Abstract
Objective:
To determine the effectiveness of dexamethasone on post tonsillectomy morbidities in patients with chronic tonsillitis.
Materials and Methods:
In this randomized double-blind study, 100 patients who underwent tonsillectomy were enrolled and were randomly allocated into control or dexamethasone group (pre operative, intra operative and post operative groups). Patients were assessed for pain nausea, vomiting and oral intake in the post operative period at 24 h.
Results:
Patients treated with dexamethasone particularly in the pre and intra operative groups (Group B, Group C) showed a general trend towards lower pain score than post operative group (Group D). The scores were about 1.72±0.84 and 2.20±1.19 in Groups B and C respectively, and 2.64±0.99 in Group D. Overall pain score was found to be more in the control Group A about 4.84±1.21 at 6 h post operatively and showed similar trend for next 24 h. Total number of patients with nausea was significantly high about 84% in control group compared to dexamethasone groups (Group B, C and D) about 20%, 8% and 24% respectively and also incidence of vomiting episodes showed a similar trend. Oral intake was significantly delayed in control group (6.16 ±1.52), P < 0.001 than dexamethasone group. Pre operative and intra operative groups showed early intake (3.68±0.68) and (3.60±1.12) respectively than the postoperative group (5.08±0.95).
Conclusions:
A single intravenous dose of dexamethasone, given following induction of anaesthesia and at the time of surgery, provided prolonged analgesia, reduced nausea and vomiting and resulted in earlier oral intake.
Keywords: Dexamethasone, tonsillectomy, pain, nausea
INTRODUCTION
Tissue injury induces acute inflammation, nerve irritation and spasm of exposed pharyngeal muscle and is known to cause post tonsillectomy pain. Oropharyngeal pain and irritation of gastric mucosa by swallowed blood are main contributors for nausea and vomiting.
Steroids inhibit production of inflammatory cell factors such as cytokines in macrophages, monocytes and lymphocytes which decrease extravasation of leucocytes, release of lysosomal enzymes and vascular permeability in areas of injury leading to reduction in edema and fibrosis during healing.
Steroids also inhibit phospholipase enzyme which blocks the cyclooxygenase and lipooxygenase pathways, and reduce prostaglandin production relieving pain.
MATERIALS AND METHODS
In this prospective double-blind study, 100 patients aged above 15 years undergoing tonsillectomies were enrolled. Patients with coagulopathy disorders, diabetes, gastritis, peptic ulcer, hypertension and cardiovascular and renal diseases or on therapy with corticosteroids, anti-emetics, anti-histaminics, aspirin undergoing additional procedure at the same time as tonsillectomy (eg:-Adenoidectomy, uvulo palatopharyngoplasty) were excluded.
Patients were randomly allocated according to a computer generated random table into control or dexamethasone group. Allotment of the subjects in clinical study is summarized in Figure 1.
Figure 1.
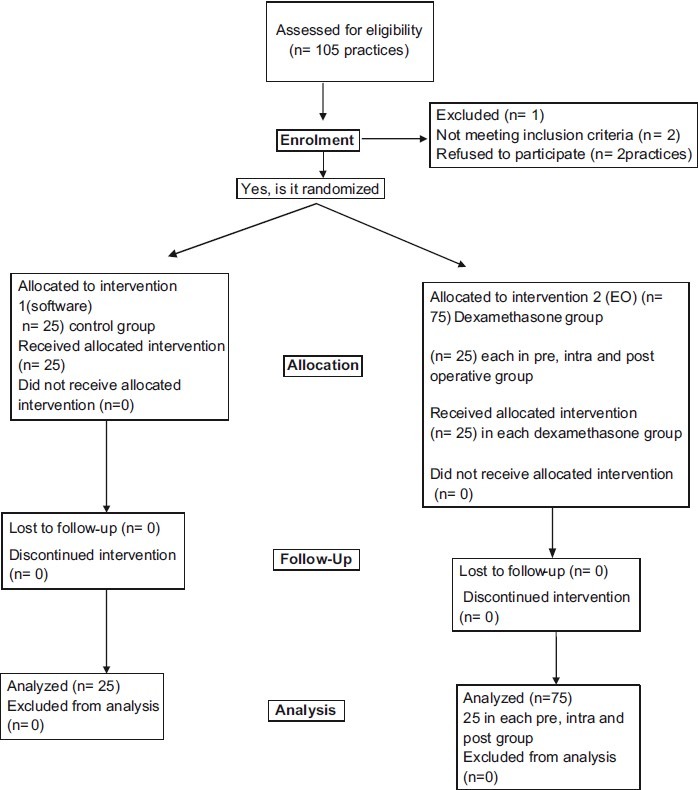
Recruitment, allocation and follow-up of participants
Out of 100 patients, 25 patients received dexamethasone preoperatively (0.5 mg/kg), immediately after IV access was established by the anesthesiologist (pre operative). And 25 patients received dexamethasone after induction and before commencement of the surgery, about 0.5 mg/kg through I V (intra operative), while another set of 25 patients received equal volume of saline as control group. Sharp dissection snare technique was used for tonsillectomy. Bleeders were ligated using ties. Hemostasis was achieved using packs or sutures. Electrocautery was not used.
Patients were monitored in the postoperative care for first 6 h and in the ward for next 6-24 h. Out of them, 25 patients received single intra venous dexamethasone dosage of 0.5 mg/kg in the postoperative care unit, just after shifting the patient to post operative recovery unit (post operative group). The same surgeon using dissection and snare technique performed tonsillectomy in all patients with same standard anesthetic technique.
Pain was assessed using visual analogue scale (VAS) from 0 – 6, every half hourly for first 2 h and second hourly for next 4 h and then at 6, 12, and 24 h. Recordings were made at 30 min, 6, 12 and 24 h. If VAS was more than 6 tramadol 1 mg/ kg was administered.
Nausea (a subjective unpleasant sensation associated with awareness of the urge to vomit) and vomiting (forceful expulsion of liquid gastric content), if occurred, were recorded. Number of episodes of vomiting was also noted. Inj. Ondansetron 0.1 mg/kg was administered, if more than two episodes of vomiting in an hour or nausea lasting for more than half an hour occurred. Temperature was recorded at 2, 4, 6, 12, and 24 h. Temperature > 37.6°C was considered as fever. Hemorrhage, if thereany, was noted.
At 4 h after the surgery, patients were asked to take oral liquids. The quality of oral intake was graded as follows:
Excellent = patient requests it;
Good = patient accepts it when offered;
Fair = patient accepts it when coaxed;
Poor = patient refuses.
The time of first acceptance of oral liquid diet was recorded.
If the oral intake was delayed, the time duration between the end of surgery and first acceptance of oral liquid was recorded. Till that time 3 ml/kg-1h-1 of lactated ringer's solution with dextrose was infused. At 24 h, patients were discharged. Patients or parents were asked to note, the time of first acceptance of solid food, fever, bleeding or other side effects if occurred.
This information was collected, when they returned on seventh day for follow up.
For analysis, patients of each group were divided into three pain groups for first 30 min, 6, 12, 24 h.
Significant pain = > 4;
Mild pain = > 2 and < 4;
Pain free = < 2.
Statistical methods
Kruskal Wallis test was used to analyse the pain scores and ANOVA was used to analyse the average time taken for liquid and solid consumption. P-values < 0.05 were considered statistically significant.
RESULTS
There were no statistically significant differences seen between the groups for age, sex and tonsillar size.
Although pain score was high at 30 min post operatively in all the groups (around 5), there was a gradual decline in the pain score in dexamethasone group (P <0.001) than control group in next 24 h post operatively. [Table 1]
Table 1.
Pain scores in four groups
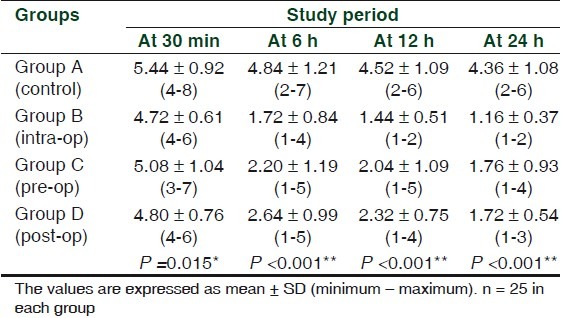
Patients treated with dexamethasone particularly the pre and intra operative (Group B, Group C) showed a general trend toward lower pain score than post operative (Group D) about 1.72 ± 0.84 and 2.20 ± 1.19 in Group B and C and 2.64 ± 0.99 in Group D. Overall pain score was found to be more in the control Group A about 4.84 ± 1.21 at 6 h post operatively and showed similar trend for next 24 h.
However, pre and intra operative groups (Group B, C) showed a lower pain scores than post operative group (Group D) throughout 24 h postoperatively. There was no significant difference noted between pre and intra operative groups.
None of the patients in the dexamethasone group required postoperative analgesic supplementation compared to four patients in the control group for whom analgesic tramadol was given.
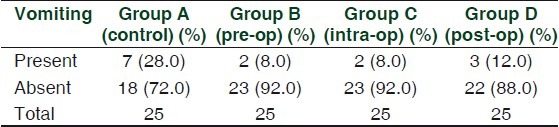
Nausea in Groups B, C and D was compared with that in Group A. Group B and Group C were significantly different when compared to control (P > 0.05). [Table 2] Vomiting in Groups B, C and D was not significantly different when compared to Group A. [Table 3]
Table 2.
Presentation of nausea

Table 3.
Presentation of vomiting
Oral intake was significantly delayed in control group (6.16 ± 1.52) P < 0.001 than dexamethasone group. Pre operative and intra operative group showed early intake (3.68 ± 0.68) and (3.60 ± 1.12) respectively than the postoperative group (5.08 ± 0.95). Time of first solid intake in control group was 2.92 ± 0.91 days and in dexamethasone group that is pre operative (Group B) was 2.64 ± 0.76 and intra operative (Group C) was 2.68 ± 0.80, whereas, post operative (Group D) was post operative (Group D) was 2.76 ± 0.59. There were no statistical differences seen between pre, intra and postoperative group. None of the patients had hemorrhage in dexamethasone group (Group B, C and D), whereas one patient had bleeding on third postoperative day in the control group (Group A). In either of these groups, there was no fever or any other adverse reactions.
DISCUSSION
Dexamethasone is one of the potent glucocorticoids available, being 25 times more potent than endogenous cortisol. It has a 36 to 72 h of biological half-life. About 10 mg of cortisol is secreted by an adult daily, which is equivalent to 0.4 mg of dexamethasone, thus the dose chosen for this study (0.5 mg/ kg, max 20 mg) is very supraphysiological. Dogma states that 1.00 to 1.5 mg/kg can be used intravenously, about 70 to 105 mg in an adult. This is probably excessive and it invites adverse drug reactions. In 1970 Papangelou[1] treated 323 patients with four days course of 0.5 mg betamethasone tablets given every 6 h in the first day, every 8 h in the second day, every 12 h in the third day and once in the fourth day. The steroid treated group had better oral intake than the control group. In our study, mean days of starting solid food were not much different in dexamethasone group compared to control group, perhaps because of the single IV dose used in our study. Administration of oral dexamethasone next day onwards might have resulted in earlier solid intake. However, randomized controlled studies are needed to find out the safety and efficacy of repeated doses.[2]
Henzi I, Walder B, Tramer MR did meta-analysis of 17 trials involving use of dexamethasone for prevention of postoperative nausea and vomiting in surgical patients. The number needed to treat to prevent early and late vomiting compared with placebo in adults and children was 7.1 (95%CL 4.5 to 18) and 3.8 (2.9 to 5) respectively, They concluded that when there is a high risk of postoperative nausea and vomiting, a single prophylactic dose of dexamethasone is anti-emetic compared with placebo, without evidence of any clinically prevalent toxicity in otherwise healthy patients. Late efficacy seems to be most pronounced.[3] Steward DL, Welge JA, Myer CM did a meta-analysis of randomized double blind placebo controlled trials of a single dose of intravenous intraoperatively steroid for pediatric patients who underwent tonsillectomy or adenotonsillectomy. Eight trials met their inclusion criteria. They concluded that routine use of steroids would result in earlier soft or solid diet intake. But, because of the missing data and varied outcome measures, pain could not be meaningfully analyzed as a distinct end point.[4]
Vosdogonis and Baines described 41 children who received 0.4 mg/kg of intravenous dexamethasone or placebo, concentrating their observations on the first 24 h postoperatively. Time taken for first liquid and solid food intake, vomiting, and pain were assessed. Postoperative vomiting was significantly decreased in the dexamethasone group (45 vs 63%; P =. 02), as was the need for administration for rescue anti emetic and intravenous fluid supplementation. Time taken to first liquid intake was similar between the groups, but first solid intake was earlier in the study group (6 vs 10 h; P <0.1). There was no difference in postoperative pain assessment or analgesic requirement.[5]
Splinter and Roberts observed 133 children who received 0.15 mg/kg of dexamethasone or placebo intravenously before outpatient tonsillectomy. Postoperative vomiting both in the hospital and at home for 24 h was recorded. The dexamethasone group had less vomiting in the immediate postoperative period as well as during the first 24 h at home (overall, 40 vs 71%, respectively; P < 0.5).[6]
Pappas ALS, Sukhani R, Hotaling AJ examined postoperative vomiting in 130 children who received 1 mg/kg of dexamethasone or placebo during outpatient tonsillectomy. Vomiting was recorded in the hospital and for the first 24 h at home. Duration of recovery-room stay was longer in the placebo group. The incidence of vomiting, need for rescue anti emetic treatment, and analgesic requirements were not different before hospital discharge, but during the 24-h period after that, fewer patients in the steroid group experienced vomiting (48 vs 88%; P < 0.5).[7]
April MM, Callan ND, Nowak DM, Hausdorff MA, studied 80 children who received 1 mg/kg of dexamethasone or placebo prior to adenotonsillectomy and measured postoperative oral intake, pain, vomiting, fever, and complications during the first 24 h. The dexamethasone group had significantly less trismus, fever, and vomiting in the first 6 h postoperatively, and took more oral food (including early acceptance of solid food) than the control group.[8]
Tom LWC, Templeton JJ, Thompson ME, Marsh RR, observed 58 children who received 1 mg/kg of dexamethasone or placebo preoperatively. Parents recorded diet, medication, activity level, pain, nausea, and vomiting everyday for 10 days. Patients who received steroids had less vomiting and less severe throat pain on the first postoperative day, and returned to soft and solid diet more quickly than the control group (P <0.01). No difference was found in analgesic use, delayed pain and/or return to normal activity.[9]
In our study there was a significant decrease in the pain in dexamethasone group than the control group P <0.001. However, in dexamethasone group, pre and intraoperative group showed better response than the postoperative group (1.72 ± 0.84 and 2.20 ± 1.19) than 2.6 4± 0.99 in group D respectively at 6 h of postoperative period, whereas at 24 h, all the dexamethasone groups (B, C and D) were found to be around 1.5±095. Pre and intra operative dexamethasone was found to be better than the postoperative group in reducing pain in the early post operative period. Overall incidence of post operative nausea and vomiting in our study was less compared to previous studies, perhaps due to avoidance of potent opioids and use of electrocautery. More severe pain and hence PONV (Post Operative Nausea and Vomiting) are known to occur with electrocautery. Previous studies using 8-10 mg of dexamethasone for other surgeries have shown decrease in incidence of PONV by 50%. Pappas et al. showed decrease in incidence of PONV from 62 to 40% Using 1 mg/kg dexamethasone for adenotonsillectomy. With 0.15-mg/kg dexamethasone, reduction in incidence of PONV from 72 to 40% was noted by Splinter et al. In our study dose of 0.5 mg/kg showed such benefit in low incidence of PONV. We found significantly better quality of oral intake with dexamethasone. Perhaps, by decreasing pain and inflammation, it improved the oral intake. In a meta-analysis Steward et al. showed that children receiving dexamethasone were more likely to advance to a soft or solid diet on post-tonsillectomy day 1 (RR= 1.69; 95% CI, 1.02-2.79; P =0.04). Catlin and Grimes studied the effect of single post induction dose of 8 mg/kg on recovery from tonsillectomy in children. On day 3, half of the steroid treated group had good appetite in contrast to 12% of the control group patients and 60% of dexamethasone treated group who could take full diet compared to 13% of the control group.
Administration of a single dose of steroid medication, even a large one, is regarded as very safe and is virtually without harmful effects.[8] No adverse effects of steroid administration were observed in any of the studies.
CONCLUSION
We conclude that a single intravenous dose of 0.5 mg/kg dexamethasone at a maximum of 20 mg, given following induction of anesthesia or at the time of surgery, provided good and prolonged analgesia, reduced nausea and vomiting and resulted in earlier and better quality of oral intake without side effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Papangelou L. Steroid therapy in tonsillectomy. Laryngoscope. 1972;82:297–302. doi: 10.1288/00005537-197202000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Shott SR. Tonsillectomy and post operative vomiting: Do steroids really work? Arch Otolaryngol Head Neck Surg. 2001;127:1009–10. doi: 10.1001/archotol.127.8.1009. [DOI] [PubMed] [Google Scholar]
- 3.Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: A quantitative systematic review. Anesth Analg. 2000;90:186–94. doi: 10.1097/00000539-200001000-00038. [DOI] [PubMed] [Google Scholar]
- 4.Steward DL, Welge JA, Myer CM. Do steroids reduce morbidity of tonsillectomy? Meta-analysis of randomized trials. Laryngoscope. 2001;111:1712–8. doi: 10.1097/00005537-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Vosdogonis F, Baines DB. The effect of single-dose intravenous dexamethasone in tonsillectomy in children. Anaesth Intensive Care. 1999;27:489–92. doi: 10.1177/0310057X9902700509. [DOI] [PubMed] [Google Scholar]
- 6.Splinter AM, Roberts DJ. Dexamethasone decreases vomiting by children after tonsillectomy. Anaesth Analg. 1996;83:913–6. doi: 10.1097/00000539-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Pappas AL, Sukhani R, Hotaling AJ, Mikat-Stevens M, Javorski JJ, Donzelli J, et al. The effect of preoperative dexamethasone on the immediate and delayed postoperative morbidity in children undergoing adenotonsillectomy. Anaesth Analg. 1998;87:57–61. doi: 10.1097/00000539-199807000-00013. [DOI] [PubMed] [Google Scholar]
- 8.April MM, Callan ND, Nowak DM, Hausdorff MA. The effect of intravenous dexamethasone in pediatric adenotonsillectomy. Arch Otolaryngol Head Neck Surg. 1996;112:117–20. doi: 10.1001/archotol.1996.01890140007003. [DOI] [PubMed] [Google Scholar]
- 9.Tom LW, Templeton JJ, Thompson ME, Marsh RR. Dexamethasone in adenotonsillectomy. Int J Ped Otorhinolaryngol. 1996;37:115–20. doi: 10.1016/0165-5876(96)01388-2. [DOI] [PubMed] [Google Scholar]


