Sir,
Extrapyramidal syndromes (EPS) are a group of neurological syndromes, resembling those seen in idiopathic Parkinson's disease, which occur following antipsychotic medication. With the introduction of second generation antipsychotics (SGAs), incidence of EPS has declined compared to the first generation agents, but not eliminated. EPS adversely impact antipsychotic efficacy and tolerability and reduce compliance. Hence, we wanted to study EPS with SGAs for detecting, assessing, understanding, and taking measures (if possible) to prevent EPS.
We studied the prevalence and patterns of EPS with SGAs and assessed for their causality, severity, and preventability by a one year retrospective analysis of outpatient case records of the psychiatric unit of our hospital. We included patients with atypical antipsychotic prescriptions. Documented adverse events were subjected to analysis of causality (Naranjo's scale), severity (modified Hartwig and Siegel scale), and preventability (modified Schumock and Thornton's Preventability scale).
Out of 222 (both old and new) patients receiving psychotropic medicines, 73 received one or more antipsychotic prescriptions. Among these 73 patients, SGAs (olanzapine, risperidone, aripiprazole, quetiapine, and ziprasidone) were prescribed to 69 patients (94.5% of patients receiving antipsychotics). Out of the 69 patients, there were 13 reports of EPS in 12 patients (18.9%). Seven were diagnosed with schizophrenia, three with severe depressive episode with psychotic symptoms, one with mixed anxiety and depressive disorder with delusional disorder and mental retardation and one with psychosis (unspecified). EPS constituted 32.5% of all adverse drug reactions (ADRs) seen with SGAs (13/40 different ADRs). In all the patients, trihexiphenidyl in a dose of 2–4 mg/day was used for relieving the EPS.
Type of EPS, causality assessment, and dose and duration of drug treatment at which EPS occurred are summarized in Tables 1 and 2. There were no co-morbid conditions or concomitant medication use in the patients, but two were on substance use (nicotine). The prevalence of EPS is higher compared to the western literature,[1] but this should be correlated to utilization of SGAs. Olanzapine was the most common antipsychotic (65/99 antipsychotic prescriptions or 65.7%) and the most common SGA associated with EPS (9/12 EPS or 72.7%), including one with concomitant risperidone.
Table 1.
Types of EPS and second generation antipsychotic (SGA) drugs
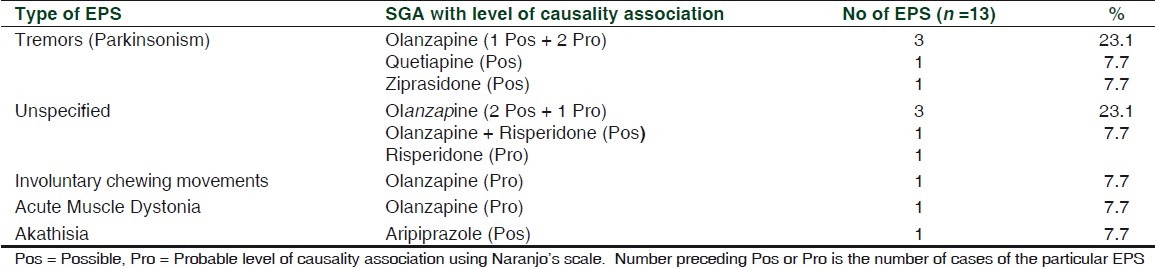
Table 2.
Dose of drug at which EPS observed and duration of treatment before developing EPS
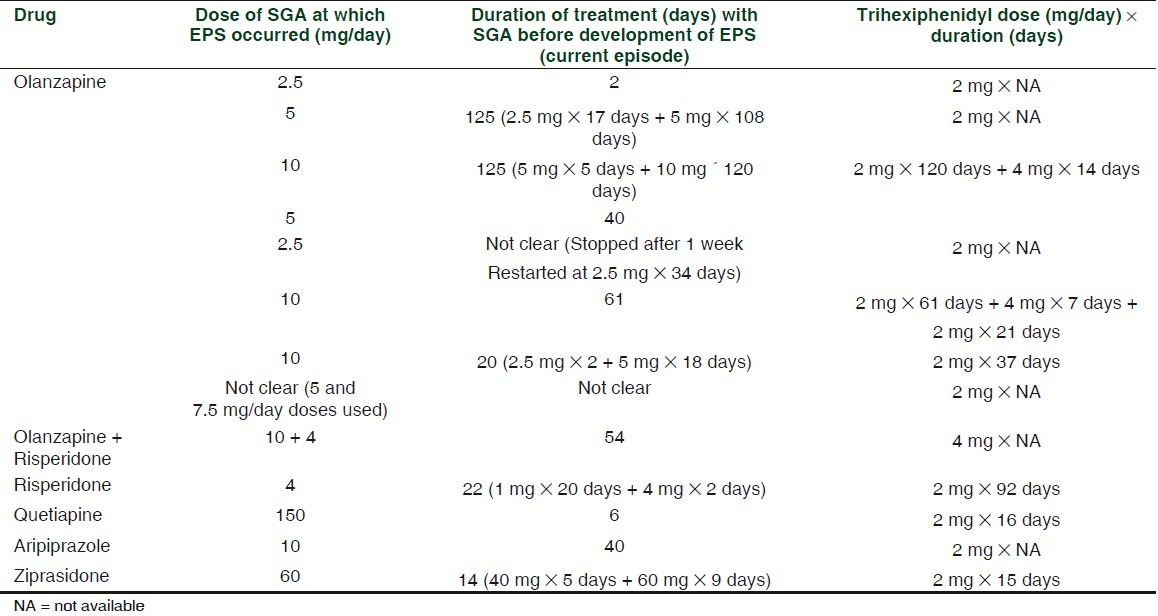
Tremors (parkinsonism) were the most common type of EPS encountered (41.7%), which follows the trend seen in other studies.[2] Olanzapine (2.5 mg × 2 days) probably caused acute muscle dystonia in a 32-year-old female, with mixed anxiety and depressive disorder with delusional disorder and mental retardation. This dose of olanzapine has been associated with acute muscle dystonia in a postencephalitis patient.[3] Co-administration of selective serotonin reuptake inhibitors (SSRIs), sertraline or escitalopram, caused tremors in this patient, as SSRI-use concurrently with antipsychotics increases risk of EPS.[4] Duloxetine, the serotonin norepinehrine reuptake inhibitor (SNRI), also causes EPS,[5] so concurrent administration may be additive in four patients. Involuntary chewing movements (tardive dyskinesia) were seen in a 47-year-old male with severe depressive episode with psychotic symptoms on olanzapine (duration not specified). Among SGAs, only risperidone has so far been clearly linked to tardive dyskinesia, but this may be due only to limitations in data collection.
Atypical antipsychotic polytherapy (olanzapine 10 mg and risperidone 4 mg) which was seen in a 42-year-old male patient diagnosed with schizophrenia, increases risk of EPS, because of higher cumulative doses. Risperidone alone in a dose of less than 6mg has limited adverse neurological effects,[6] even though we observed one patient developing EPS at 4 mg/day.
In our study, olanzapine was associated with most EPS, but out of 65 olanzapine prescriptions, nine cases of EPS were observed (13.8%). Risperidone was prescribed 19 times with one case of EPS (5.3%), aripiprazole seven times with one EPS (14.3%), quetiapine three times with one EPS (33.3%), and ziprasidone, once with one EPS (100%). A recent meta-analysis[6] reported that descending order in which EPS occurs with the use of SGAs is risperidone ≥ aripiprazole > ziprasidone > olanzapine > quetiapine.
Tremors were most commonly seen with olanzapine. Akathisia was attributed to aripiprazole, consistent with other studies.[6]
All ADRs were of Moderate Level 3 severity. The ADRs were not preventable in six cases, but in the remaining six, with concurrent administration of two antipsychotics (olanzapine and risperidone) in one patient, olanzapine with escitalopram/sertraline in another and olanzapine with duloxetine in four patients, EPS due to drug interaction was probably preventable.
Strengths of the study were detection of prevalence and type of EPS with SGA drugs, for which Indian data were scarce. Moreover, because SGAs were prescribed to 94.5% of the patients receiving antipsychotics, it helped in accurately calculating prevalence of EPS among SGAs.
Limitations of the study were small sample of prescriptions, low number of prescriptions of drugs other than olanzapine, absence of stronger causality association for EPS, missing/unclear data in recording type, and dose and duration of treatment of EPS.
In conclusion, prevalence of EPS in our psychiatric outpatient setting was high. Most common type of EPS seen with SGAs was tremor (parkinsonism). Most common SGA associated with EPS was olanzapine. Risk of EPS increased with concurrent administration of SSRIs and SNRIs. All cases of EPS were of Moderate Level 3 severity. The ADRs were not preventable in seven cases of monotherapy, but were probably preventable in the remaining six when prescribed with another SGA, SSRI, or SNRI. This study highlights the need for raising awareness among psychiatrists about the risk of development of EPS with the use of SGAs in Indian population, especially with polytherapy. It also throws light on the frequency, pattern, severity, and preventability of EPS in Indian population and the dose and duration of treatment that induced EPS, which will help psychiatrists in optimizing treatment regimens with SGAs to avoid EPS; this will improve patients’ compliance.
REFERENCES
- 1.Stübner S, Rustenbeck E, Grohmann R, Wagner G, Engel R, Neundörfer G, et al. Severe and uncommon involuntary movement disorders due to psychotropic drugs. Pharmacopsychiatry. 2004;37(Suppl 1):S54–64. doi: 10.1055/s-2004-815511. [DOI] [PubMed] [Google Scholar]
- 2.Ghaemi NS, Hsu DJ, Rosenquist KJ, Pardo TB, Goodwin FK. Extrapyramidal side effects with atypical neuroleptics in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:209–13. doi: 10.1016/j.pnpbp.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Mendhekar DN. Recurrent oculogyric crisis and retrocollis after re-exposure with a low dose of olanzapine. Indian J Pharmacol. 2005;37:337. [Google Scholar]
- 4.Schillevoort I, van Puijenbroek EP, de Boer A, Roos RA, Jansen PA, Leufkens HG. Extrapyramidal syndromes associated with selective serotonin reuptake inhibitors: A case-control study using spontaneous reports. Int Clin Psychopharmacol. 2002;17:75–9. doi: 10.1097/00004850-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Madhusoodanan S, Alexeenko L, Sanders R, Brenner R. Extrapyramidal symptoms associated with antidepressants–a review of the literature and an analysis of spontaneous reports. Ann Clin Psychiatry. 2010;22:148–56. [PubMed] [Google Scholar]
- 6.Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Kissling W, et al. Second-Generation Antipsychotic Drugs and Extrapyramidal Side Effects: A Systematic Review and Meta-analysis of Head-to-Head Comparisons. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq042. doi: 10.1093/schbul/sbq042. [DOI] [PMC free article] [PubMed] [Google Scholar]


