INTRODUCTION
Intravenous anesthetic agents are mainly used for induction and maintenance of general anesthesia. Drugs such as thiopental, methohexital, etomidate, ketamine, and propofol are mainly used for induction of anesthesia as they have quick onset of action.[1] Recovery is also sufficiently rapid with most intravenous (IV) drugs to permit their use for short ambulatory surgical procedures.[1] Since more preference nowadays is being given to outpatient surgery, the trend is shifting from general anesthesia to monitored anesthesia care (MAC).[2] Many diagnostic and minor therapeutic surgical procedures can be performed without general anesthesia using sedation-based techniques. A wide variety of IV anesthetic agents have proved to be useful in these techniques such as diazepam, midazolam, and propofol.[1] Fospropofol disodium is the recently approved agent for MAC by FDA in December 2008.[3] It is a water-soluble prodrug of propofol.[4] According to the American Society of Anesthesiologists (ASA), MAC is a planned procedure during which the patient undergoes local anesthesia together with sedation and analgesia.[5] It allows for the safe administration of a maximal depth of sedation in an excess of that provided during moderate sedation.[6] Moderate sedation (also known as conscious sedation) as defined by the ASA requires that the patient should be arousable to verbal commands or light tactile stimulation. Patent airways, as well as stable cardiac and respiratory functions are maintained throughout the period of sedation.[7] For the purpose of the emergency department practitioner, the term procedural sedation is used in the place of moderate sedation.[8] Sedation is actually a continuum. Moderate sedation may lead to deep sedation, which, in turn, may lead to general anesthesia, which may further progress to cardiorespiratory compromise and loss of airway protective reflexes.[8]
Fospropofol (FP), also known as GPI15715 or Aquavan, is a new molecular entity with sedative-hypnotic properties, to be administered intravenously and proposed for the indication of sedation in adult patients undergoing diagnostic or therapeutic procedures.[9] The aqueous solubility of fospropofol provides an advantage over propofol which is available only as a lipid containing oil–water emulsion.[4] However, propofol has become the most popular intravenous anesthetic for making the patients subjectively feel better in the immediate post-operative period because of reduced nausea and vomiting. However, when administered for prolonged infusions, propofol can lead to delayed arousal due to its cumulative effects. It also causes a marked decrease in blood pressure during induction of anesthesia by reducing the peripheral arterial resistance and causing venodilation.[1] FP may ameliorate some of the problems associated with administration of propofol which are mentioned in Table 1.[10]
Table 1.
Differences between propofol and fospropofol[11]

MECHANISM AND ONSET OF ACTION
Fospropofol disodium being a prodrug of propofol is hydrolyzed by endothelial alkaline phosphatases in vivo after intravenous administration releasing propofol, phosphate, and formaldehyde. Propofol derived from FP is the active compound which helps in providing sedation.[11] Its mechanism of action is uncertain, but it is postulated that its primary effect is to enhance the activity of gamma-aminobutyric acid (GABA) and glycine which are the chief inhibitory neurotransmitters of central nervous system (CNS) acting on the GABA-A and glycine receptors, respectively. Hence, it helps in potentiating the inhibitory GABA synaptic currents.[12] Formaldehyde released upon hydrolysis is rapidly metabolized to formate. The accumulation of formate is generally responsible for toxicity as seen after methanol ingestion. But so far, there has been no report of toxicity due to administration of fospropofol or other phosphate ester prodrugs, such as fosphenytoin.[13]
The onset of action is fundamental for selection of a MAC sedation agent, but both rapid and slow onset of action present distinct aspects that may be clinically useful. A MAC sedation agent with a rapid onset of action allows for more immediate and probably more comfortable sedation. Fospropofol is generally considered to have a slow onset of action from 4 to 13 min compared to propofol, which has a rapid onset of action of about 40 s.[14] This slower onset of action can be attributed to the fact that fospropofol is a prodrug which must first be metabolized to release propofol. This slower onset of action may make it possible, in some instances, to offer fewer boluses of medication for a short operation, possibly making fospropofol practical for use in an outpatient clinic performing very brief diagnostic or therapeutic procedures. However, the slow onset of action may require consideration in dosing, recognizing that there is a “lag time” between infusion and effect.[2]
Pharmacokinetics
The half-life for hydrolysis of fospropofol is 8 min.[15] It has a small volume of distribution and a terminal half-life of around 46 min.[15] The currently published pharmacokinetic data on fospropofol were derived using an analytical method that has now been shown to be inaccurate; correct pharmacokinetic data are not yet available.[15]
This analytical inaccuracy in the propofol assay was discovered by the investigators after publication of the data regarding the pharmacokinetic and pharmacodynamic properties of fospropofol and its tolerability.[16]
Clinical trials
There are few published studies regarding the clinical efficacy of fospropofol in MAC sedation. [Table 2].
Table 2.
Evaluation of fospropofol as a MAC sedation agent in various clinical trials
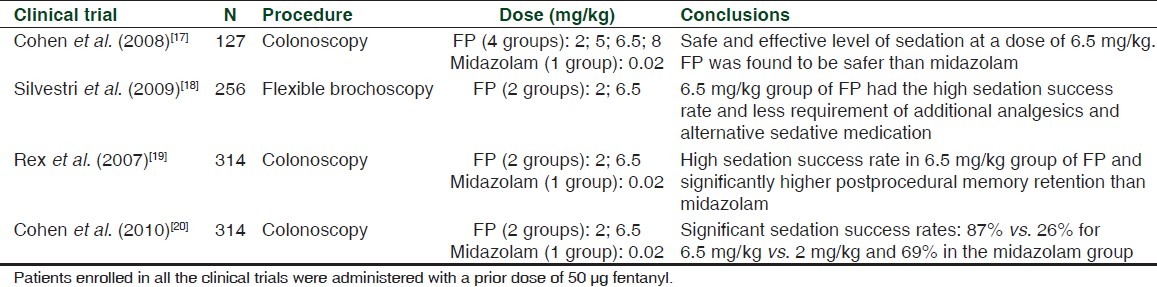
Patients undergoing colonoscopy were randomized to one of four fospropofol treatment arms: 2, 5, 6.5, or 8 mg/kg in the Cohen et al. study.[17] A fifth treatment group served as a sensitivity reference for measurements of clinical benefits and safety which received midazolam 0.02 mg/kg. All patients received a dose of fentanyl prior to the study treatment. Sedation success was dose-dependent across all the fospropofol treatment groups. Patients were more satisfied with the feeling of adequate sedation and memory retention in the 6.5 mg/kg fospropofol group compared to the reference group receiving midazolam therapy. This study demonstrates that administration of 6.5 mg/kg dose of fospropofol provides a level of sedation that is safe and effective for patients undergoing colonoscopy. In addition, the safety profile of fospropofol compares favorably with that of other sedatives such as midazolam. The limitations were the preprocedure dose of fentanyl which could have had an additive effect in combination with fospropofol. The study was not designed or intended to compare midazolam to fospropofol but rather, midazolam was included as a reference therapy. However, the conclusions were made comparing the fospropofol arms to the midazolam reference arm.
Patients undergoing flexible bronchoscopy in the Silvestri et al. study[18] were randomly assigned to one of two treatment groups: fospropofol 2 mg/kg or fospropofol 6.5 mg/kg in a 2:3 ratio, respectively. All patients received 50 μg fentanyl prior to the first dose of fospropofol, as well as supplemental oxygen (4 l/min). Topical lidocaine was administered for cough suppression. Sedation success rates were 88.7% (fospropofol 6.5 mg/kg group) and 27.5% (fospropofol 2 mg/ kg group).
Rex et al.[19] evaluated sedation success and clear-headed recovery in the patients undergoing colonoscopy. Cognitive testing at the baseline (Hopkins Verbal Learning Test-Revised) was performed to evaluate recall and memory. Patients were randomized to receive fospropofol (2.0 mg/kg), fospropofol (6.5 mg/kg), or midazolam (0.02 mg/kg). The patients receiving 6.5 mg/kg fospropofol had high rates of sedation success with better memory retention than patients receiving midazolam.
Cohen et al.[20] also evaluated significant sedation success rates of 87% vs. 26% for 6.5 mg/kg vs. 2 mg/kg and 69% in the midazolam group in patients undergoing colonoscopy.
In general, fospropofol was observed to provide adequate and better MAC sedation using the 6.5 mg/kg dose vs. the 2 mg/ kg dose. In all of the studies, a 50-μg dose of fentanyl was administered prior to fospropofol. At this time, there are no studies comparing the safety and efficacy of fospropofol to other agents for MAC sedation making it difficult to conclude whether certain advantages or disadvantages of fospropofol exist in comparison to these other agents. Furthermore, there are no published clinical trials investigating the use of fospropofol for the induction and maintenance of general anesthesia or for the induction and maintenance of sedation in intubated, mechanically ventilated patients in the intensive care unit.
Dose
The recommended maximum dose of fospropofol is 12.5 mg/ kg leading to a loss of consciousness in about 4 min. The recommended effective dose is 6.5 mg/kg.[2]
Approved indication
Fospropofol disodium injection is Food and Drug administration (FDA) approved for use as an intravenous sedative-hypnotic agent in adult patients undergoing diagnostic or therapeutic procedures for MAC sedation.[3] Fospropofol is classified as a schedule four (C-IV) controlled substance by the FDA.[4]
Adverse effects
The adverse effect profile of fospropofol is almost similar to that of propofol. However, fospropofol has a lower incidence of hypotension, respiratory depression, apnea, and loss of airway patency because of its slower onset of action. However still, unintended deep levels of sedation can occur with fospropofol. Therefore, the drug should be used where one can maintain an adequate airway and support cardiorespiratory function.[15]
The most common adverse events seen with this drug are paresthesias and pruritis. Paresthesias (burning, stinging or tingling) and pruritis generally occur in the perineal and perianal regions within 5 min after the initial dose of fospropofol. They are usually mild-to-moderate in intensity, transient, and self-limiting.[21]
Propofol can lead to a syndrome termed propofol infusion syndrome (PRIS), which is a rare but potentially fatal complication. The syndrome is characterized by metabolic acidosis, hyperlipidemia, rhabdomyolysis, and an enlarged liver.[22] It is currently not clear whether fospropofol can also result in PRIS.[13] However, there is a theoretical risk of formic acid accumulation, a metabolic product of fospropofol in patients who have a folate deficiency as folic acid is required as a cofactor for the conversion of formic acid into carbon dioxide and water by an enzyme tetrahydrofolate dehydrgenase.[15]
Contraindications
According to the manufacturer labeling, no contraindications are documented with fospropofol use. Applicable contraindications to fospropofol include hypersensitivity to propofol and contraindication to general anesthesia or sedation.[3]
STATUS IN SPECIFIC POPULATIONS[3]
Pregnancy
Animal studies using fospropofol have not revealed impaired fertility or harm to the fetus. However, it should be used in pregnancy, only if clearly indicated.
Pediatric use
It is not recommended for use in patients younger than 18 years of age.
Geriatric use
The modified dosing regimen is recommended in patients greater than 65 years of age. However, the incidence of hypoxemia is more in patients with age more than 75 years as compared to the age group between 65 and 74 years of age.
Patients with renal impairment
Dosage adjustments are not required in patients with mild-to-moderate renal insufficiency (creatinine clearance, CrCl ≥ 30 ml/min). Limited data are available for patients with a CrCl < 30 ml/min.
Patients with hepatic impairment
Caution is advised with the use of fospropofol in patients with hepatic impairment.
Abuse potential
There is currently no abuse data for fospropofol, but it can lead to physical and psychological dependence.[3]
CONCLUSION
MAC provides a valuable bridge between moderate sedation (may be inadequate for a given procedure) and general anesthesia (may be unnecessary).[7] Moreover, the expansion of outpatient procedures has raised interest in MAC sedation and appropriate MAC sedation agents. This may be due to the fact that the already existing agents are having few drawbacks which need to be taken care of. Fospropofol, a prodrug of propofol, has been introduced to the market as a potential MAC sedation agent.[2] The patient can be both medically managed and safely sedated to allow for successful completion of the procedure under the direction of the anesthesiologist.[7]
However, the recent retraction of six studies of the pharmacokinetic and pharmacodynamic properties of fospropofol due to possible errors in propofol assays has clouded discussion about the PK/PD of fospropofol. In addition, the published safety and efficacy studies of colonoscopies and bronchoscopies have clinical values, but are limited in that they report on the use of fospropofol in relatively short procedures, rather than longer procedures where repeated dosing might be required.[2]
The commonly occurring adverse effects of fospropofol, i.e. genital and perianal itching have not interfered with the widespread clinical use of other phosphorylated prodrugs (e.g. fosphenytoin) which share the same adverse effect profile.[23] Fospropofol injections are associated with less pain than propofol injections and are well tolerated. Fospropofol appears to be a promising new agent for MAC sedation, but further studies are necessary to better assess its PK/PD properties and its suitable role in the outpatient background.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.White PF, Trevor AJ. General anesthetics. In: Katzung BC, Masters SB, Trevor AJ, editors. Basic and Clinical Pharmacology. 11th ed. New Delhi: Tata McGraw-Hill Education Private Limited; 2009. pp. 423–38. [Google Scholar]
- 2.Pergolizzi JV, Gan TJ, Plavin S, Labhsetwar S, Taylor R. Perspectives on the role of fospropofol in the monitored anesthesia care setting. Anesthesiol Res Pract. 2011;2011:458920. doi: 10.1155/2011/458920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lusedra official FDA information, side effects and uses. [Last accessed on 2012 Feb 02]. Available from: http://www.drugs.com/pro/lusedra.html .
- 4.Mueller SW, Moore GD, MacLaren R. Fospropofol Disodium for Procedural Sedation: Emerging Evidence of its Value? Clinical Medicine Insights: Therapeutics. 2010;2:513–22. [Google Scholar]
- 5.Ghisi D, Fanelli A, Tosi M, Nuzzi M, Fanelli G. Monitored anesthesia care. Minerva Anestesiol. 2005;71:533–8. [PubMed] [Google Scholar]
- 6.Committee of origin: Economics. Distinguishing monitored anesthesia care (“MAC”) from moderate sedation/analgesia (conscious sedation) Approved by ASA house of delegates on Oct. 27, 2004 and last amended on Oct. 21,2009 [Google Scholar]
- 7.Harris EA, Lubarsky DA, Candiotti KA. Monitored anesthesia care (MAC) sedation: Clinical utility of fospropofol. Ther Clin Risk Manag. 2009;5:949–59. doi: 10.2147/tcrm.s5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hom J, Burg J. Pediatrics, Sedation. [Last accessed on 2011 Oct 07]. Available from: http://emedicine.medscape.com/article/804045-overview .
- 9.Food and Drug Administration. Centre for drug evaluation and research, Division of anesthesia, analgesia and rheumatology products, Memorandum. [Last accessed on 2011 Oct 07]. Available from: http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4354b1-01-FDA.pdf .
- 10.Atlas G. Fospropofol: Is there an infusion regimen for propofol equivalence? J Anesthesiol Clin Pharmacol. 2011;27:303–6. doi: 10.4103/0970-9185.83671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fechner J, Ihmsen H, Hatterscheid D, Schiessl C, Vornov JJ, Burak E, et al. Pharmacokinetics and clinical pharmacodymamics of the new propofol prodrug GPI 15715 in volunteers. Anesthesiology. 2003;99:303–13. doi: 10.1097/00000542-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Drug Name: Lusedra (fospropofol disodium) [Last accessed on 2011 Oct 08]. Available from: http://www.centerwatch.com/drug-information/fda-approvals/drug-details.aspx?DrugID=1011 .
- 13.Fechner J, Schwilden H, Schuttler J. Pharmacokinetics and pharmacodynamics of GPI 15715 or fospropofol (Aquavan injection) - a water-soluble propofol prodrug. Handb Exp Pharmacol. 2008;182:253–66. doi: 10.1007/978-3-540-74806-9_12. [DOI] [PubMed] [Google Scholar]
- 14.Tong D, Chung F, Wong D. Predictive factors in global and anesthesia satisfaction in ambulatory surgical patients. Anesthesiology. 1997;87:856–64. doi: 10.1097/00000542-199710000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Patel PM, Patel HH, Roth DM. General anesthetics and therapeutic gases. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilmans: The pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill Medical Publishing Division; 2011. pp. 527–64. [Google Scholar]
- 16.Struys MM, Fechner J, Schuttler J, Schwilden H. Erroneously published fospropofol pharmacokinetic–pharmacodynamic data and retraction of the affected publications. Anesthesiology. 2010;112:4. doi: 10.1097/ALN.0b013e3181d536df. [DOI] [PubMed] [Google Scholar]
- 17.Cohen LB. Clinical trial: A dose-response study of fospropofol disodium for moderate sedation during colonoscopy. Aliment Pharmacol Ther. 2008;27:597–608. doi: 10.1111/j.1365-2036.2008.03598.x. [DOI] [PubMed] [Google Scholar]
- 18.Silvestri G, Vincent B, Wahidi M, Robinette E, Hansbrough J, Downie G. A phase 3, randomized, double-blind study to assess the efficacy and safety of fospropofol disodium injection for moderate sedation in patients undergoing flexible broncoscopy. Chest. 2009;135:41–7. doi: 10.1378/chest.08-0623. [DOI] [PubMed] [Google Scholar]
- 19.Rex D, Cohen L, Klein J, Wang C. Fospropofol disodium for sedation during colonoscopy produces clearheaded recovery: Results of a phase 3, randomized, doubleblind trial. Digestive Disease Week, Washington, DC, USA. 2007 [Google Scholar]
- 20.Cohen L, Cattau E, Goetsch A, Shah A, Weber J, Rex D, et al. A randomized, double-blind, phase 3 study of fospropofol disodium for sedation during colonoscopy. J Clin Gastroenterol. 2010;44:345–53. doi: 10.1097/MCG.0b013e3181c2987e. [DOI] [PubMed] [Google Scholar]
- 21.VA Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. Fospropofol (Lusedra) National Drug Monograph. 2011 Jan [Google Scholar]
- 22.Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62:690–701. doi: 10.1111/j.1365-2044.2007.05055.x. [DOI] [PubMed] [Google Scholar]
- 23.Luer M. Phosphenytoin. Neurol Res. 1998;20:178–82. doi: 10.1080/01616412.1998.11740502. [DOI] [PubMed] [Google Scholar]


