Abstract
Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN) are rare but serious dermatologic disorders. These grave conditions present as medical emergency, requiring prompt diagnosis and management. These are often drug induced and various groups of drugs, such as sulfa drugs, NSAIDS, etc., have been implicated as to cause TEN. Fluconazole is a commonly used drug with mild side effects. TEN caused by fluconazole is rare, and till now only few cases have been reported in the literature. We present a case of TEN in a human immunodeficiency virus infected man following fluconazole therapy in view of its rare occurrence.
Keywords: Fluconazole, HIV infection, toxic epidermal necrolysis
INTRODUCTION
Toxic epidermal necrolysis (TEN) is a severe and life-threatening condition involving skin and mucous membrane. Drugs are the most important cause of TEN. The drugs most commonly causing TEN are sulfonamides, penicillins, other antibiotics, non-steroidal anti-inflammatory drugs, anti convulsants, etc. These drugs reaction are more common in individuals having human immunodeficiency virus (HIV) infection/AIDS than the general population.[1]
Fluconazole, an antifungal drug of the azole group, is frequently used for management of candidial infections, coccidiodal meningitis, cryptococcal meningitis, and in empiric treatment of critically ill HIV-infected patients. Surveillance studies show a low incidence of adverse drug reactions to fluconazole.[2] The usual side effects of fluconazole mentioned in the literature are mild like nausea, vomiting, headache, elevation of hepatic transaminases, etc.[3] TEN/Stevens-Johnson Syndrome (SJS) caused by fluconazole in immunesuppressed patients is very rare and reported in only three reports previously.[4–6] We present an additional case of this type in view of lack of such reports in the Indian literature.
CASE REPORT
A 28 year old male patient, taking anti-tuberculosis treatment for last 3 months for pulmonary tuberculosis, now presented with oral thrush. He was a truck driver and was addicted to tobacco and alcohol. He was not married but gave history of multiple unprotected roadside sexes. On examination, the body mass index was 18. There was pallor and extensive oral thrush over tongue extending up to tonsil, and posterior pharyngeal wall. Other general physical examination was unremarkable. Respiratory system examination revealed crepitations over right upper lung fields. Other systemic examination revealed no abnormality.
In view of high risk behavior and the presence of oral thrush, his sera were subjected to HIV1 and HIV2 testing that turned out to be reactive. His other investigations revealed hemoglobin 8 g% with normal leukocyte count, differential count, fasting blood sugar, liver function test, renal function test, etc. A skiagram chest showed regression of pulmonary infiltrates in comparison to previous chest X-ray. His CD4 count was 625 per mm3. Throat swab smear and culture confirmed the presence of candidial infection.
The antituberculosis treatment was continued and oral fluconazole 150 mg daily was started with povidone iodine gargles. On the second day of fluconazole therapy, the patient developed generalized body ache along with pruritic rash over face, trunk, and extremities. The rashes first appeared over the trunk and then spread to involve extremities and face. Next day, these maculopapular eruptions became necrotic and surrounded by erythema. Later, vesiculobullous lesions appeared over face and trunk along with conjunctivitis. Nikolsky's sign was positive. The lesions subsequently ruptured with sloughing of large sheets of skin, leaving behind erythematous areas involving more than 70% of the body surface area. [Figure 1]. The patient also became febrile. Skin biopsy could not be taken. Based on clinical course following fluconazole therapy, a diagnosis of fluconazole-induced TEN (Naranjo Score 6) was made. Laboratory investigations this time revealed normal total leukocyte counts, but slight elevation of liver enzyme (ALT—50 IU/L, AST—64 IU/L). Fluconazole was stopped immediately. The patient was managed conservatively with local soothing agents, analgesics, azithromycin, antihistaminics, and eye care with adequate parenteral hydration. The patient improved with above therapy. Skin lesions resolved gradually and disappeared completely after 3 weeks.
Figure 1.
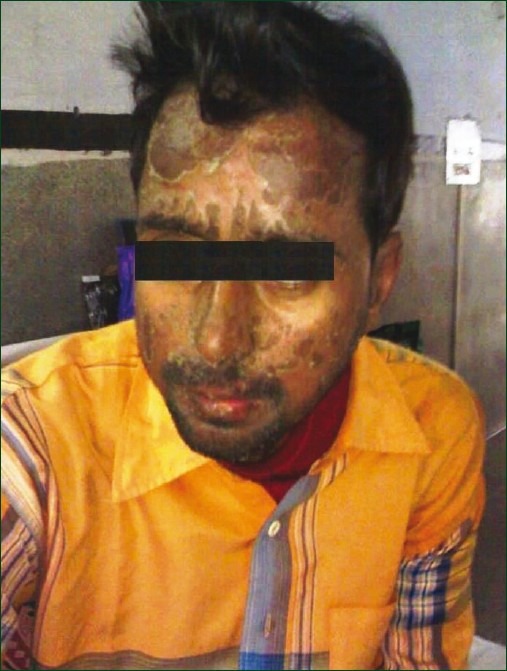
Photograph of a patient showing erythematous lesions over face with sloughing of skin.
DISCUSSION
TEN and SJS are acute and life-threatening disorder of unclear pathophysiology, characterized by epidermal necrosis, erosions of mucous membrane, and detachment of epidermis with constitutional symptoms.[7] Cases having less than 10% of epidermis involvement are designated as SJS, while those having 30% epidermis involvement are labeled as TEN. Cases with between 10% and 30% involved areas are defined as overlap SJS–TEN.[8]
The estimated incidence of TEN is about 1.17–1.89 cases per million inhabitants per year in observational studies.[9] The risk of TEN is much higher in individuals having HIV infection and other autoimmune disorders.[10–12] TEN is almost always induced by drugs. Although a long list of drugs have been implicated as a cause for TEN, the drugs commonly associated with TEN are sulfonamides, anticonvulsants, allopurinol, non-steroidal anti-inflammatory drugs, etc.[1,7] Another drug commonly causing SJS/TEN in HIV patients is thioacetazone sodium. This drug has thence been contraindicated in HIV patients.[13]
TEN/SJS induced by fluconazole is very rare and till date only three cases have been reported. The very first case involved a 30-year-old gay, HIV positive man developing SJS following fluconazole therapy for oral candidiasis.[4] The second case involved a 33-year-old HIV positive male who developed TEN following treatment with fluconazole for dysphagia and recurrent oral thrush.[5] The third one was a 52-year-old immunosuppressive woman developing TEN after fluconazole for the treatment of esophageal candidasis.[6] Our case also developed TEN following fluconazole therapy for severe oral candidiasis.
The exact mechanism for drug-induced TEN is unclear but immunological mechanisms, reactive drug metabolites and interactions between the two have been proposed.[14] TEN is also linked to genetic differences in patient's metabolite reactions to drugs.[1] Multiple prior cutaneous drug reaction or prior reaction to a specific drug is also found to be a risk factor for TEN in HIV infected individuals.[10] The higher incidence of TEN among HIV-infected patients may also be related to glutathione deficiency.[15] Further, patients with AIDS also have increased number of abnormal polyclonaly activated ‘B’ cells which are defectively activated to secrete immunoglobulins leading to circulatory immune complexes and autoimmune phenomenon.[16] All these mechanism makes HIV-infected individuals more vulnerable to TEN due to drugs. Therefore in all patients with drug-induced TEN, an underlying HIV infection should always be ruled out. To our knowledge this is the fourth reported case and probably be the first one in India of TEN secondary to fluconazole in a patient having HIV infection.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Backer DS. Toxic epidermal necrolysis. Lancet. 1998;351:1417–20. doi: 10.1016/S0140-6736(97)11369-1. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury BD, Jick SS. Itraconazole and fluconazole and certain rare, serious adverse events. Pharmacotherapy. 2002;22:697–700. doi: 10.1592/phco.22.9.697.34072. [DOI] [PubMed] [Google Scholar]
- 3.Bennett JE. Antimicrobial Agents – Antifungal agents. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. New York: Mc Graw – Hill; 2006. pp. 1225–41. [Google Scholar]
- 4.Gussenhoven MJ, Haak A, Peereboom-Wynia JD, van’t Wout JW. Stevens-Johnson syndrome after fluconazole. Lancet. 1991;338:120. doi: 10.1016/0140-6736(91)90112-3. [DOI] [PubMed] [Google Scholar]
- 5.Azon-Masoliver A, Vilaplana J. Fluconazole-induced toxic epidermal necrolysis in a patient with human immunodeficiency virus infection. Dermatology. 1993;187:268–9. doi: 10.1159/000247261. [DOI] [PubMed] [Google Scholar]
- 6.Ofoma UR, Chapnic EK. Fluconazole induced toxic epidermal necrolysis: A case report. Cases J. 2009;2:9071. doi: 10.1186/1757-1626-2-9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roujeau JC, Stern RS. Severe cutaneous adverse reactions to drugs. N Engl J Med. 1994;331:1272–82. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]
- 8.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–6. [PubMed] [Google Scholar]
- 9.Rzany B, Mockenhaupt M, Baur S, Schröder W, Stocker U, Mueller J, et al. Epidemiology of erythema: Exsudativum multiformr majus, Stevens Johnson Syndrome and toxic epidermal necrolysis in Germany (1990-92). Structure and results of a population based registry. J Clin Epidemiol. 1996;49:769–73. doi: 10.1016/0895-4356(96)00035-2. [DOI] [PubMed] [Google Scholar]
- 10.Proteous DM, Berger TG. Severe cutaneous drug reactions (stevon Jhonson Syndrome and toxic epidermal necrolysis) in human immunodeficiency virus infection. Arch Dermatol. 1991;127:740–1. [PubMed] [Google Scholar]
- 11.Burge SM, Dawber RP. Stevens-Johnson syndrome and toxic epidermal necrolysis in a patient with systemic lupus erythematosus. J Am Acad Dermatol. 1985;13:665–6. doi: 10.1016/s0190-9622(85)80447-3. [DOI] [PubMed] [Google Scholar]
- 12.Dixit R, Sharma S. Toxic epidermal necrolysis caused by cotrimoxazole in a patient with human immunodeficiency virus infection. Indian J Allergy Asthma Immunol. 2008;22:19–21. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Treating opportunistic infections among HIV-exposed and infected children: Recommendations from CDC, the National Institutes of Health, and the Infectious Diseases Society of America. MMWR. 2004;53(RR-14):65. [PubMed] [Google Scholar]
- 14.Roujeau JC, Chosidow O, Saiag P, Guillaume JC. Toxic epidermal necrolysis (Lyell syndrome) J Am Acad Dermatol. 1990;23:1039–58. doi: 10.1016/0190-9622(90)70333-d. [DOI] [PubMed] [Google Scholar]
- 15.Paquet P, Pierard GE, Quatresooz P. Novel treatments for drug-induced toxic epidermal necrolysis (Lyell's Syndrome) Int Arch Allergy Immunol. 2005;136:205–16. doi: 10.1159/000083947. [DOI] [PubMed] [Google Scholar]
- 16.Bowen DL, Lane HC, Fauci AS. Immunopathogenesis of the acquired immunodeficiency syndrome. Ann Intern Med. 1985;103:704–9. doi: 10.7326/0003-4819-103-5-704. [DOI] [PubMed] [Google Scholar]


