Abstract
Objective: The objective of this study was to evaluate the effects of a 405 nm diode laser on bleaching reaction of H2O2 and VL-TiO2 on methylene blue (MB) dye. Background data: Visible light activating titanium dioxide photocatalyst (VL-TiO2) may improve efficacy of hydrogen peroxide (H2O2) bleaching agents used in dentistry while contributing to their safety by lowering the required concentration of peroxide. Methods: The experimental solution was prepared with H2O2, VL-TiO2, MB, and pure water. The final concentration of H2O2 was 3.5% and that of MB was 10 ppm. The experimental solution of 3 mL in a quartz cell was irradiated by a 405 nm diode laser with various powers, duty cycles, and pulse durations for 7 min. Results: In all irradiation conditions, the increase in laser irradiation time gradually decreased the MB concentration. Irradiation by higher output power showed more reduction of MB concentration. Pulse durations as short as 5 ms with duty cycle reduced to 25% did not affect the degree of the reduction in MB concentration compared with continuous wave irradiation at the same average output power. Conclusions: It was concluded that using 405 nm diode laser, the bleaching effects of VL-TiO2 depended upon the irradiation time and the average output power, regardless of pulse duration or duty cycle.
Introduction
Over the past two decades, tooth whitening or bleaching has become one of the most popular esthetic dental treatments.1 Tooth bleaching is a conservative and cost-effective dental treatment to improve or enhance a person's smile.1 For vital-tooth bleaching, two methods are provided; namely, office bleaching and home bleaching. The office bleaching is applied in a dental office, and one or several visits are required.
Generally, hydrogen peroxide (H2O2) at a high concentration is the active ingredient of office bleaching products.1 H2O2 is an oxidizing agent and has the ability to produce free radicals. The free radicals are unstable molecules that will attack the organic molecules in the tooth substrate to achieve stability. The radicals react with double bonds of the pigmented organic molecules and break down pigments to smaller molecules. As a result, simpler molecules that diffuse out of the tooth or reflect less light are formed, creating a successful bleaching action.2 The bleaching reaction depends upon the type of discoloration involved and the chemical and physical environment present at the time of action, that is, concentrations,2,3 heating,4 lightening,5 pH,6 co-catalysts,7–10 times of application,3 and other conditions.
Most current office bleaching products employ high concentration H2O2 to produce higher amounts of free radicals.11 However, a number of safety issues have been raised regarding the effects of bleaching on the tooth structure,12,13 pulp tissues,14–17 and the mucosal tissues of the mouth, as well as systemic ingestion.1 High concentration H2O2 irritates soft tissue; therefore, these products require strict isolation of the teeth.2,6 In addition, the high concentration often causes postoperative dentin hypersensitivity.1 In view of safety, an efficient bleaching product with lower concentration of H2O2 is desirable.
Recently, a new office bleaching material (Pyrenees, Mitsubishi Gas Chemical, Tokyo, Japan) has been introduced, which contains a lower concentration of H2O2 (3.5%) and visible light-activating titanium dioxide (VL-TiO2) as a photocatalyst.7,9 VL-TiO2 can accelerate H2O2 reaction at the visible light range, especially in the range of 380–450 nm.9 However, the peak energy of common light sources in dental offices is not in this wavelength range.
The laser is widely used for not only industry, but also in medical fields, including dental medicine. In clinical dentistry, lasers are applied for cavity preparation,18 photopolymerization of resinous materials,19 caries prevention,20 endodontic treatments,21 pulp capping and pulpotomy,22 periodontal therapy,23 dentin hypersensitivity,24 tooth bleaching,11,25–27 and oral surgery.28 Nd:YAG laser,25 argon laser,26 CO2 laser,27 diode laser11,25 and KTP laser25 have been used for tooth bleaching. Most of them would raise the temperature of applied bleaching gel and accelerate the bleaching reaction. However, the activation of bleaching agents by heat, light, or laser may have an adverse effect on pulpal tissue because of an increase of intrapulpal temperature.29 An experimental violet diode laser with a wavelength of 405 nm was developed for oral surgery.30 The wavelength of this laser is in the lowest part of the visible spectrum and appeared to be suitable for activating VL-TiO2 of the bleaching agent.9 In addition to the wavelength, other laser parameters may affect the outcome of irradiation; however, the effects of laser parameters on the performance of VL-TiO2-containing bleaching agent are not known.
Given this background, the objective of this study was to evaluate the effect of the condition of 405 nm diode laser irradiation and VL-TiO2 on the bleaching effect using methylene blue (MB) in vitro.
Materials and Methods
Preparation of experimental solutions
An experimental solution was made by mixing 1.0 g of 0.1 wt% of VL-TiO2 solution (Mitsubishi Gas Chemical), 1.0 g of 35 wt% H2O2 (Mitsubishi Gas Chemical), 1.0 g of 100 ppm MB solution (Methylenblau med. Puriss; Chroma-Gesellschaft, Muenster, Germany) and 7.0 g of pure water. The concentration of H2O2 in this solution was 3.5%. The VL-TiO2 was the same catalyst used for the Pyrenees but its concentration was 10% of that in the commercial product, as determined from a previous study.7
Laser irradiation and bleaching
A 405 nm diode laser (experimental HH-02, Sumitomo Electric Industries, Osaka, Japan) was used in this study. The spectrum of the radiation was in the interval of 400–410 nm, with the peak on the 405 nm wavelength, and the range of output power was 100–1000 mW. This laser is variable at pulse duration in the range of 5–500 ms, and duty cycle in the range of 5–50%.
The irradiation conditions of the laser are shown in Table 1; in total, 19 conditions categorized in three experimental groups according to the oscillation method with continuous wave (CW) or pulse wave (PW) modes under different parameters (power, duty cycle, or pulse duration), including a non-irradiation (control) group were investigated. These parameters of laser irradiation were determined within the specification of the laser, referring previous studies.8 The output power was monitored and adjusted using a laser power meter (FieldMate; Coherent, Newberg, OR) for every irradiation.
Table 1.
The Condition of Laser Irradiation
| Category | Code | Oscillation method | Power, mW | Duty, % | Pulse duration, ms | Average output power, mW |
|---|---|---|---|---|---|---|
| A | Non | Non | 0 | – | – | 0 |
| 100 CW | CW | 100 | – | – | 100 | |
| 200 CW | CW | 200 | – | – | 200 | |
| 300 CW | CW | 300 | – | – | 300 | |
| 400 CW | CW | 400 | – | – | 400 | |
| 500 CW | CW | 500 | – | – | 500 | |
| 600 CW | CW | 600 | – | – | 600 | |
| 700 CW | CW | 700 | – | – | 700 | |
| 800 CW | CW | 800 | – | – | 800 | |
| 1000 CW | CW | 1000 | – | – | 1000 | |
| B | 800 D25 W5 | PW | 800 | 25 | 5 | 200 |
| 800 D25 W50 | PW | 800 | 25 | 50 | 200 | |
| 800 D25 W500 | PW | 800 | 25 | 500 | 200 | |
| 800 D50 W5 | PW | 800 | 50 | 5 | 400 | |
| 800 D50 W50 | PW | 800 | 50 | 50 | 400 | |
| 800 D50 W500 | PW | 800 | 50 | 500 | 400 | |
| C | 100 CW | CW | 100 | – | – | 100 |
| 200 CW | CW | 200 | – | – | 200 | |
| 400 CW | CW | 400 | – | – | 400 | |
| 200 D50 W50 | PW | 200 | 50 | 50 | 100 | |
| 400 D25 W50 | PW | 400 | 25 | 50 | 100 | |
| 400 D50 W50 | PW | 400 | 50 | 50 | 200 | |
| 800 D25 W50 | PW | 800 | 25 | 50 | 200 | |
| 800 D50 W50 | PW | 800 | 50 | 50 | 400 |
Non, non-irradiation; CW, continuous wave; PW, pulse wave; D, duty; W, pulse duration (width of pulse).
Irradiation was performed on 3.0 mL of freshly prepared experimental solution poured into a quartz cell (1.0×1.0×4.5 cm). The distance between the tip of the fiber and the cell was maintained at 5 mm, and the diameter of the radiation area was ∼2.5 mm, which covered 4.9 mm2. The solution in the cell was then irradiated for 7 min at 30 sec intervals. After each irradiation interval, the solution was stirred using a magnetic stirrer (Mini Stealer; GL Sciences, Tokyo, Japan) for 30 sec to ensure homogeneity of the solution and even distribution of color pigments. Following that, the absorbance of solution at 660 nm was measured using a spectrophotometer (Mini Photo 5; Sanshin, Tokyo, Japan). The laser irradiation, stirring, and measurement cycle was repeated 14 times for each sample to reach a total irradiation time of 7 min. The experiment was conducted on three samples under each condition, which was determined based on a previous study7 and a pilot study.
The concentration of the remaining MB in the solution after laser irradiation was determined using a standard curve previously drawn in the pilot study by plotting the known concentrations and the determined absorbance of the sample solution of MB at various concentrations.7 The concentration was calculated according to the following equation:
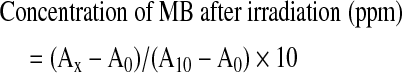 |
A10: absorbance of experimental solution before irradiation (10 ppm of MB)
A0: absorbance of blank solution; 1.0 g of VL-TiO2 and 9.0 g of the pure water without MB
AX: absorbance of experimental solution after irradiation
Statistical analysis
Statistical analysis of the concentration of MB in the solution at periods of 1, 4, and 7 min was performed among the groups in each category using two-way analysis of variance (ANOVA) and t-test with Bonferroni correction at a 95% level of confidence. The factors analyzed were irradiation condition and time of laser irradiation. Statistical tests were performed using a computerized statistical program (SPSS for Windows release Version 11, IBM, Armonk, NY).
Results
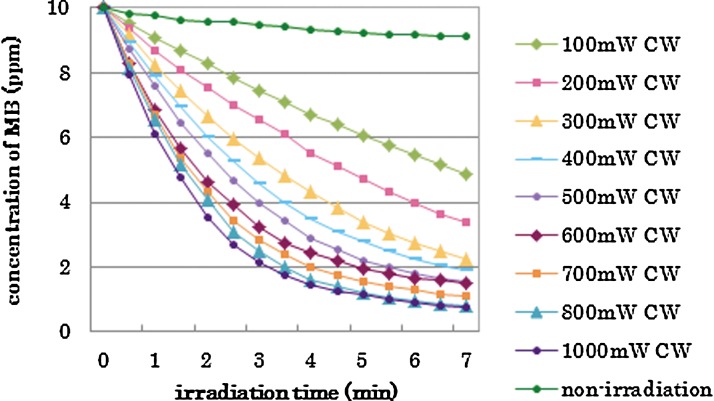
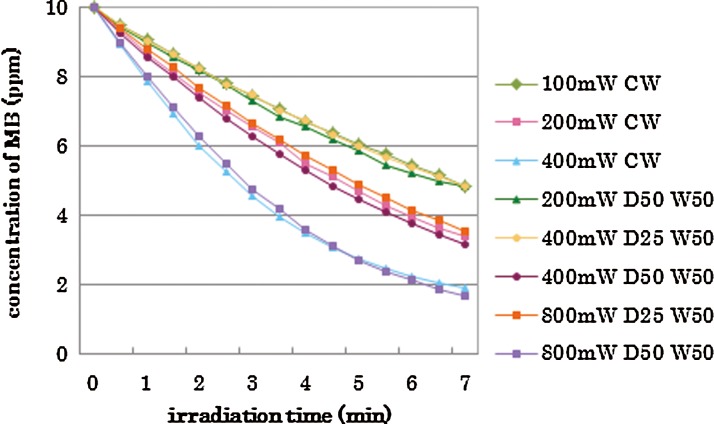
The concentration of MB at each experimental period in the experimental groups is shown in Figs. 1–3, respectively, by category. The repeated laser irradiation in each group reduced the concentration of MB gradually, and the color change could be confirmed with the naked eye. The group without laser irradiation (Non) also reduced the MB concentration, but the reduction was less than those of irradiated groups, as seen in Fig. 1. There were statistical differences in MB concentration among 1, 4, and 7 min in each group except the “Non” (no laser irradiation) group.
FIG. 1.
Changes of methylene blue (MB) concentration in the different power (100–1000 mW) with continuous wave; category A.
FIG. 3.
Changes of methylene blue (MB) concentration with fixed pulse duration (50 ms); category C.
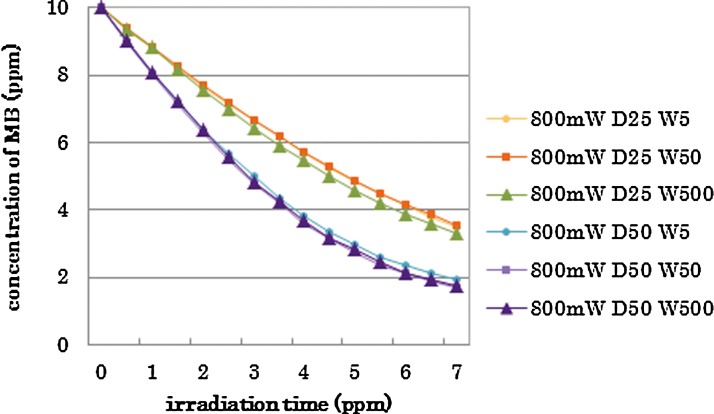
The results of statistical analysis are shown by the category in Tables 2–4. There was no interaction between two factors, “irradiation conditions” and “irradiation times” in each category. The two-way ANOVA showed significant differences among “irradiation conditions” and among “irradiation times.”. Generally, higher power irradiation with CW showed more reduction of MB concentration, as shown in Table 2, and the differences were statistically significant among groups (p<0.05). There were significant differences between the non group and all irradiated groups (p<0.05). On the other hand, pulse duration change did not significantly affect the MB concentration in 800 mW 25% duty cycle groups and 800 mW 50% duty cycle groups (p>0.05) as is shown in Table 3. Likewise, there were no statistical differences in MB concentration between the 400 mW 25% duty cycle group and the 100 mW CW group; among the 400 mW 50% duty cycle group, the 800 mW 25% duty cycle group, and the 200 mW-CW group; or between the 800 mW 50% duty cycle group and the 400 mW CW group (p>0.05) as is shown in Table 4.
Table 2.
Mean and Standard Deviation of MB Concentrations in Category A for 1, 4, and 7 Min Light Irradiation
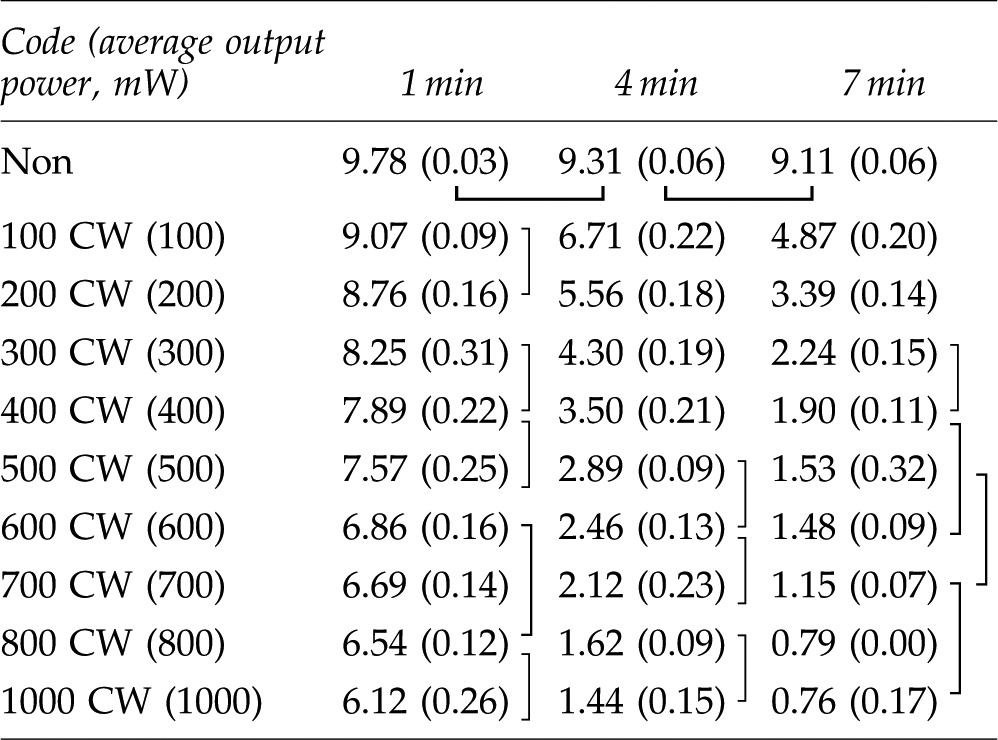 |
The data connected with lines have no statistically significant difference (p>0.05). Mean (±SD), n=3.
MB, methylene blue; CW, continuous wave.
Table 4.
Mean and Standard Deviation of MB Concentrations in Category C for 1, 4, and 7 Min Light Irradiation
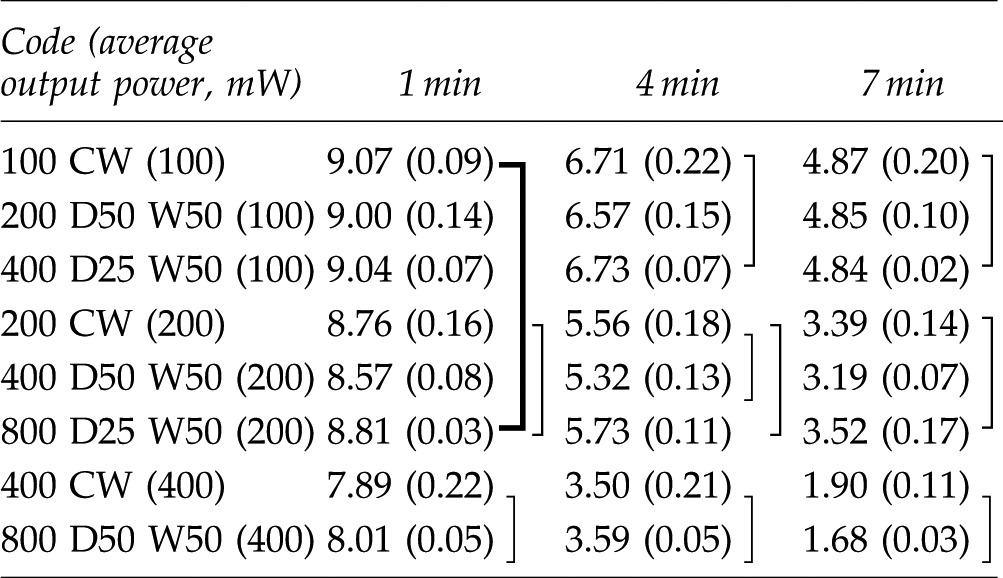 |
The data connected with lines have no statistically significant difference (p>0.05). Mean (±SD), n=3.
MB, methylene blue; CW, continuous wave; D, duty; W, pulse duration (width of pulse).
Table 3.
Mean and Standard Deviation of MB Concentrations in Category B for 1, 4, and 7 Min Light Irradiation
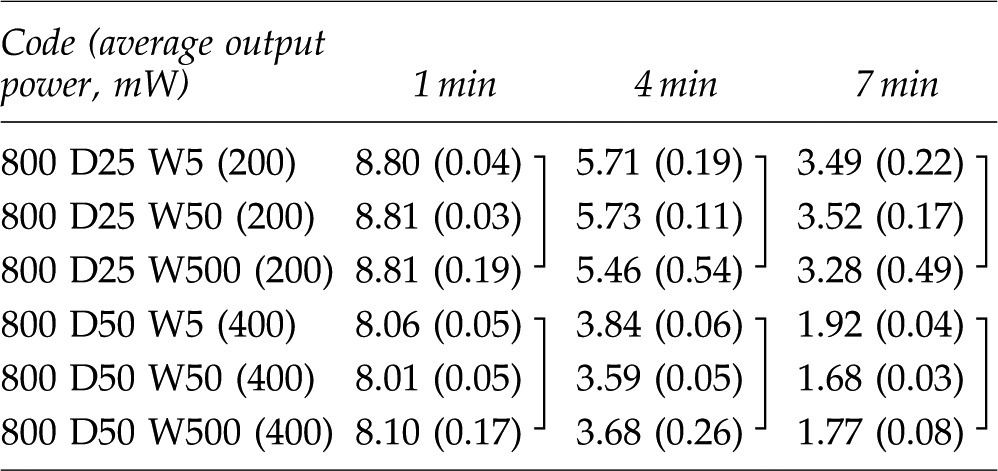 |
The data connected with lines have no statistically significant difference (p>0.05). Mean (±SD), n=3.
MB, methylene blue; D, duty; W, pulse duration (width of pulse).
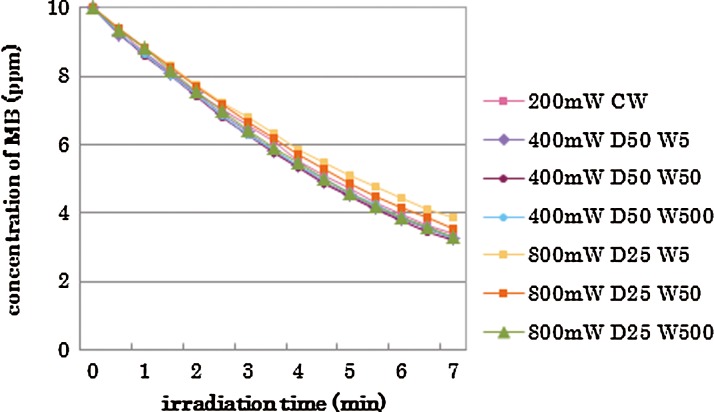
Collectively, it was seen that the peak power did not affect the MB concentration, if the irradiation energy (average power) was the same. Figure 4 shows the results of irradiation with an average power of 200 mW with different peak powers, duty cycles, and pulse durations. There were no statistical differences among these groups (p>0.05).
FIG. 4.
Changes of methylene blue (MB) concentration with same average output power and various peak power, duty cycle, and pulse duration.
Discussion
In the previous in vitro studies, several methods have been employed for evaluating tooth bleaching effects.4,7–10,31,32 Those studies used various substrates, such as extracted human teeth,4,31,32 bovine teeth,8,10 hematoporphyrin-stained paper9 and dye solution.7 Teeth were used after staining with tea,4,10 blood,31 or dye.32 It should be noted that using extracted teeth, results of a study would be more clinically relevant compared with other in vitro studies in which teeth were not involved. However, it seems to be very difficult to collect extracted human teeth as standard samples for experiments of large-sample size, considering the factors affecting results such as age, shade, mineralization, and thickness of enamel.7 In this study, MB solution was used to evaluate the efficacy of bleaching as previously recommended.7 Considering the results, the in vitro design seems to be more reproducible and accurate than those tooth-model studies; and, therefore, it is more suitable for a screening test.7
The use of 3.5% H2O2, as in the experimental agent used in this study, is expected to exert several advantages when compared to other agents that routinely use 25–35% H2O2. It is known that an agent containing ≥10% concentrations of H2O2 can be corrosive to mucous membranes or skin, causing a burning sensation and tissue damage, especially to soft tissue surrounding the tooth, if gingival protection is inadequate.33 Clinical studies have also observed a higher prevalence of gingival irritation in patients using bleaching materials with higher peroxide concentrations.2,34
It was previously shown that addition of VL-TiO2 to the H2O2-based bleaching agent could enhance the bleaching efficacy of the agent when exposed to the visible light from a dental light unit. Moreover, in the previous work effect of irradiation was less when H2O2 was used without VL-TiO2.7 It was suggested that bleaching was enhanced by hydroxyl radical generation through the photocatalytic action of VL-TiO2.7 The effects of irradiation on VL-TiO2-H2O2 agent was confirmed in the current study, in which laser irradiation significantly increased the bleaching efficacy as previously reported.10 The temperature of the experimental solution was increased <2.4 °C during 7 min of laser irradiation in this study. It was suggested that the heat could not play a major role in bleaching reaction.
The 405 nm diode laser has been tried for surgery.30,35 The use of 405 nm diode laser with its narrow spectra has an advantage over common halogen light or light-emitting diode (LED) curing units with the peak at ∼470 nm;10 the laser wavelength is within the specific visible range required for the photocatalyst, and unnecessary higher wavelengths emitted from incandescent lamps or blue LEDs that may be absorbed by the live tissue are avoided.27
In this study, the parameter of the laser irradiation was determined within the specification of the laser and referring to previous studies.8 Among these conditions of irradiation, it was shown that a higher power resulted in a stronger bleaching effect (Fig. 1), which confirmed the assumption that the higher power irradiation produced more radicals.8 However, there were no statistical differences between 800 and 1000 mW groups, and among 700, 800, and 1000 mW groups at 7 min, indicating that under the current setup, there was a limitation for optimum efficiency at ∼800 mW. This limitation may be related to the availability of H2O2, VL-TiO2 or MB for reaction in the solution.
The change of pulse duration with the same peak power and duty cycle did not affect the bleaching effect in the 800 mW 25% duty cycle and the 800 mW-50% duty cycle groups (Fig. 2). Moreover, the experimental groups with average output power of 200 mW with different pulse durations and duty cycles showed the same bleaching effects (Fig. 4). This finding may be important from a dental point of view; generally, longer pulse durations show more heat accumulation. In terms of thermal damage, the pulsed laser irradiation of the tissue is preferred over CW. If the number of pulses per second is sufficiently low, and the interval between pulses is sufficiently long to permit delivery of peak powers without exceeding the thermal relaxation time of the irradiated tissue, increased temperature within the tissue may be negligible when compared with the CW emitting the same amount of total energy.36 In this study, pulse durations of 5, 50, and 500 ms did not affect the bleaching results when the power peak was similar and the same average output power was maintained; therefore, the shortest pulse duration should be preferred. Similarly, the change of duty cycle with same power did not affect the bleaching effect (Fig. 3); a smaller duty cycle can be expected to generate less heat. Further study is required to directly evaluate the effect of laser irradiation conditions on the temperature of dental tissue using the new generation bleaching solution.
FIG. 2.
Changes of methylene blue (MB) concentration in the different pulse duration (5, 50, 500 ms) with fixed output power and duty (25% 200 mW or 50% 400 mW); category B.
Considering a spot size of 8 mm, 400 mW output power of the 405 nm diode laser unit would result in a power density of ∼800 mW/cm2, which is in the same range as that for common LED or halogen units.9 The diameter of the laser tip used in this study was 150 μm, and it seems to be difficult to apply several anterior teeth simultaneously; therefore, the shape of the tip should be modified to improve the clinical usage of this device for bleaching. Moreover, the cost of the laser will be a problem for marketing. Recently, a 405 nm LED unit was marketed at an affordable cost compared with the laser. Given the wavelength of this LED, a similar bleaching effect to that of the laser can be expected.10 The results of this study should also contribute to determining the irradiation conditions of the LED unit for bleaching with H2O2 and VL-TiO2.
Conclusions
From this in vitro study, it was concluded that the 405 nm diode laser was an effective light source for bleaching using 3.5% H2O2 and VL-TiO2, and that the bleaching effect using the 405 nm diode laser depended upon the average output power and irradiation time. At the same average output power, pulsed laser irradiation with short pulse durations should be preferred over CW irradiation.
Acknowledgments
This work was partially supported by Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (C) 21592414, and Global Center of Excellence (GCOE) Program, “International Research Center for Molecular Science in Tooth and Bone Diseases.”
Author Disclosure Statement
No competing financial interests exist.
References
- 1.ADA Council on Scientific Affairs. Tooth whitening/bleaching: treatment considerations for dentists and their patients. Chicago: American Dental Association; 2009. [Google Scholar]
- 2.Sulieman M.A. An overview of bleaching techniques: chemistry, safety and efficacy. Periodontol 2000. 2008;48:148–169. doi: 10.1111/j.1600-0757.2008.00258.x. [DOI] [PubMed] [Google Scholar]
- 3.Matis B.A. Cochran M.A. Eckert G. Review of the effectiveness of various tooth whitening systems. Oper. Dent. 2009;34:230–235. doi: 10.2341/08-74. [DOI] [PubMed] [Google Scholar]
- 4.Davidi M.P. Hadad A. Weiss E.I. Domb A. Mizrahi B. Sterer N. The effect of a mild increase in temperature on tooth bleaching. Quintessence Int. 2008;39:771–775. [PubMed] [Google Scholar]
- 5.Buchalla W. Attin T. External bleaching therapy with activation by heat, light or laser – A systematic review. Dent. Mater. 2007;23:586–596. doi: 10.1016/j.dental.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Sun G. The role of lasers in cosmetic dentistry. Dent. Clin. North Am. 2000;44:831–850. [PubMed] [Google Scholar]
- 7.Suyama Y. Otsuki M. Ogisu S., et al. Effects of light sources and visible light-activated titanium dioxide photocatalyst on bleaching. Dent. Mater. J. 2009;28:693–699. doi: 10.4012/dmj.28.693. [DOI] [PubMed] [Google Scholar]
- 8.Sakai K. Kato J. Nakazawa T. Hirai Y. Bleaching effect of a 405-nm diode laser irradiation used with titanium dioxide and 3.5% hydrogen peroxide. Laser Phys. 2007;17:1166–1170. [Google Scholar]
- 9.Suemori T. Kato J. Nakazawa T., et al. Effects of light irradiation on bleaching by a 3.5% hydrogen peroxide solution containing titanium dioxide. Laser Phys. Lett. 2008;5:379–383. [Google Scholar]
- 10.Kishi A. Otsuki M. Alireza Sadr A. Ikeda M. Tagami J. Effect of light units on tooth bleaching with visible-light activating titanium dioxide photocatalyst. Dent. Mater. J. 2011;30:723–729. doi: 10.4012/dmj.2010-210. [DOI] [PubMed] [Google Scholar]
- 11.Verheyen P. Walsh L.J. Wernisch J., et al. Laser-assisted bleaching. In: Moritz A., editor. Oral Laser Application. Berlin: Quintessnz Verlags-GmbH; 2006. pp. 407–448. [Google Scholar]
- 12.Kawamoto K. Tsujimoto Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J. Endod. 2004;30:45–50. doi: 10.1097/00004770-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Basting R.T. Rodrigues A.L., Jr. Serra M.C. The effects of seven carbamide peroxide bleaching agents on enamel microhardness over time. J. Am. Dent. Assoc. 2003;134:1335–1342. doi: 10.14219/jada.archive.2003.0047. [DOI] [PubMed] [Google Scholar]
- 14.Dias Ribeiro A.P. Sacono N.T. Lessa F.C., et al. Cytotoxic effect of a 35% hydrogen peroxide bleaching gel on odontoblast-like MDPC-23 cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009;108:458–464. doi: 10.1016/j.tripleo.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Costa C.A. Riehl H. Kina J.F. Sacono N.T. Hebling J. Human pulp responses to in-office tooth bleaching. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010;109:e59–e64. doi: 10.1016/j.tripleo.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 16.De Lima A.F. Lessa F.C. Gasparoto Mancini M.N. Hebling J. de Souza Costa C.A. Marchi G.M. Cytotoxic effects of different concentrations of a carbamide peroxide bleaching gel on odontoblast-like cells MDPC-23. J. Biomed. Mater. Res. B Appl. Biomater. 2009;90:907–912. doi: 10.1002/jbm.b.31362. [DOI] [PubMed] [Google Scholar]
- 17.Lima A.F. Lessa F.C. Hebling J. de Souza Costa C.A. Marchi G.M. Protective effect of sodium ascorbate on MDPC-23 odontoblast-like cells exposed to a bleaching agent. Eur. J. Dent. 2010;4:238–244. [PMC free article] [PubMed] [Google Scholar]
- 18.Hibst R. Keller U. Experimental studies of the application of the Er:YAG laser on dental hard substances: I. Measurement of the ablation rate. Lasers Surg. Med. 1989;9:338–344. doi: 10.1002/lsm.1900090405. [DOI] [PubMed] [Google Scholar]
- 19.Powell G.L. Kelsey W.P. Blankenau R.J. Barkmeier W.W. The use of an argon laser for polymerization of composite resin. J. Esthet. Dent. 1989;1:34–37. doi: 10.1111/j.1708-8240.1989.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto H. Sato K. Prevention of dental caries by acousto-optically Q-switched Nd: YAG laser irradiation. J. Dent. Res. 1980;59:137. doi: 10.1177/00220345800590020801. [DOI] [PubMed] [Google Scholar]
- 21.Zakariasen K.L. Dederich D.N. Tulip J. DeCoste S. Jensen S.E. Pickard M.A. Bactericidal action of carbon dioxide laser radiation in experimental dental root canals. Can. J. Microbiol. 1986;32:942–946. doi: 10.1139/m86-174. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi Z. Laser applications in endodontics: an update review. Int. Dent. J. 2009;59:35–46. [PubMed] [Google Scholar]
- 23.Schwarz F. Aoki A. Sculean A. Becker J. The impact of laser application on periodontal and peri-implant wound healing. Periodontol 2000. 2009;51:79–108. doi: 10.1111/j.1600-0757.2009.00301.x. [DOI] [PubMed] [Google Scholar]
- 24.Sgolastra F. Petrucci A. Gatto R. Monaco A. Effectiveness of laser in dentinal hypersensitivity treatment: a systematic review. J. Endod. 2011;37:297–303. doi: 10.1016/j.joen.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 25.Goharkhay K. Schoop U. Wernisch J. Hartl S. De, Moor R. Moritz A. Frequency doubled neodymium:yttrium–aluminum–garnet and diode laser-activated power bleaching—pH, environmental scanning electron microscopy, and colorimetric in vitro evaluations. Lasers Med. Sci. 2009;24:339–346. doi: 10.1007/s10103-008-0567-x. [DOI] [PubMed] [Google Scholar]
- 26.Luk K. Tam L. Hubert M. Effect of light energy on peroxide tooth bleaching. J. Am. Dent. Assoc. 2004;135:194–201. doi: 10.14219/jada.archive.2004.0151. [DOI] [PubMed] [Google Scholar]
- 27.Smigel I. Laser tooth whitening. Dent. Today. 1996;15:32–36. [PubMed] [Google Scholar]
- 28.Pick R.M. Colvard M.D. Current status of lasers in soft tissue dental surgery. J. Periodontol. 1993;64:589–602. doi: 10.1902/jop.1993.64.7.589. [DOI] [PubMed] [Google Scholar]
- 29.Wolfgang B. Thomas A. External bleaching therapy withactivation by heat, light or laser–A systematic review. Dent. Mater. 2007;23:586–596. doi: 10.1016/j.dental.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Kato J. Hatayama H. Miyazaki H. Akashi G. Moriya K. Hirai Y. Surgical performance of a 405-nm diode laser in treatment of soft tissue. Laser Phys. Lett. 2008;5:316–320. [Google Scholar]
- 31.Yui K.C.K. Rodrigues J.R. Mancini M.N.G. Balducci I. Goncalves S.E.P. Ex vivo evaluation of the effectiveness of bleaching agents on the shade alteration of blood-stained teeth. Int. Endod. J. 2008;41:485–492. doi: 10.1111/j.1365-2591.2008.01379.x. [DOI] [PubMed] [Google Scholar]
- 32.Joiner A. Philpotts C.J. Alonso C. Ashcroft A.T. Sygrove N.J. A novel optical approach to achieving tooth whitening. J. Dent. 2008;36:S8–S14. doi: 10.1016/j.jdent.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Giusti G.V. Fatal poisoning with hydrogen peroxide. Forensic Sci. 1973;2:99–100. doi: 10.1016/0300-9432(73)90016-2. [DOI] [PubMed] [Google Scholar]
- 34.Kugel G. Aboushala A. Zhou X. Gerlach R.W. Daily use of whitening strips on tetracycline-stained teeth: comparative results after 2 months. Compend. Contin. Educ. Dent. 2002;23:29–34. [PubMed] [Google Scholar]
- 35.Miyazaki H. Kato J. Kawai S., et al. Surgical effects on soft tissue produced by a 405-nm violet diode laser in vivo. Laser Phys. 2011;21:2128–2131. [Google Scholar]
- 36.Gutknecht N. Franzen R. Meister J. Vanweersch L. Mir M. Temperature evolution on human teeth root surface after diode laser assisted endodontic treatment. Lasers Med. Sci. 2005;20:99–103. doi: 10.1007/s10103-005-0347-9. [DOI] [PubMed] [Google Scholar]