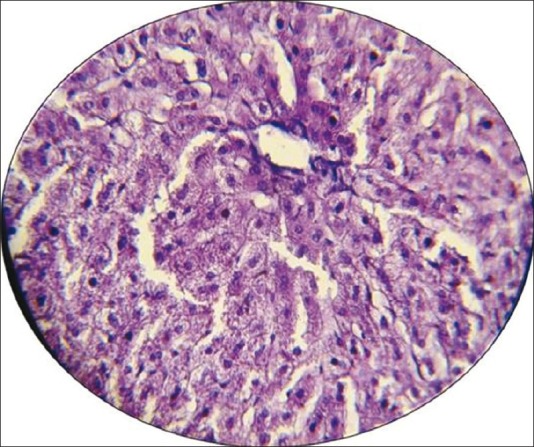
Photomicrograph of the liver section of normal rabbits (no abnormality) shows the normal architecture. Stained with hematoxylin and eosin (× 40)
Figure 2.
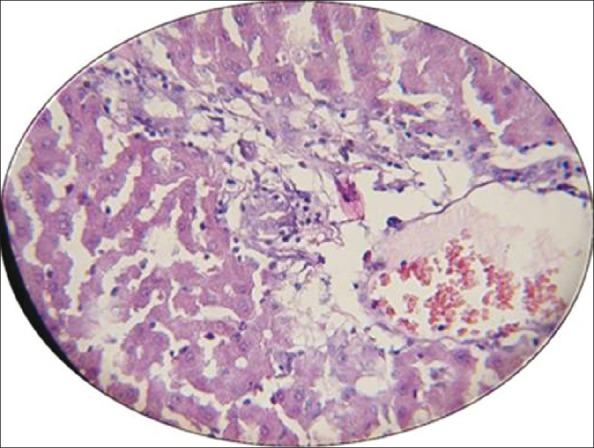
Photomicrograph of histological liver sections of rabbits (severe changes shows large area of necrosis) treated with MTX alone. Stained with hematoxylin and eosin (×10)
Table 3.
The difference in median histopathological grading of abnormal hepatic changes between the three treatment groups
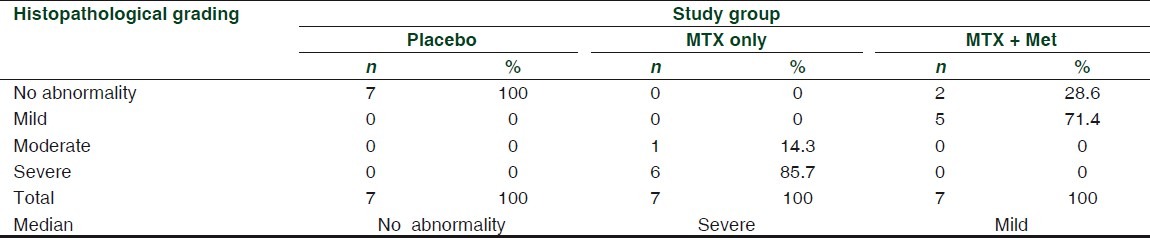
Figure 3.
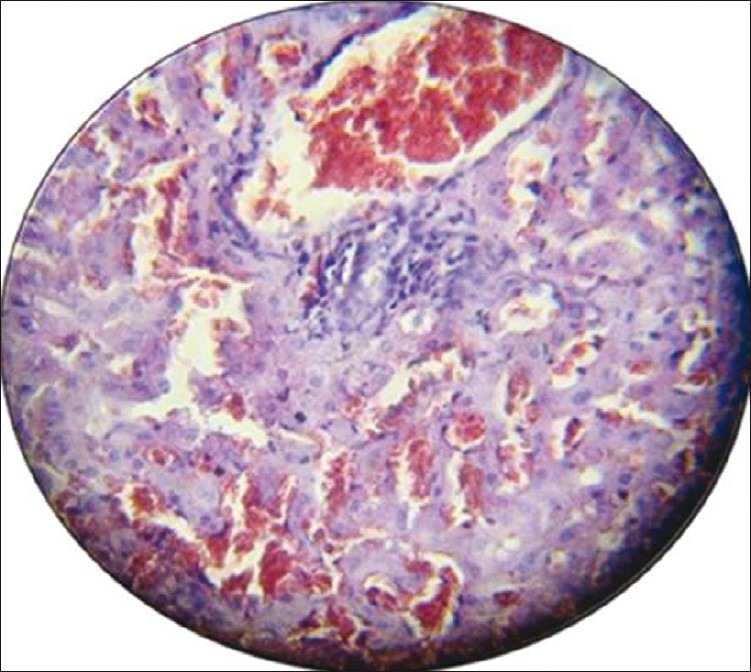
Photomicrograph of the liver section with moderate histological findings (portal tract dilatation and congestion inflammatory cell infiltration, sinusoidal dilatation congestion). Stained with hematoxylin and eosin (×40)
Figure 4.
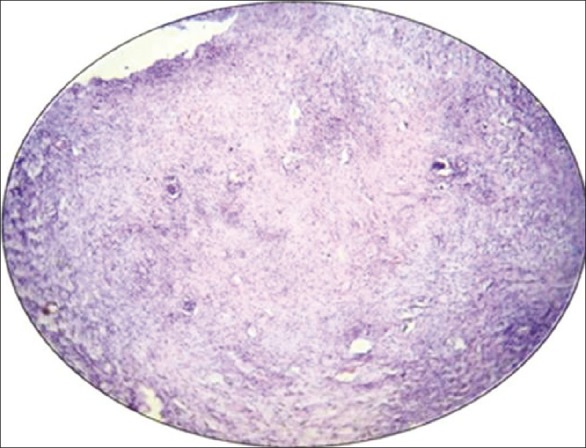
Photomicrograph of histological liver sections of rabbits (severe changes shows large area of necrosis) treated with MTX alone. Stained with hematoxylin and eosin (×10)
DISCUSSION
In this study, it was shown that keeping the rabbits on a MTX treatment for 8 weeks caused a significant increase in the levels of bilirubin with significant increase in the activities of SGPT, SGOT, and ALP, but significant decrease in the levels of protein. These findings are in agreement with the reports of other studies.[4,20,21]
The elevation of enzyme activities could be attributed to the damaged structural integrity of the liver (possibly by oxidative stress and lipid peroxidation). The lipid peroxidation causes disruption of the memberane bilayer and cell integrity and eventually necrosis that leads to leakage of these cytoplasmic enzymes into the blood.[21]
The altered levels of bilirubin and protein are possibly due to increased production of free radicals. The reduction in the protein concentration in animals treated with MTX for 8 weeks may be due to disruption in protein structures and formation of protein adducts with ROS and also the possible involvement of nephrotoxicity that caused loss of protein through the renal route.[22]
In this study, we observed that adding metformin to MTX was found to produce a significant decrease in liver enzyme activities, bilirubin level, and shortening in PT and a significant increase in TSP levels. Consistent with this finding was the observation of other studies[16,18,23,24] which reported that metformin has no effect on liver function parameters. Concerning oxidation parameters, the results of this study demonstrated that adding metformin to MTX significantly attenuated lipid peroxidation as evident by significant decreases in the serum MDA level. Also it was found that combination treatment caused a significant decrease in serum SOD activity and a significant increase in the serum GSH level. Similar observations were obtained by others.[18,25] Another previous study[15] has shown that combination treatment with metformin did not exert any significant alteration on oxidation parameters. Reduction in the serum MDA level, SOD activity and increase in the GSH level in the group supplemented with metformin show a potential protective effect of metformin against oxidative damage to the hepatic tissue and improvement in the hepatic antioxidant enzyme system. A reasonable explanation is that metformin significantly decreases radical oxygen species production in cells. This possible antioxidant property of metformin might be explained by its ability to cause inhibition in JNK activation. Moreover, metformin might lead to overexpression of HO-1 that leads to protection against oxidative stress-induced hepatic damage.[10]
Histopathological changes of the group treated with metformin showed significant improvement in architecture. Although necrotic changes were still evident, the severity of the damage less intense significantly. This appears wise to speculate that metformin possess hepatoprotection against MTX-induced hepatotoxicity. The antioxidant properties, antiapoptotic, and anti-inflammatory effect of metformin were probably the contributing factors for this hepatoprotection.[10]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gram TE, Gillette JR. Fundamental of Biochemical Pharmacology. New York: Pergamen Press; 1971. Bio-transformation of drugs; pp. 571–609. [Google Scholar]
- 2.Friis H, Andreasen PB. Drug-induced hepatic injury: An analysis of 1,100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med. 1992;232:133–8. doi: 10.1111/j.1365-2796.1992.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 3.Farrel GC. Drug-induced liver disease. Edinburgh: Churchill Livingstone; 1994. [Google Scholar]
- 4.Uraz S, Tahan V. Role of Uredodeoxycholic Acid in prevention of methotrexate-induced liver toxicity. Dig Dis Sci. 2008;53:1071–7. doi: 10.1007/s10620-007-9949-3. [DOI] [PubMed] [Google Scholar]
- 5.Naldi L, Griffiths CE. Traditional therapies in the management of moderate to severe chronic plaque psoriasis: An assessment of the benefits and risks. Br J Dermatol. 2005;152:597–615. doi: 10.1111/j.1365-2133.2005.06563.x. [DOI] [PubMed] [Google Scholar]
- 6.Wu JJ, Schiff KR. Sarcoidosis. Am Fam Physician. 2004;70:312–22. [PubMed] [Google Scholar]
- 7.Hopwood D, Nyfors A. Effect of methotrexate therapy in psoriatics on the Ito cells in liver biopsy. J Clin Pathol. 2004;29:198–703. doi: 10.1136/jcp.29.8.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi SR. Metformin: Old wine in new bottle-evolving technology and therapy in diabetes. J Assoc Physicians India. 2005;53:963–72. [PubMed] [Google Scholar]
- 9.Bennett PN, Brown MJ. Clinical Pharmacology. 9th ed. Edinburgh: Churchill Livingstone; 2003. [Google Scholar]
- 10.Scheen AJ. Clinical pharmacokinetics of metformin. J Clin Pharm. 1996;30:359–71. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 11.Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, et al. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: Involvement of JNK and ERK MAP kinases. J Hepatol. 2006;44:918–29. doi: 10.1016/j.jhep.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Hadi NR, Swadi AA, Mohammad BI. Hepatoprotective Potential of Metformin against Methotrexate Induced Hepatotoxicity. Asian J Exp Biol Sci. 2012;3:110–7. [Google Scholar]
- 13.Bonnefont-Rousselot D, Raji B, Walrand S, Gardès-Albert M, Jore D, Legrand A, et al. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism. 2003;52:586–9. doi: 10.1053/meta.2003.50093. [DOI] [PubMed] [Google Scholar]
- 14.Pavlović D, Kocić R, Kocić G, Jevtović T, Radenković S, Mikić D, et al. Effect of four week metformin treatment on obese patients with type 2 diabetes. Diabetes Obes Metab. 2000;2:251–6. doi: 10.1046/j.1463-1326.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz M, Bukan N, Ayvaz G, Karakoç A, Törüner F, Cakir N, et al. The effects of rosiglitazone and metformin on oxidative stress and homocysteine levels in lean patients with polycystic ovary syndrome. Hum Reprod. 2005;20:3333–40. doi: 10.1093/humrep/dei258. [DOI] [PubMed] [Google Scholar]
- 16.Marchesini G. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–5. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 17.Novaes GS, Mello SB, Laurindo IM, Cossermelli W. Low dose methotrexate decreases intraarticular prostaglandin and interleukin 1 levels in antigen induced arthritis in rabbits. J Rheumatol. 1996;23:2092–7. [PubMed] [Google Scholar]
- 18.Poon MK, Chiu PY, Mak DH, Ko KM. Metformin protects against carbon tetrachloride hepatotoxicity in mice. J Pharmacol Sci. 2003;93:501–4. doi: 10.1254/jphs.93.501. [DOI] [PubMed] [Google Scholar]
- 19.Kiersztan A, Modzelewska A, Jarzyna R, Jagielska E, Bryła J. Inhibition of gluconeogenesis by vanadium and metformin in kidney-cortex tubules isolated from control and diabetic rabbits. Biochem Pharmacol. 2002;63:1371–82. doi: 10.1016/s0006-2952(02)00861-4. [DOI] [PubMed] [Google Scholar]
- 20.Kremer JM, Alarcón GS, Lightfoot RW, Jr, Willkens RF, Furst DE, Williams HJ, et al. Methotrexate for rheumatoid arthritis: Suggested guidelines for monitoring liver toxicity. Am Coll Rheumatol Arthritis Rheum. 1994;37:316–28. doi: 10.1002/art.1780370304. [DOI] [PubMed] [Google Scholar]
- 21.Walker TM, Rhodes PC, Westmoreland C. The differential cytotoxicity of methotrexate in rat hepatocyte monolayer and spheroid cultures. Toxicol In Vitro. 2000;14:475–85. doi: 10.1016/s0887-2333(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 22.Sallie R, Tredger JM, William R. Drugs and liver. Part, Testing liver function. Biopharm Drug Dis. 1991;12:251–9. doi: 10.1002/bdd.2510120403. [DOI] [PubMed] [Google Scholar]
- 23.Lin HZ, Yang SQ, Chuckaree C. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 24.de Oliveira CP, Stefano JT, de Siqueira ER, Silva LS, de Campos Mazo DF, Lima VM, et al. Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non alcoholic steatohepatitis. Hepatol Res. 2007;38:159–65. doi: 10.1111/j.1872-034X.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 25.Tandon VR. Metformin therapy: Benefits beyond glycemic control. Int J Diab Ctries. 2008;27:87–95. [Google Scholar]


