Abstract
Right ventricular (RV) function is a strong independent predictor of outcome in a number of distinct cardiopulmonary diseases. The RV has a remarkable ability to sustain damage and recover function which may be related to unique anatomic, physiologic, and genetic factors that differentiate it from the left ventricle. This capacity has been described in patients with RV myocardial infarction, pulmonary arterial hypertension, and chronic thromboembolic disease as well as post-lung transplant and post-left ventricular assist device implantation. Various echocardiographic and magnetic resonance imaging parameters of RV function contribute to the clinical assessment and predict outcomes in these patients; however, limitations remain with these techniques. Early diagnosis of RV function and better insight into the mechanisms of RV recovery could improve patient outcomes. Further refinement of established and emerging imaging techniques is necessary to aid subclinical diagnosis and inform treatment decisions.
Keywords: right ventricular function, right ventricular failure, pulmonary arterial hypertension, echocardiography, cardiac magnetic resonance imaging
The importance of the right ventricle (RV) in global cardiopulmonary function remains imperfectly understood and historically underappreciated despite increasingly detailed investigations of RV function as it applies to long-term outcomes and clinical trial endpoints.[1,2] This is highlighted by a recognized disconnect between therapeutic improvement in pulmonary pressures and long-term outcomes. In patients with pulmonary arterial hypertension (PAH), for example, long-term outcomes more closely follow RV function and reverse remodeling than reversal of pulmonary hypertension and vascular pathology.[1] In light of these findings, some have called for clinical trial endpoints to be more focused on measures of global RV function. Therefore, urgency exists for the development of clinically useful and universal definitions of RV function derived from both invasive and noninvasive means.
The RV is understudied in relation to its hemodynamic and prognostic importance in a number of distinct cardiac and pulmonary diseases. As we learn more about RV function, it is clear that it exhibits remarkable plasticity in its ability to recover after injury (for example, after RV myocardial infarction (RVMI), left ventricular assist device (LVAD) placement, and lung transplantation). This may reflect a combination of the unique physiology of the RV and genetic and neurohormonal modifiers of RV function. With the expansion of LVAD implantation and lung transplantation, there is an exigent need to reliably measure the functional reserve of the RV to help determine a patient's suitability for these procedures. A more reliable measure of the recovery capacity, or plasticity, of a dysfunctional RV would help clinicians predict which patients will have improved function after being “unloaded.” Although noninvasive imaging and invasive hemodynamic assessment are helpful, they are limited by the complexity of RV anatomy and the often dramatic load dependence of many indices of RV function as well as lack of widespread availability. Here we review the prognostic significance of RV function, its plasticity in the face of injury, and the unique physiology which contributes to those features. We also discuss the current noninvasive assessment of RV function, the limitations therein, and future directions for research.
DEVELOPMENT, GENETICS, AND ANATOMY
Unlike the other three chambers of the heart (derived from the primary heart field), the RV and both outflow tracts are derived from the anterior heart field with its own unique genetic pathways and chamber-specific transcription factors.[3,4]
The macroscopic anatomy of the RV includes three parts: the inlet, which includes the tricuspid valve and papillary attachments; the trabeculated apex; and the infundibulum. This arrangement results in a crescentic shape which contracts with a peristaltic “bellows” action. This contraction pattern is the result of muscle fibers arranged mostly longitudinally with lesser contributions from circumferential fibers and LV attachments to the RV free wall.[5,6] The complex shape of the RV has led to historical difficulty in creating models to determine RV volume, a necessary component of measuring function. Using contrast angiography and different derivations of the area-length method, the RV has been modeled as a parallelepiped, ellipsoid of revolution, triangular prism, and pyramid, all of which are less accurate than modern methods using cardiac magnetic resonance imaging (CMR) or three-dimensional echocardiography (3DE).[7,8]
During fetal development, the wall thickness and force generated by the RV and LV are equal. In the first year after birth, RV thickness regresses, increasing compliance. These changes, in concert with a low impedance pulmonary bed, allow the RV to produce the same cardiac output as the LV with one-fourth the stroke work and one-sixth the muscle mass.[1,9] This regression is not observed in patients with congenital heart disease associated with pulmonary hypertension, which may contribute to their relatively good outcome compared to patients with PAH.[10–12] Some have postulated, improved outcomes which may also be due to persistence of the fetal gene program (for example, a decrease in α-myosin heavy chain and an increase in fetal β-myosin heavy chain gene expression).[13]
The RV is subject to unique neurohormonal signaling as indicated by increased RV gene expression of endothelin-1 and endothelin receptors in RV tissue in a rat model of PAH.[14] This observation may explain in part why endothelin receptors lead to improved exercise capacity and reverse RV remodeling in PAH patients but no such improvements in patients with left heart failure.[15,16] In addition, patients with RVH of disparate etiologies show upregulation of phosphodiesterase type 5 mRNA and protein in the RV myocardium which is not present in the normal RV.[17]
The unique embryological, genetic, and neurohormonal processes that occur in the RV serve as the basis for the development of RV-specific therapies. It has also led to the hypothesis that there exists a “permissive genotype” that leads to early failure of some RVs while others tolerate the same hemodynamic conditions with impunity. One piece of supporting evidence for this hypothesis lies in the angiotensin-converting enzyme DD polymorphism. Among patients with PAH and equal PA pressures, those with DD polymorphism had normal RAP pressure and cardiac output whereas non-DD patients had elevated RAP and decreased cardiac output;[18] however, these findings have not been replicated to date.
RV PHYSIOLOGY AND PLASTICITY
Global RV function is determined by a dynamic combination of preload, afterload, and contractility. Preload is determined by volume status, tricuspid valve (TV) gradient, and return from the vena cava. RV afterload is a combination of resistance at the level of the pulmonary valve (usually negligible), pulsatile flow reflected from the main pulmonary arteries (PAs) and early bifurcations, impedance of the proximal PAs, and arterioles (pulmonary vascular resistance, PVR). Using PVR as a surrogate for total RV afterload, therefore, may be inaccurate, especially in patients with non-compliant pulmonary vasculature (as in PAH) or proximal disease involvement such as with chronic thromboembolic pulmonary hypertension (CTEPH). In clinical practice, contractility of the RV, theoretically independent of loading conditions, reflects dynamic changes in preload and afterload, calcium loading, heart rate, adrenergic state, pharmacologic milieu, and ventricular interdependence.
It is important to make a distinction between RV dysfunction and RV failure. RV dysfunction may occur in the absence of clinical signs or symptoms. RV failure implies clinical sequelae as a result of RV dysfunction. This distinction is critical because one of the goals of RV imaging is to detect RV dysfunction before irreparable damage develops.
Under resting conditions, metabolic demand is low compared to supply. Compared to the LV, the RV has a lower mass as well as lower preload and afterload, resulting in lower overall oxygen demand.[9] These circumstances allow the RV to produce the same cardiac output as the LV with one-fourth the stroke work and one-sixth the muscle mass.[9] As a result, at times of stress, the RV is better prepared for increasing oxygen extraction.[19,20] RV physiology creates a favorable situation to maintain RV perfusion. As much as one-third of the RV free wall is partially perfused by left coronary artery branches creating a dual blood supply.[21] The thin RV wall results in lower intramyocardial and intracavitary pressures allowing coronary flow throughout the cardiac cycle. Further, a transcoronary gradient from left to right favors the formation of acute collateral vessels during RV ischemia.[22] This may explain why patients with isolated RV infarcts often do not experience angina.[23]
Like the LV, the RV tolerates volume overload better than pressure overload. Isolated volume-overload states such as tricuspid regurgitation or an atrial septal defect can be sustained for years without a measureable decrement in RV function, though emerging evidence suggests an increase in morbidity and mortality with untreated tricuspid regurgitation.[24] The RV exhibits greater sensitivity to increases in afterload. While acute pressure overload (as in massive pulmonary embolism) results in failure of RV compensation and cardiovascular collapse, chronic pressure overload leads to RV dilation, a decrease in cardiomyocyte density, and an increase in connective tissue.[25,26] In the setting of normal pulmonary resistance, RV failure rarely occurs. In the unconditioned RV, small increases in pulmonary pressure result in dramatic decreases in systemic pressure and cardiac output.[27] Modest increases in right atrial pressure can generate an adequate transpulmonary gradient to maintain cardiac output.[28] In the unconditioned RV, small increases in pulmonary pressure result in dramatic decreases in system pressure and cardiac output.
Ventricular interdependence (VI) has critical hemodynamic implications in patients with right heart failure, particularly due to pressure overload or RV infarction. The septum is shared by both ventricles and normally bows toward the RV. When the RV dilates, the septum moves toward the LV and can dramatically affect LV filling compromising cardiac output (Fig. 1). Up to one-third of RV stroke work is performed by septal contraction, and when RV dysfunction is severe LV septal contraction into the RV can compensate by providing pulmonary perfusion.[29] RV contractility improves with increased systemic mean arterial pressure (and thus peak-developed LV pressure), likely due to increased coronary perfusion pressure. Inactivation of septal contraction decreases peak-developed RV pressure by one-half, indicating the important contribution of LV septal contraction to RV function.[30] RV enlargement decreases pericardial compliance, placing constraint on diastolic filling of both ventricles. Decreasing RV afterload or improving RV systolic function can result in the movement of the septum back toward the RV and resolution of deranged hemodynamics.
Figure 1.
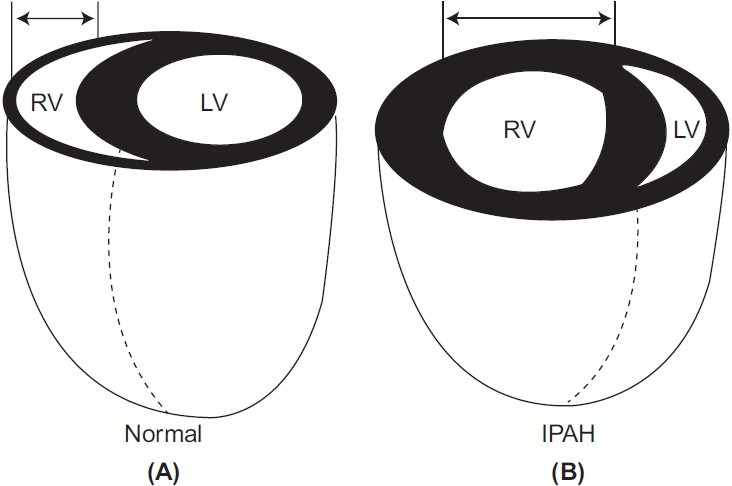
Reproduced with permission from Voelkel et al.[1] RV dilation changes LV geometry decreasing LV preload and worsening diastolic function. If acute, RV dilation may contribute to pericardial constraint, limiting filling of both ventricles. IPAH = idiopathic pulmonary arterial hypertension; LV = left ventricle; RV = right ventricle.
NONINVASIVE ASSESSMENT OF RV FUNCTION AND HEMODYNAMICS
Invasive and noninvasive assessment of RV function and pulmonary hemodynamics provides a wealth of information but is limited by inherent characteristics of the RV including retrosternal position, complex geometry, and load dependence of many measurements. Invasive measurements are more accurate by virtue of direct measurement but come with the attendant risks of catheter-based procedures. Due to morphological considerations discussed above, multiple tomographic views are necessary to obtain a complete appraisal of RV structure and function. Unlike the LV in which the biplane Simpson's method is generally accepted as a global assessment of systolic function, no single parameter is considered a sufficient global measure of RV function. Cardiac MRI is emerging as a valuable tool in the assessment of RV and pulmonary vascular function and is considered the gold standard for measurement of RV volume.[31] A proposed algorithm for the assessment of RV function is found in Table 1.
Table 1.
Proposed algorithm for the assessment of RV function

Echocardiography
Echocardiography is favored as a screening test for RV dysfunction and pulmonary hypertension due to ease of acquisition and reproducibility. Technical aspects of the RV echocardiographic assessment, including normal values, acquisition tips, and limitations are found in Table 2. Examples of important parameters of RV function are found in Figure 2. Representative echocardiographic findings in RV dysfunction are found in Figure 3.
Table 2.
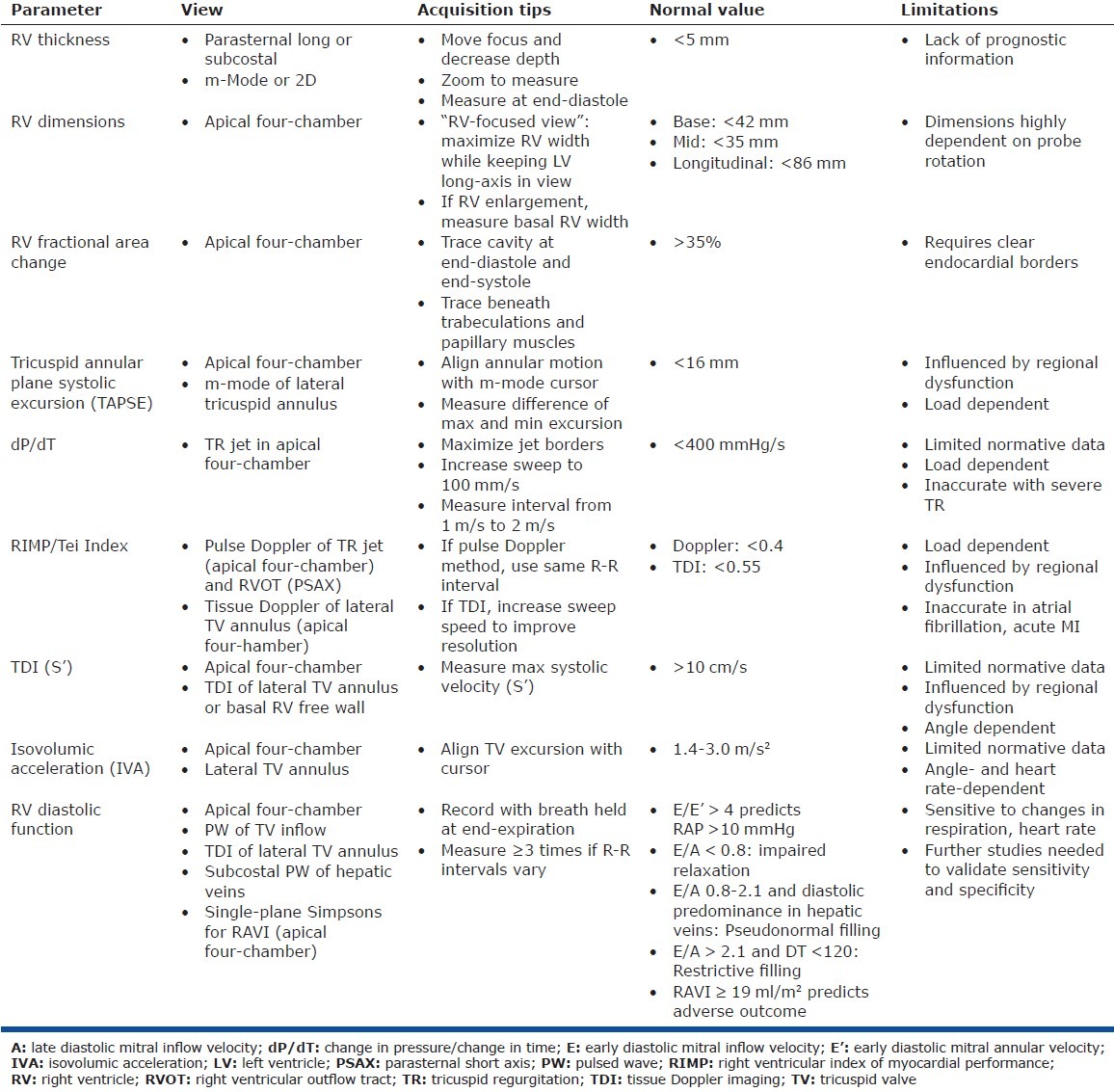
Figure 2.

Reproduced with permission from Mertens and Friedberg.[180] (A) %FAC, calculated from measures of the apical four-chamber view. The importance of longitudinal shortening can be appreciated in this image. (B) MPI, calculated by measuring the ejection time on the pulmonary artery tracing and the time between closure and opening of the tricuspid valve on the tricuspid inflow tracing. MPI = (TCOT - ET)/ET. In this patient, the MPI was normal after tetralogy of Fallot repair. (C) TAPSE. An M-mode echocardiogram through the tricuspid annulus is obtained and the excursion of the tricuspid annulus is measured as illustrated. This index enables assessment of longitudinal RV function. (D) Tissue Doppler velocities of the tricuspid annulus. Pulse tissue Doppler measurements can be used to calculate tissue velocities. Systolic velocities can be used as a parameter for systolic longitudinal RV function. (E) Longitudinal strain measurements of the right ventricle, made using speckle tracking technology. By convention, systolic longitudinal shortening is represented as a negative value and can be measured in six different segments. The mean values of these segments are used to trace a mean longitudinal strain curve (white dotted line). The value at end-systole is then measured. (F) Color tissue Doppler echocardiogram at the lateral tricuspid valve annulus and measurement of IVA. Aortic valve opening and closure are depicted by green lines for event timing. The timing of these events may be taken as that of pulmonary valve opening and closure. The slope of IVA is shown. Note that IVA occurs within the QRS complex and peaks before pulmonary valve opening in the isovolumic period. A’= late-diastolic tissue velocity; AVC = aortic valve closure; AVO = aortic valve opening; E’ = early-diastolic tissue velocity; ET = ejection time; %FAC = percentage fraction area change; IVA = isovolumic acceleration; MPI = myocardial performance index; S’ = systolic tissue velocity; TAPSE = tricuspid annular systolic plane excursion; TCOT = tricuspid valve closure-opening time.[179]
Figure 3.
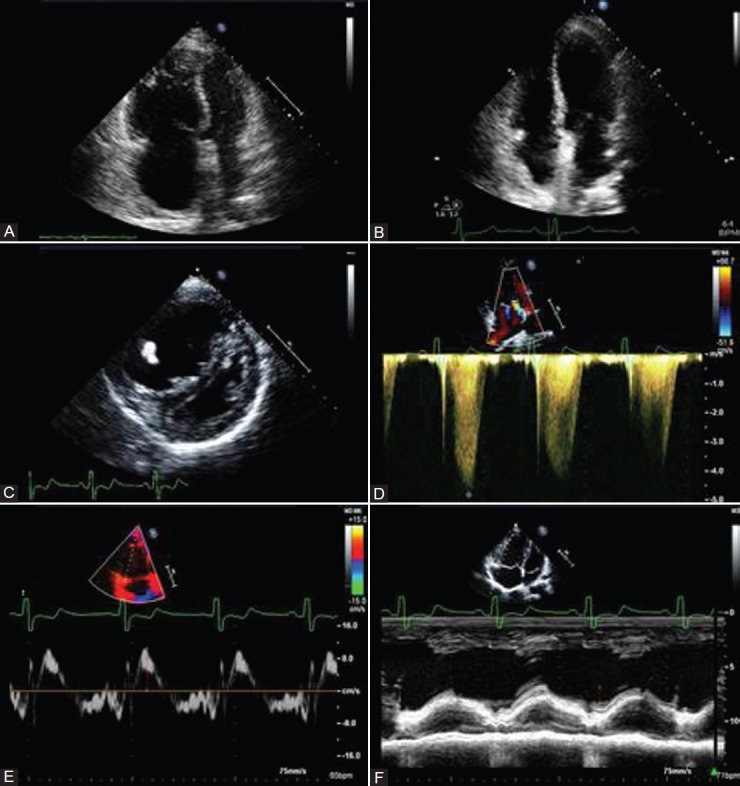
(A) RV enlargement and hypertrophy in patient with CTEPH. (B) Normal RV size 6 months after PTE in the same patient as in A. (C) Parasternal short-axis view demonstrating RV dilation and hypertrophy and displacement of septum. (D) Tricuspid regurgitation jet demonstrating severe PH in patient with PAH. (E) Peak systolic velocity of tricuspid annulus borderline depressed at 10 cm/s. (F) TAPSE in patient with PAH measured at 14 mm consistent with RV hypokinesis.
The accuracy of echo-derived PASP is variable when referenced to right-heart catheterization.[32] This variability precludes the use of echo as a sole diagnostic modality, and, as such, all patients with suspected PH should undergo invasive testing if such a diagnosis will change the patient's management.
In patients without intrinsic lung disease, Doppler estimation of PASP has a fair correlation with invasive measurements.[33,34] In patients with intrinsic lung disease, however, the accuracy of DE estimation was found to correlate poorly.[35,36] This discrepancy may be explained by sub-optimal visualization of the TR jet in patients with lung disease whose chests are hyperinflated and whose hearts are rotated rightward. In a study of 65 patients with various forms of PH, Fisher et al. found that DE estimated PASP accurately in only 48% of patients, over- and under-estimating PASP in the remaining patients equally.[37] The mean pulmonary artery systolic pressure can be estimated by Doppler as well and used to corroborate the Doppler estimate of PASP. The time to peak velocity of PW Doppler in the RV outflow tract (RVOT; pulmonary acceleration time, PAC) of <70 ms indicates a high likelihood of mPAP > 40 mmHg whereas PAC > 100 ms indicated that PH is unlikely.[38] The presence or absence of PH should not be diagnosed based on sub-optimal TR signals, and PASP should not be used in isolation to make a diagnosis of pulmonary hypertension (PH) or guide management decisions.
The interaction between RV function and pulmonary hemodynamics and the concept of RV-PA coupling is becoming increasingly important to our understanding of RV dysfunction. As discussed above, PVR is an imperfect surrogate for total RV afterload but the gold standard, pulmonary input impedance, cannot currently be assessed noninvasively. PVR can be qualitatively estimated by the formula ([TR velocity/RV outflow tract velocity - time integral] × 10 + 0.16) which accurately stratifies patients with high- and low-PVR. A cutoff value of 0.175 predicts PVR > 2WU with a sensitivity and specificity of 77% and 81%, respectively,[39] but is less accurate at PVR > 8WU.[40]
While echocardiographic measurement of RV ejection fraction is unreliable and not recommended by the American Society of Echocardiography, there are a number of other ways to assess RV systolic function.[41] The most basic is the fractional area change (FAC) in which endocardial borders are traced at end-systole and end-diastole and a per cent area change is calculated. FAC correlates with RV ejection fraction (EF) measured by MRI and predicts heart failure and stroke in patients with pulmonary embolism and MI.[42–44]
The RV index of myocardial performance (RIMP) or Tei index is a Doppler or tissue Doppler-derived measure of global function defined as the sum of isovolumic relaxation and contraction times divided by the ejection time. A normal RV has essentially no isovolumic contraction time (IVCT). Equations for pulse Doppler and tissue Doppler-derived RIMP are in Table 2. RIMP is thought to be preload independent and accurate across most physiologic heart rates but may be falsely low when RA pressure is elevated due to shortening of the isovolumic contraction time.[45] RIMP is prognostically valuable in patients with PH, RVMI, and congenital heart disease.[46–48]
One of the most widely studied indices of RV function is the tricuspid annular plane systolic excursion (TAPSE), an M-mode measurement that takes advantage of the fact that most of the RV output is produced by longitudinal (base to apex) contraction. Thus, low TAPSE indicates low contractile motion within the RV. It is easily and reproducibly measured on M mode and is prognostic in a number of cardiac and pulmonary diseases in 46 different studies.[41,49] Limitations include angle dependence and the assumption that basal RV function reflects global function.
Tissue Doppler imaging (TDI) of the RV measures regional myocardial velocity. Pulsed TDI can be used to measure the velocity of myocardial excursion (S’) at the tricuspid annulus or lateral RV basal segment. It has been validated in a population-based study with a wide age range and discriminates normal and abnormal EFs well when compared to radionuclide angiography.[41,50] Like TAPSE, TDI is angle-dependent and extrapolates basal RV function to assume global function.
Myocardial acceleration during isovolumic contraction (IVA) is considered the least load-dependent index of RV contractility by echocardiography. It is derived by dividing the peak isovolumic velocity by the time to reach peak velocity as measured by TDI at the lateral tricuspid annulus. IVA is a prognostic marker in patients with OSA, mitral stenosis, and congenital heart disease.[51–53] The normal value for IVA varies with age and is unaffected by wide changes in preload and afterload.[54]
Strain and strain rate are emerging echocardiographic technologies which quantify myocardial deformation. These techniques are relatively load independent and can provide both regional and global assessment of RV function.[55] The utility of strain analysis to detect subclinical RV dysfunction and predict outcomes in patients with PH has recently been demonstrated.[56,57] Current limitations include lack of normal values and the requirement of offline software for analysis; however, when further refined, these techniques hold promise for the routine detection of early and subclinical RV systolic dysfunction.
Three-dimensional echocardiography to measure RV volumes and function correlates well with MRI measurements in a number of studies, particularly those using semi-automated border detection,[58–62] but recent studies found systemic under-estimation of RV volumes and EF on 3DE.[63,64] ASE recommendations currently limit the use of 3DE to serial measurements, but as normative data accumulate and analysis software improves 3DE will become a practical tool for assessment of RV size and function.[41]
RV diastolic function is increasingly recognized as having a significant hemodynamic influence in a number of cardiopulmonary diseases.[41] It is easily assessed using pulsed Doppler of TV inflow, TDI of the lateral tricuspid annulus, and Simpson's method of RA volume. Measurements should be taken at end-expiration and may be inaccurate in patient with atrial fibrillation. Given the load dependence of RV function, diastolic parameters may be affected by heart rate, respiration, and loading conditions.[65–67] In a multivariate model, RA volume index strongly predicted a composite of death, transplant, or heart failure admission.[68] RV E/E’ predicts elevated RA pressure with high sensitivity and is a prognostic marker in patients with secondary pulmonary hypertension.[69,70] Notably, RV diastolic function has repeatedly been shown to predate clinical RV systolic dysfunction. With further refinement of RV diastolic parameters, this may represent a screening tool in patients at risk of developing RV dysfunction.
Cardiac MRI
With advances in image acquisition protocols, cardiac MRI (CMR) is emerging as the technique of choice in the assessment of RV structure and function (Table 3). Representative CMR findings in RV dysfunction are found in Figure 4. Volumes are usually obtained and images oriented in short-axis stacks from which endomyocardial contours are traced at end-diastole and end-systole. Because up to 30% of the RV volume is contained in the infundibulum, this structure should be included in volume measurements. Myocardial mass is calculated from tracing epicardial and endocardial borders and multiplying by myocardial density. Because of its good spatial and temporal resolution, CMR-derived volumes and EF are increasingly considered the gold standard against which other imaging modalities are compared.[71] The MESA-RV study recently demonstrated significant differences among age, race, and sex in values of RV mass, RV volume, and RVEF; the clinical implications of these differences is unknown.[72] Intra-observer and inter-observer variability in RV measurements ranges from 3% to 6% and 4% to 9%, respectively.[73,74] Valvular regurgitant volumes and shunt severity can be calculated with precision using velocity-encoded cine images.[75]
Table 3.
RV morphology and functional MRI parameters

Figure 4.
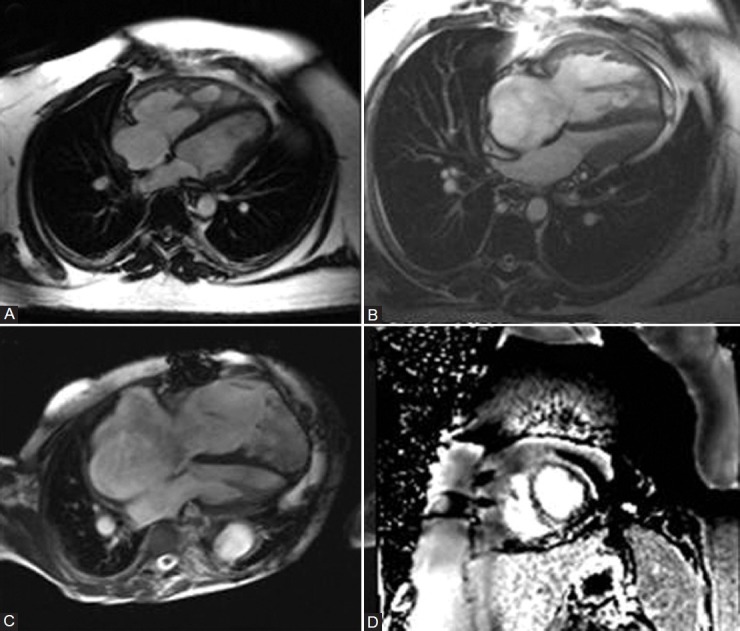
(A) Mild RV and RA dilation with RV hypertrophy and prominent hypertrophy of moderator band. (B) Severe RV and RA dilation with RVH and compression of left-sided chambers by septal deviation. (C) End-stage RV failure with marked RV and RA dilation and thinned out RV free wall. (D) Late gadolinium-enhanced short-axis image demonstrating fibrosis at the RV septal insertion point.
Tissue characterization is another advantage of CMR over echocardiography. In arrhythmogenic RV cardiomyopathy (AVRC), MRI can detect dysplastic areas of myocardial thinning, wall motion abnormalities, and fibrofatty replacement; however, fatty infiltration is seen in normal adults and can be obscured by epicardial fat, limiting the utility of MRI in this aspect of diagnosis.[76] Gadolinium enhancement of myocardial fibrosis may be more sensitive than Task Force Criteria for the detection of AVRC carrier-gene status, but is not currently a diagnostic criterion.[77,78] The identification of RV fibrosis on MRI is also of value in patients after Tetralogy of Fallot repair in whom the degree of fibrosis correlates with global RV function and risk of ventricular arrhythmias.[79] The extent of myocardial fibrosis also correlates with RVEF in PAH and survival after RVMI.[80,81] Cardiac MRI is particularly valuable in assessing diseases which involve the RV including arrhythmogenic RV dysplasia, response to therapy in PAH, congenital heart disease with shunt lesions, differentiating constrictive and restrictive physiology, and diagnosing RVMI by late gadolinium enhancement.[78,81–83] Evolving clinical applications include strain analysis using myocardial tagging and diastology assessment using phase-contrast CMR to measure deceleration time and IVRT.[84,85]
Metabolic imaging
Just as tomographic imaging techniques have improved our understanding of RV function, so has molecular imaging advanced the concepts of a metabolic phenotype and metabolic remodeling. PET imaging allows measurement of the relative use of glucose (FDG) and fatty acids (C-11 palmitate as surrogate) as myocardial energy substrates. In the RV in PAH, there is a well-described switch from predominant fatty-acid oxidation to glycolysis as a source of fuel,[86] which, although less efficient, may produce a cellular survival advantage similar to cancer cells.[87] In patients with PAH, FDG accumulation in the RV free wall correlates with PVR and mean PAP.[88,89] After three months of epoprostenol, FDG uptake decreased significantly in responders in proportion to reduction in PVR.[88] Increased RV FDG uptake correlates with RV dysfunction in patients with left heart failure,[90] though the response to treatment is unknown. PET may thus allow assessment of RV-specific metabolic therapies.
Magnetic resonance spectroscopy is an emerging tool which can quantify intracellular triglyceride content. Myocardial lipid accumulation has been observed in diabetics and patients with dilated cardiomyopathy.[91,92] In diabetics, the degree of triglyceride content strongly correlates with RV systolic and diastolic dysfunction as assessed by strain and strain rate.[92]
ASSESSMENT AND PROGNOSTIC IMPLICATIONS OF RV FUNCTION IN SPECIFIC DISEASE STATES
In many cardiopulmonary diseases, RV function is an independent prognostic predictor (Table 4). Right heart dysfunction is associated with poor outcomes in patients with valve disease, congenital heart disease, ischemic and dilated cardiomyopathy, pulmonary embolism, post-cardiac transplantation, post-LVAD implantation, and post-valve surgery.[93–102] In patients with PAH, outcomes parallel RV function more so than PA pressure, in part because PA pressure falls as RV dysfunction becomes severe (Fig. 5).[103,104] Conversely, preserved RV function even in the setting of elevated PA pressure is associated with improved survival, decreased hospitalization, and improved exercise capacity in patients with chronic heart failure.[94,105]
Table 4.
Prognostic importance of right ventricular function in various cardiopulmonary diseases
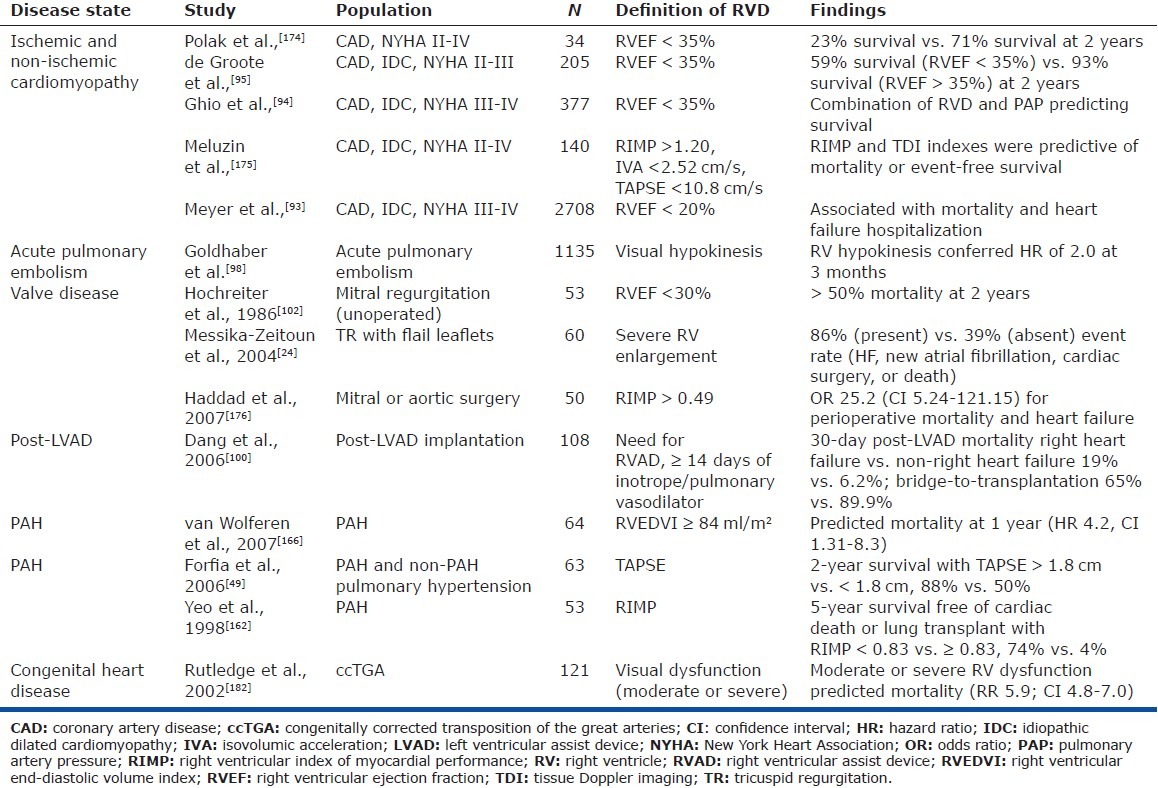
Figure 5.
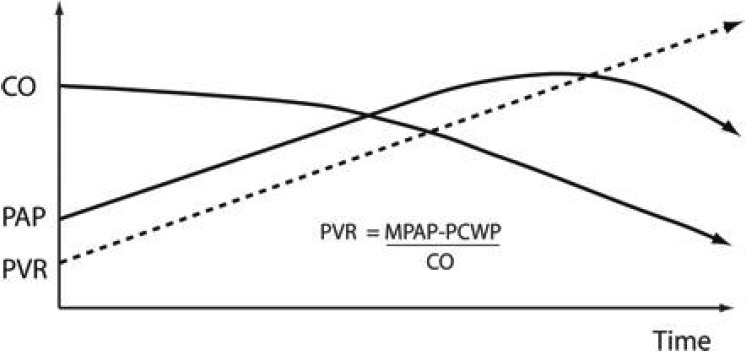
Reproduced with permission from Haddad et al.[181] A decrease in pulmonary arterial pressure in patients with PH may indicate low cardiac output and severe right ventricular failure. CO = cardiac output; MPAP = mean pulmonary artery pressure; PAP = pulmonary artery pressure; PCWP = pulmonary artery capillary wedge pressure; PVR = pulmonary vascular resistance.[180]
EVIDENCE FOR RV PLASTICITY AND IMAGING
In a number of diseases, the RV can sustain damage and recover to a remarkable degree. This plasticity should serve as the impetus to better understand RV physiology and pathology and to develop clinical techniques to help predict whether or not an individual RV will recover from insult. With this knowledge, for example, clinicians could exercise more parsimony in referral for LVAD or lung transplantation and better prognosticate after RVMI. In the absence of a tool to predict RV recovery, early diagnosis of RV dysfunction and appropriate therapy remain paramount.
Table 5 describes useful functional and morphologic parameters assessed by echocardiography or CMR in each of the diseases discussed below.
Table 5.
Imaging in specific disease states

Right ventricular MI
RV myocardium is involved in up to 50% of inferior myocardial infarctions (MI) and ischemic RV dysfunction is associated with increased morbidity and mortality.[106–109] Despite this high prevalence, long-term damage to RV function after RVMI is exceedingly rare.[106] Acute hemodynamic compromise may result during RV ischemia due to cavity dilation, diastolic and systolic dysfunction, ventricular interactions, and arrhythmia.[22,27,29,110] Even if revascularization is not performed, hemodynamic improvement usually manifests within 3-10 days and RV function returns to normal within several weeks.[111–114] If culprit artery revascularization is achieved expeditiously, short-term dysfunction is infrequent and the detrimental effects of RV dilation on left ventricular diastolic filling can be mitigated.[22,115] Laster and colleagues showed in a canine model that preservation of RV function after RCA occlusion is in part due to favorable development of collateral circulation to the RCA territory.[22] In some patients with chronic RCA occlusion, RV function may be preserved at rest but exhibit significant dysfunction on stress echocardiography.[116] Long-term outcomes in patients with RVMI likely depend primarily on the degree of concomitant LV dysfunction.[117]
While echocardiography in acute MI is most informative for assessing LV function, evidence of RV dysfunction adds prognostic value and can inform treatment decisions. Visual assessment of RV free wall motion by experienced readers is a sensitive qualitative measure of RV function and can serve to confirm or provoke clinical suspicion of RV involvement.[112] RV dilation and septal bowing can be seen on echocardiography and the inter-atrial septum may bow leftward due to increased right atrial pressure transmitted from the RV. As outlined in Table 5, a number of quantitative measures of RV function have been shown to be diagnostic or predictive of adverse outcomes. More quantitative measures of RV function such as decreased TAPSE and lateral tricuspid annular velocity on TDI indicate RV involvement in acute MI.[118,119] Antoni et al. studied 621 patients within 48 hours of acute MI and found that RV FAC (≤32%), TAPSE (≤15 mm), and RV strain (≤-22.1%) independently predicted mortality, reinfarction, and hospitalization at 2 years follow-up.[120] RIMP was found to have a sensitivity and specificity of 94% and 80%, respectively, for the diagnosis of RVMI.[121]
Advanced techniques such as strain imaging and 3D echocardiography may eventually prove more accurate than current 2D methods at detecting RV dysfunction. CMR has evolved as the gold standard for assessing RVEF and morphology; however, in the setting of acute MI it is currently limited to post-reperfusion assessment of systolic function and scar burden.
Post-lung transplantation and post-thromboendarterectomy
There are two interesting experiments of nature that demonstrate the plasticity of the RV in response to correction of pulmonary vascular disease: lung transplantation and pulmonary thromboendarterectomy (PTE) for chronic thromboembolic pulmonary hypertension (CTEPH). In both cases, the cause of elevated PVR is removed and thus load stress is essentially eliminated.
In patients with end-stage pulmonary hypertension, heart-lung transplantation was initially felt to be the only viable surgical option due to profound RV dilation and dysfunction.[122] Based on observations of significant RV functional recovery in patients with chronic thromboembolic disease who underwent thromboendarterectomy, lung transplantation alone has been pursued with success.[123,124]
In the immediate post-operative period, RV function may not improve,[125] but RVEF and PA systolic pressure (PASP) normalize within two to three months post-lung transplant; however, LV recovery may take up to a year.[126,127] Toyooka et al. showed similar results in 33 patients in whom RV function normalized within two months but LV functional recovery was delayed for 6-12 months after living-donor lobar lung transplantation.[127] Echocardiography in post-lung transplant patients frequently demonstrates evidence of marked RV reverse remodeling: RV and right atrial (RA) size decrease, the tricuspid valve annulus shrinks with a decrease in TR, and the interventricular septum resumes its normal position.[25,128–130] Residual elevation in pulmonary vascular resistance with a single lung transplant is frequent and may be accompanied by some degree of post-operative RV dysfunction.[25,128,130] Magnetic resonance imaging (MRI) in post-lung transplant patients indicates that while RV performance is improved in the short term, reduction in RV mass can take up to one year, demonstrating the utility of MRI as a complementary tool for assessing RV remodeling.[131]
CTEPH causes increased RV afterload, RV hypertrophy, and dilation as well as a shift of the IVS toward the left decreasing LV filling and cardiac output.[132,133] Preoperative findings on CMR or echocardiography are virtually indistinguishable from other forms of elevated pulmonary vascular disease.[132,134] Successful PTE results in dramatic improvements in mean pulmonary pressure, PVR, and cardiac output.[135] Reverse remodeling can be seen on echocardiography and MRI as evidenced by reduction in RV chamber size and mass, improvement of RV systolic and diastolic function to near normal, and a decrease in tricuspid regurgitation (Fig. 3).[136–140]
Post- LVAD implantation
RV dysfunction is both an obstacle to LVAD placement and an independent prognostic indicator. Right heart failure occurs in 15-20% of patients after LVAD implantation and confers a worse outcome.[141,142] In the largest randomized trial to date, post-operative RV failure was less frequent with a continuous-flow device (Heart Mate II) compared to a pulsatile device (Heart Mate I).[143]
LV unloading may favorably alter RV function through decreased RV afterload and normalization of the neurohormonal milieu characteristic of heart failure.[144–146] LV unloading can adversely affect RV function as well. A leftward shift of the interventricular septum distorts RV geometry and leads to depressed RV contractile efficiency as well as increased tricuspid regurgitation. RV preload also increases following LVAD implantation, which may further exacerbate RV failure.[147]
Despite significant unloading, the degree of RV remodeling post-LVAD implant is unclear. Explanted hearts of patients with pulsatile LVAD support showed no evidence of RV remodeling as measured by volume reduction, myocyte diameter, force-frequency relationship, and SERCA2a content.[148] However, improvement in RV mass, myocyte size, and RV end-diastolic pressure-volume relationship was observed with biventricular devices compared to no or left ventricle only support.[149] In a series of continuous-flow LVADs, pre-existing RV dysfunction remained stable over 4.5 months despite a decrease in right-sided pressures and chamber size.[150] These findings suggest that reduction in RV afterload must be coupled with preload reduction for RV reverse remodeling to occur.
Accurate assessment of RV function with echocardiographic and hemodynamic assessment prior to LVAD implantation is crucial. Qualitative assessment of RV contractility in the apical four-chamber and RV inflow views provides a general sense of RV function. Quantitatively, preimplant FAC, RV-to-LV end-diastolic diameter ratio (R/L), and TAPSE predict post-implant RV failure.[151] The duration of TR corrected for heart rate (TDRc = TR flow time/√RR-interval) predicts RV failure and mortality in patients with a Heart Mate II LVAD. In a study of 83 patients from the Mayo Clinic implanted with the Heart Mate II LVAD demonstrated that the TDRc predicted right heart failure and mortality. Patients with a TDRc ≤ 461 ms had a significantly lower 1- and 2-year survival compared with patients with TDRc ≥ 461 ms.[147] Recently, S’ and leftward deviation of the septum at 30-day post-implant were associated with adverse outcomes.[152] RVFAC < 20% has been shown to predict right heart failure following LVAD implant.[147] Puwanant et al. reported that tricuspid annular motion < 7.5 mm predicts RV failure following LVAD with a sensitivity of 91% and specificity of 46%.[153] Finally, it is important to assess the severity of tricuspid regurgitation as valves with more than moderate regurgitation require operative repair or replacement at the time of LVAD implant.
The same protocol and parameters to determine RV function pre-implant should be utilized and assessed post-implant. Right atrial and inferior vena cava size can be used to estimate right atrial pressure, and the estimated RVSP can be monitored in patients with pre-implant pulmonary hypertension. Ramped speed studies are useful to optimize LVAD rotor speed by determining the speed with optimal LV unloading while maintaining a midline interventricular septum.[147]
Pulmonary arterial hypertension
Evidence of RV plasticity in the surgical management of PAH is discussed in the post-lung transplantation. In regard to medical therapy, the addition of sildenafil to bosentan therapy in PAH resulted in a significant decrease in RV mass and increase in cardiac index compared to bosentan alone.[154,155] Other studies have confirmed that RV hypertrophy can regress and RV function can improve acutely on sildenafil.[156,157]
Although echocardiography is the most commonly used modality for assessment of the RV in PAH, superior quantitative abilities of CMR with respect to the RV have driven interest in this technique both for the diagnosis of PAH and for the determination of response to therapy. Nevertheless, because of widespread availability and challenges with pumps associated with prostacyclin delivery and cost, echocardiography remains most widely used. Familiarity with both techniques is essential in the assessment of PAH.
The primary role of imaging in suspected pulmonary hypertension is determination of the severity of RV compromise, not subcategorization of pulmonary hypertension (e.g., PAH vs. pulmonary venous hypertension [PVH] vs. CTEPH).[158,159] Conformational changes in the RV include RV and RA dilation,[2] RV wall and moderator band hypertrophy, reduced RV fractional area change,[160] and septal bowing to the left ventricle.[161] As functional assessments, TAPSE and RIMP are broadly useful in all forms of PH and have been shown in PAH specifically to correlate with survival.[49,162] RV strain imaging has recently been shown to correlate with survival and RVEF measured by CMR in PAH patients and will likely become more clinically useful as normative data emerge.[56,57]
The shape of the RV outflow tract Doppler envelope has been demonstrated to discriminate patients with high pulmonary vascular resistance from those without,[163] with mid-systolic notching indicating high PVR and uncoupling between the RV and PA. As described earlier, PVR can be estimated as greater or less than 2WU based on the ratio of the peak TR velocity to velocity-time integral of the RVOT.[39]
CMR allows accurate assessment of RV mass, volume, and EF which have been shown to correlate with disease severity and death in various types of PAH.[82] CMR has also allowed more specific determination of regional abnormalities in PH including patterns of myocardial delayed enhancement. The degree of late gadolinium enhancement (LGE) at the RV septal insertions, thought to be due to local fibrosis,[80,164] has been observed in patients with scleroderma-associated PAH and IPAH to correlate inversely with RVEF.[165] RV mass strongly predicts the presence or absence of PAH in patients with scleroderma, while increased RV volume and decreased LV volume predicts death in IPAH.[165,166] Recent evidence using adenosine stress CMR demonstrated that reduced biventricular myocardial perfusion reserve correlates with RVEF in PAH, suggesting role of ischemia in RV dysfunction in these patients.[167] In both conditions, RV mass has been studied as a predictor of death. While in idiopathic PAH RV mass was not found to predict death, it is a strong predictor of death in scleroderma-associated PAH. In idiopathic PAH, the primary CMR predictors of death are large RV volume, low stroke volume, and reduced LV volume. In echocardiographic analysis, TAPSE remains an important marker of poor prognosis in both idiopathic and scleroderma-associated PAH.
Unfortunately, there are few imaging techniques for diagnosis of PH subtype, particularly discerning PAH from PVH. Recently, the shape of the RV Doppler envelope has been demonstrated to discriminate patients with high pulmonary vascular resistance from those without.[163] The flow velocity envelope of the RV outflow tract is altered in response to pathologic wave reflection in the setting of increased pulmonary arterial impedance; mid-systolic wave reflection is present in elevated pulmonary vascular resistance while normal notching pattern or late systolic notching is present in lower PVR patients.[39] The primary limitation of both echocardiography and CMR is their inability to accurately measure pulmonary arterial pressures, cardiac output, or pulmonary artery occlusion pressure, essential features in the diagnosis and assessment of pulmonary vascular disease.[32,37,134] Until better diagnostic modalities or techniques are available, current recommendations are to obtain right heart catheterization in order to confirm etiology of pulmonary hypertension as this cannot be reliably discerned through echocardiography, CMR, or other noninvasive imaging techniques[134,168]
Once PAH is confirmed, little is known about differences in RV imaging between the various subtypes, including heritable, idiopathic, connective-tissue disease-associated, or associated with congenital left-to-right shunt. There is a clear indication for CMR in the diagnosis of congenital heart disease-associated PAH, but the utility of echocardiography or CMR for the other subtypes is under investigation. Since idiopathic and scleroderma-associated PAH make up the bulk of patients, these are the primary subgroups studied, though rarely compared.
CONCLUSION AND FUTURE DIRECTIONS
RV dysfunction is a strong contributor to poor outcomes across a broad range of cardiopulmonary diseases, and clinical appreciation of this fact is widening. The RV displays remarkable resiliency in the face of insult, at least in part due to its unique physiology. The development of noninvasive imaging techniques to assess RV function is growing apace with investigation into genetic and neurohormonal modifiers. A biomarker profile diagnostic or predictive of RV failure would also be valuable as a routine part of clinical assessment. Given the dynamic beat-to-beat changes in RV loading conditions, imaging techniques that are load independent should be the focus of future advances. Development of exercise imaging protocols may also shed light on RV functional reserve and help predict response to therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, et al. Right ventricular function and failure: Report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–91. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 2.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: State of the art and clinical and research implications. Circulation. 2009;120:992–1007. doi: 10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- 3.Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circ Res. 2004;95:261–8. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]
- 4.McFadden DG, Barbosa AC, Richardson JA, Schneider MD, Srivastava D, Olson EN. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- 5.Ho SY, Nihoyannopoulos P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart. 2006;92(Suppl 1):i2–13. doi: 10.1136/hrt.2005.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kukulski T, Hubbert L, Arnold M, Wranne B, Hatle L, Sutherland GR. Normal regional right ventricular function and its change with age: a Doppler myocardial imaging study. J Am Soc Echocardiogr. 2000;13:194–204. doi: 10.1067/mje.2000.103106. [DOI] [PubMed] [Google Scholar]
- 7.Sheehan F, Redington A. The right ventricle: anatomy, physiology and clinical imaging. Heart. 2008;94:1510–5. doi: 10.1136/hrt.2007.132779. [DOI] [PubMed] [Google Scholar]
- 8.Sheehan FH, Bolson EL. Measurement of right ventricular volume from biplane contrast ventriculograms: Validation by cast and three-dimensional echo. Catheter Cardiovasc Interv. 2004;62:46–51. doi: 10.1002/ccd.20003. [DOI] [PubMed] [Google Scholar]
- 9.Dell’Italia LJ. The right ventricle: Anatomy, physiology, and clinical importance. Curr Probl Cardiol. 1991;16:653–720. doi: 10.1016/0146-2806(91)90009-y. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins WE, Ochoa LL, Richardson GW, Trulock EP. Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or Eisenmenger syndrome. J Heart Lung Transplant. 1996;15:100–5. [PubMed] [Google Scholar]
- 11.Hopkins WE, Waggoner AD. Severe pulmonary hypertension without right ventricular failure: The unique hearts of patients with Eisenmenger syndrome. Am J Cardiol. 2002;89:34–8. doi: 10.1016/s0002-9149(01)02159-2. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins WE. The remarkable right ventricle of patients with Eisenmenger syndrome. Coron Artery Dis. 2005;16:19–25. doi: 10.1097/00019501-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lowes BD, Minobe W, Abraham WT, Rizeq MN, Bohlmeyer TJ, Quaife RA, et al. Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest. 1997;100:2315–24. doi: 10.1172/JCI119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno M, Miyauchi T, Sakai S, Kobayashi T, Goto K, Yamaguchi I. Effects of physiological or pathological pressure load in vivo on myocardial expression of ET-1 and receptors. Am J Physiol. 1999;277:R1321–30. doi: 10.1152/ajpregu.1999.277.5.R1321. [DOI] [PubMed] [Google Scholar]
- 15.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: A randomised placebo-controlled study. Lancet. 2001;358:1119–23. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 16.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 17.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–48. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 18.Abraham WT, Raynolds MV, Badesch DB, Wynne KM, Groves BM, Roden RL, et al. Angiotensin-converting enzyme DD genotype in patients with primary pulmonary hypertension: Increased frequency and association with preserved haemodynamics. J Renin Angiotensin Aldosterone Syst. 2003;4:27–30. doi: 10.3317/jraas.2003.003. [DOI] [PubMed] [Google Scholar]
- 19.Hess DS, Bache RJ. Transmural right ventricular myocardial blood flow during systole in the awake dog. Circ Res. 1979;45:88–94. doi: 10.1161/01.res.45.1.88. [DOI] [PubMed] [Google Scholar]
- 20.Kusachi S, Nishiyama O, Yasuhara K, Saito D, Haraoka S, Nagashima H. Right and left ventricular oxygen metabolism in open-chest dogs. Am J Physiol. 1982;243:H761–6. doi: 10.1152/ajpheart.1982.243.5.H761. [DOI] [PubMed] [Google Scholar]
- 21.Farrer-Brown G. Vascular pattern of myocardium of right ventricle of human heart. Br Heart J. 1968;30:679–86. doi: 10.1136/hrt.30.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laster SB, Shelton TJ, Barzilai B, Goldstein JA. Determinants of the recovery of right ventricular performance following experimental chronic right coronary artery occlusion. Circulation. 1993;88:696–708. doi: 10.1161/01.cir.88.2.696. [DOI] [PubMed] [Google Scholar]
- 23.Shiraki H, Yoshikawa T, Anzai T, Negishi K, Takahashi T, Asakura Y, et al. Association between preinfarction angina and a lower risk of right ventricular infarction. N Engl J Med. 1998;338:941–7. doi: 10.1056/NEJM199804023381402. [DOI] [PubMed] [Google Scholar]
- 24.Messika-Zeitoun D, Thomson H, Bellamy M, Scott C, Tribouilloy C, Dearani J, et al. Medical and surgical outcome of tricuspid regurgitation caused by flail leaflets. J Thorac Cardiovasc Surg. 2004;128:296–302. doi: 10.1016/j.jtcvs.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 25.Kasimir MT, Seebacher G, Jaksch P, Winkler G, Schmid K, Marta GM, et al. Reverse cardiac remodelling in patients with primary pulmonary hypertension after isolated lung transplantation. Eur J Cardiothorac Surg. 2004;26:776–81. doi: 10.1016/j.ejcts.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 26.Marino TA, Kent RL, Uboh CE, Fernandez E, Thompson EW, Cooper Gt. Structural analysis of pressure versus volume overload hypertrophy of cat right ventricle. Am J Physiol. 1985;249:H371–9. doi: 10.1152/ajpheart.1985.249.2.H371. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein JA, Barzilai B, Rosamond TL, Eisenberg PR, Jaffe AS. Determinants of hemodynamic compromise with severe right ventricular infarction. Circulation. 1990;82:359–68. doi: 10.1161/01.cir.82.2.359. [DOI] [PubMed] [Google Scholar]
- 28.Brooks H, Kirk ES, Vokonas PS, Urschel CW, Sonnenblick EH. Performance of the right ventricle under stress: relation to right coronary flow. J Clin Invest. 1971;50:2176–83. doi: 10.1172/JCI106712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein JA, Harada A, Yagi Y, Barzilai B, Cox JL. Hemodynamic importance of systolic ventricular interaction, augmented right atrial contractility and atrioventricular synchrony in acute right ventricular dysfunction. J Am Coll Cardiol. 1990;16:181–9. doi: 10.1016/0735-1097(90)90477-7. [DOI] [PubMed] [Google Scholar]
- 30.Klima UP, Lee MY, Guerrero JL, Laraia PJ, Levine RA, Vlahakes GJ. Determinants of maximal right ventricular function: Role of septal shift. J Thorac Cardiovasc Surg. 2002;123:72–80. doi: 10.1067/mtc.2002.118683. [DOI] [PubMed] [Google Scholar]
- 31.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–48. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 32.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988–93. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 33.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6:359–65. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 34.Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, et al. Continuous wave Doppler determination of right ventricular pressure: A simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–6. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 35.Fisher MR, Criner GJ, Fishman AP, Hassoun PM, Minai OA, Scharf SM, et al. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. Eur Respir J. 2007;30:914–21. doi: 10.1183/09031936.00033007. [DOI] [PubMed] [Google Scholar]
- 36.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–40. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 37.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–21. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitabatake A, Inoue M, Asao M, Masuyama T, Tanouchi J, Morita T, et al. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation. 1983;68:302–9. doi: 10.1161/01.cir.68.2.302. [DOI] [PubMed] [Google Scholar]
- 39.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41:1021–7. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 40.Rajagopalan N, Simon MA, Suffoletto MS, Shah H, Edelman K, Mathier MA, et al. Noninvasive estimation of pulmonary vascular resistance in pulmonary hypertension. Echocardiography. 2009;26:489–94. doi: 10.1111/j.1540-8175.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 41.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Anavekar NS, Gerson D, Skali H, Kwong RY, Yucel EK, Solomon SD. Two-dimensional assessment of right ventricular function: An echocardiographic-MRI correlative study. Echocardiography. 2007;24:452–6. doi: 10.1111/j.1540-8175.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 43.Zornoff LA, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GA, et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39:1450–5. doi: 10.1016/s0735-1097(02)01804-1. [DOI] [PubMed] [Google Scholar]
- 44.Nass N, McConnell MV, Goldhaber SZ, Chyu S, Solomon SD. Recovery of regional right ventricular function after thrombolysis for pulmonary embolism. Am J Cardiol. 1999;83:804–6. doi: 10.1016/s0002-9149(98)01000-5. A10. [DOI] [PubMed] [Google Scholar]
- 45.Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–47. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 46.Sebbag I, Rudski LG, Therrien J, Hirsch A, Langleben D. Effect of chronic infusion of epoprostenol on echocardiographic right ventricular myocardial performance index and its relation to clinical outcome in patients with primary pulmonary hypertension. Am J Cardiol. 2001;88:1060–3. doi: 10.1016/s0002-9149(01)01995-6. [DOI] [PubMed] [Google Scholar]
- 47.Chockalingam A, Gnanavelu G, Alagesan R, Subramaniam T. Myocardial performance index in evaluation of acute right ventricular myocardial infarction. Echocardiography. 2004;21:487–94. doi: 10.1111/j.0742-2822.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- 48.Eidem BW, O’Leary PW, Tei C, Seward JB. Usefulness of the myocardial performance index for assessing right ventricular function in congenital heart disease. Am J Cardiol. 2000;86:654–8. doi: 10.1016/s0002-9149(00)01047-x. [DOI] [PubMed] [Google Scholar]
- 49.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–41. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 50.Lindqvist P, Waldenstrom A, Henein M, Morner S, Kazzam E. Regional and global right ventricular function in healthy individuals aged 20-90 years: A pulsed Doppler tissue imaging study: Umea General Population Heart Study. Echocardiography. 2005;22:305–14. doi: 10.1111/j.1540-8175.2005.04023.x. [DOI] [PubMed] [Google Scholar]
- 51.Tugcu A, Guzel D, Yildirimturk O, Aytekin S. Evaluation of right ventricular systolic and diastolic function in patients with newly diagnosed obstructive sleep apnea syndrome without hypertension. Cardiology. 2009;113:184–92. doi: 10.1159/000193146. [DOI] [PubMed] [Google Scholar]
- 52.Sade LE, Ozin B, Ulus T, Açikel S, Pirat B, Bilgi M, et al. Right ventricular contractile reserve in mitral stenosis: Implications on hemodynamic burden and clinical outcome. Int J Cardiol. 2009;135:193–201. doi: 10.1016/j.ijcard.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 53.Toyono M, Harada K, Tamura M, Yamamoto F, Takada G. Myocardial acceleration during isovolumic contraction as a new index of right ventricular contractile function and its relation to pulmonary regurgitation in patients after repair of tetralogy of Fallot. J Am Soc Echocardiogr. 2004;17:332–7. doi: 10.1016/j.echo.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 54.Vogel M, Schmidt MR, Kristiansen SB, Cheung M, White PA, Sorensen K, et al. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: Comparison with ventricular pressure-volume relations in an animal model. Circulation. 2002;105:1693–9. doi: 10.1161/01.cir.0000013773.67850.ba. [DOI] [PubMed] [Google Scholar]
- 55.Tayyareci Y, Nisanci Y, Umman B, Oncul A, Yurdakul S, Altun I, et al. Early detection of right ventricular systolic dysfunction by using myocardial acceleration during isovolumic contraction in patients with mitral stenosis. Eur J Echocardiogr. 2008;9:516–21. doi: 10.1016/j.euje.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest. 2011;139:1299–309. doi: 10.1378/chest.10-2015. [DOI] [PubMed] [Google Scholar]
- 57.Fukuda Y, Tanaka H, Sugiyama D, Ryo K, Onishi T, Fukuya H, et al. Utility of Right Ventricular Free Wall Speckle-Tracking Strain for Evaluation of Right Ventricular Performance in Patients with Pulmonary Hypertension. J Am Soc Echocardiogr. 2011;24:1101–8. doi: 10.1016/j.echo.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Nesser HJ, Tkalec W, Patel AR, Masani ND, Niel J, Markt B, et al. Quantitation of right ventricular volumes and ejection fraction by three-dimensional echocardiography in patients: comparison with magnetic resonance imaging and radionuclide ventriculography. Echocardiography. 2006;23:666–80. doi: 10.1111/j.1540-8175.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 59.Grapsa J, O’Regan DP, Pavlopoulos H, Durighel G, Dawson D, Nihoyannopoulos P. Right ventricular remodelling in pulmonary arterial hypertension with three-dimensional echocardiography: Comparison with cardiac magnetic resonance imaging. Eur J Echocardiogr. 2010;11:64–73. doi: 10.1093/ejechocard/jep169. [DOI] [PubMed] [Google Scholar]
- 60.Jenkins C, Chan J, Bricknell K, Strudwick M, Marwick TH. Reproducibility of right ventricular volumes and ejection fraction using real-time three-dimensional echocardiography: Comparison with cardiac MRI. Chest. 2007;131:1844–51. doi: 10.1378/chest.06-2143. [DOI] [PubMed] [Google Scholar]
- 61.Niemann PS, Pinho L, Balbach T, Galuschky C, Blankenhagen M, Silberbach M, et al. Anatomically oriented right ventricular volume measurements with dynamic three-dimensional echocardiography validated by 3-Tesla magnetic resonance imaging. J Am Coll Cardiol. 2007;50:1668–76. doi: 10.1016/j.jacc.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 62.van der Zwaan HB, Geleijnse ML, McGhie JS, Boersma E, Helbing WA, Meijboom FJ, et al. Right ventricular quantification in clinical practice: Two-dimensional vs.three-dimensional echocardiography compared with cardiac magnetic resonance imaging. Eur J Echocardiogr. 2011;12:656–64. doi: 10.1093/ejechocard/jer107. [DOI] [PubMed] [Google Scholar]
- 63.Shimada YJ, Shiota M, Siegel RJ, Shiota T. Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: A meta-analysis study. J Am Soc Echocardiogr. 2010;23:943–53. doi: 10.1016/j.echo.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 64.Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, et al. Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc Imaging. 2010;3:10–8. doi: 10.1016/j.jcmg.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 65.Berman GO, Reichek N, Brownson D, Douglas PS. Effects of sample volume location, imaging view, heart rate and age on tricuspid velocimetry in normal subjects. Am J Cardiol. 1990;65:1026–30. doi: 10.1016/0002-9149(90)91008-t. [DOI] [PubMed] [Google Scholar]
- 66.Dell’Italia LJ, Walsh RA. Right ventricular diastolic pressure-volume relations and regional dimensions during acute alterations in loading conditions. Circulation. 1988;77:1276–82. doi: 10.1161/01.cir.77.6.1276. [DOI] [PubMed] [Google Scholar]
- 67.Guazzi M, Maltagliati A, Tamborini G, Celeste F, Pepi M, Muratori M, et al. How the left and right sides of the heart, as well as pulmonary venous drainage, adapt to an increasing degree of head-up tilting in hypertrophic cardiomyopathy: Differences from the normal heart. J Am Coll Cardiol. 2000;36:185–93. doi: 10.1016/s0735-1097(00)00698-7. [DOI] [PubMed] [Google Scholar]
- 68.Sallach JA, Tang WH, Borowski AG, Tong W, Porter T, Martin MG, et al. Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging. 2009;2:527–34. doi: 10.1016/j.jcmg.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 69.Sade LE, Gulmez O, Eroglu S, Sezgin A, Muderrisoglu H. Noninvasive estimation of right ventricular filling pressure by ratio of early tricuspid inflow to annular diastolic velocity in patients with and without recent cardiac surgery. J Am Soc Echocardiogr. 2007;20:982–8. doi: 10.1016/j.echo.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 70.Sundereswaran L, Nagueh SF, Vardan S, Middleton KJ, Zoghbi WA, Quiñones MA, et al. Estimation of left and right ventricular filling pressures after heart transplantation by tissue Doppler imaging. Am J Cardiol. 1998;82:352–7. doi: 10.1016/s0002-9149(98)00346-4. [DOI] [PubMed] [Google Scholar]
- 71.Greyson CR. Evaluation of Right Ventricular Function. Curr Cardiol Rep. 2011;13:194–202. doi: 10.1007/s11886-011-0174-5. [DOI] [PubMed] [Google Scholar]
- 72.Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, Tandri H, et al. Sex and race differences in right ventricular structure and function: The multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–51. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luijnenburg SE, Robbers-Visser D, Moelker A, Vliegen HW, Mulder BJ, Helbing WA. Intra-observer and interobserver variability of biventricular function, volumes and mass in patients with congenital heart disease measured by CMR imaging. Int J Cardiovasc Imaging. 2010;26:57–64. doi: 10.1007/s10554-009-9501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maceira AM, Prasad SK, Khan M, Pennell DJ. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur Heart J. 2006;27:2879–88. doi: 10.1093/eurheartj/ehl336. [DOI] [PubMed] [Google Scholar]
- 75.Goetschalckx K, Rademakers F, Bogaert J. Right ventricular function by MRI. Curr Opin Cardiol. 2010;25:451–5. doi: 10.1097/HCO.0b013e32833b78e6. [DOI] [PubMed] [Google Scholar]
- 76.Bluemke DA, Krupinski EA, Ovitt T, Gear K, Unger E, Axel L, et al. MR Imaging of arrhythmogenic right ventricular cardiomyopathy: Morphologic findings and interobserver reliability. Cardiology. 2003;99:153–62. doi: 10.1159/000070672. [DOI] [PubMed] [Google Scholar]
- 77.Sen-Chowdhry S, Prasad SK, Syrris P, Wage R, Ward D, Merrifield R, et al. Cardiovascular magnetic resonance in arrhythmogenic right ventricular cardiomyopathy revisited: comparison with task force criteria and genotype. J Am Coll Cardiol. 2006;48:2132–40. doi: 10.1016/j.jacc.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 78.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 79.Babu-Narayan SV, Kilner PJ, Li W, Moon JC, Goktekin O, Davlouros PA, et al. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405–13. doi: 10.1161/CIRCULATIONAHA.105.548727. [DOI] [PubMed] [Google Scholar]
- 80.McCann GP, Gan CT, Beek AM, Niessen HW, Vonk Noordegraaf A, van Rossum AC. Extent of MRI delayed enhancement of myocardial mass is related to right ventricular dysfunction in pulmonary artery hypertension. AJR Am J Roentgenol. 2007;188:349–55. doi: 10.2214/AJR.05.1259. [DOI] [PubMed] [Google Scholar]
- 81.Kumar A, Abdel-Aty H, Kriedemann I, Schulz-Menger J, Gross CM, Dietz R, et al. Contrast-enhanced cardiovascular magnetic resonance imaging of right ventricular infarction. J Am Coll Cardiol. 2006;48:1969–76. doi: 10.1016/j.jacc.2006.05.078. [DOI] [PubMed] [Google Scholar]
- 82.Benza R, Biederman R, Murali S, Gupta H. Role of cardiac magnetic resonance imaging in the management of patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;52:1683–92. doi: 10.1016/j.jacc.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 83.Francone M, Dymarkowski S, Kalantzi M, Rademakers FE, Bogaert J. Assessment of ventricular coupling with real-time cine MRI and its value to differentiate constrictive pericarditis from restrictive cardiomyopathy. Eur Radiol. 2006;16:944–51. doi: 10.1007/s00330-005-0009-0. [DOI] [PubMed] [Google Scholar]
- 84.Rathi VK, Biederman RW. Expanding role of cardiovascular magnetic resonance in left and right ventricular diastolic function. Heart Fail Clin. 2009;5:421–35. doi: 10.1016/j.hfc.2009.02.005. vii. [DOI] [PubMed] [Google Scholar]
- 85.Shehata ML, Basha TA, Tantawy WH, Lima JA, Vogel-Claussen J, Bluemke DA, et al. Real-time single-heartbeat fast strain-encoded imaging of right ventricular regional function: normal versus chronic pulmonary hypertension. Magn Reson Med. 2010;64:98–106. doi: 10.1002/mrm.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michelakis ED, Wilkins MR, Rabinovitch M. Emerging concepts and translational priorities in pulmonary arterial hypertension. Circulation. 2008;118:1486–95. doi: 10.1161/CIRCULATIONAHA.106.673988. [DOI] [PubMed] [Google Scholar]
- 87.Nagendran J, Gurtu V, Fu DZ, Dyck JR, Haromy A, Ross DB, et al. A dynamic and chamber-specific mitochondrial remodeling in right ventricular hypertrophy can be therapeutically targeted. J Thorac Cardiovasc Surg. 2008;136:168–78. doi: 10.1016/j.jtcvs.2008.01.040. 78 e1-3. [DOI] [PubMed] [Google Scholar]
- 88.Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, et al. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol. 2005;45:1849–55. doi: 10.1016/j.jacc.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 89.Bokhari S, Raina A, Berman Rosenweig E, et al. Positron emission tomography imaging may provide a novel biomarker and understanding of right ventricular dysfunction in patients with idiopathic pulmonary arterial hypertension. Circ Cardiovasc Imaging. 2011;4:641–647. doi: 10.1161/CIRCIMAGING.110.963207. [DOI] [PubMed] [Google Scholar]
- 90.Mielniczuk LM, Birnie D, Ziadi MC, deKemp RA, DaSilva JN, Burwash I, et al. Relation between right ventricular function and increased right ventricular [18F]fluorodeoxyglucose accumulation in patients with heart failure. Circ Cardiovasc Imaging. 2011;4:59–66. doi: 10.1161/CIRCIMAGING.109.905984. [DOI] [PubMed] [Google Scholar]
- 91.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 92.Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Hooi Ewe S, et al. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation. 2010;122:2538–44. doi: 10.1161/CIRCULATIONAHA.110.955542. [DOI] [PubMed] [Google Scholar]
- 93.Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121:252–8. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–8. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 95.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–54. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 96.Borer JS, Bonow RO. Contemporary approach to aortic and mitral regurgitation. Circulation. 2003;108:2432–8. doi: 10.1161/01.CIR.0000096400.00562.A3. [DOI] [PubMed] [Google Scholar]
- 97.Wencker D, Borer JS, Hochreiter C, Devereux RB, Roman MJ, Kligfield P, et al. Preoperative predictors of late postoperative outcome among patients with nonischemic mitral regurgitation with ‘high risk’ descriptors and comparison with unoperated patients. Cardiology. 2000;93:37–42. doi: 10.1159/000007000. [DOI] [PubMed] [Google Scholar]
- 98.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–9. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 99.Kaul TK, Fields BL. Postoperative acute refractory right ventricular failure: Incidence, pathogenesis, management and prognosis. Cardiovasc Surg. 2000;8:1–9. doi: 10.1016/s0967-2109(99)00089-7. [DOI] [PubMed] [Google Scholar]
- 100.Dang NC, Topkara VK, Mercando M, Kay J, Kruger KH, Aboodi MS, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. 2006;25:1–6. doi: 10.1016/j.healun.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 101.Hosenpud JD, Bennett LE, Keck BM, Boucek MM, Novick RJ. The Registry of the International Society for Heart and Lung Transplantation: seventeenth official report-2000. J Heart Lung Transplant. 2000;19:909–31. doi: 10.1016/s1053-2498(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 102.Hochreiter C, Niles N, Devereux RB, Kligfield P, Borer JS. Mitral regurgitation: relationship of noninvasive descriptors of right and left ventricular performance to clinical and hemodynamic findings and to prognosis in medically and surgically treated patients. Circulation. 1986;73:900–12. doi: 10.1161/01.cir.73.5.900. [DOI] [PubMed] [Google Scholar]
- 103.Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: Prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–8. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 104.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 105.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–53. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 106.Goldstein JA. Right heart ischemia: Pathophysiology, natural history, and clinical management. Prog Cardiovasc Dis. 1998;40:325–41. doi: 10.1016/s0033-0620(98)80051-0. [DOI] [PubMed] [Google Scholar]
- 107.Pfisterer M, Emmenegger H, Soler M, Burkart F. Prognostic significance of right ventricular ejection fraction for persistent complex ventricular arrhythmias and/or sudden cardiac death after first myocardial infarction: Relation to infarct location, size and left ventricular function. Eur Heart J. 1986;7:289–98. doi: 10.1093/oxfordjournals.eurheartj.a062066. [DOI] [PubMed] [Google Scholar]
- 108.Zehender M, Kasper W, Kauder E, Schönthaler M, Geibel A, Olschewski M, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med. 1993;328:981–8. doi: 10.1056/NEJM199304083281401. [DOI] [PubMed] [Google Scholar]
- 109.Mehta SR, Eikelboom JW, Natarajan MK, Diaz R, Yi C, Gibbons RJ, et al. Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J Am Coll Cardiol. 2001;37:37–43. doi: 10.1016/s0735-1097(00)01089-5. [DOI] [PubMed] [Google Scholar]
- 110.Goldstein JA, Vlahakes GJ, Verrier ED, Schiller NB, Tyberg JV, Ports TA, et al. The role of right ventricular systolic dysfunction and elevated intrapericardial pressure in the genesis of low output in experimental right ventricular infarction. Circulation. 1982;65:513–22. doi: 10.1161/01.cir.65.3.513. [DOI] [PubMed] [Google Scholar]
- 111.Goldstein JA. Pathophysiology and management of right heart ischemia. J Am Coll Cardiol. 2002;40:841–53. doi: 10.1016/s0735-1097(02)02048-x. [DOI] [PubMed] [Google Scholar]
- 112.Dell’Italia LJ, Starling MR, Crawford MH, Boros BL, Chaudhuri TK, O’Rourke RA. Right ventricular infarction: identification by hemodynamic measurements before and after volume loading and correlation with noninvasive techniques. J Am Coll Cardiol. 1984;4:931–9. doi: 10.1016/s0735-1097(84)80053-4. [DOI] [PubMed] [Google Scholar]
- 113.Steele P, Kirch D, Ellis J, Vogel R, Battock D. Prompt return to normal of depressed right ventricular ejection fraction in acute inferior infarction. Br Heart J. 1977;39:1319–23. doi: 10.1136/hrt.39.12.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yasuda T, Okada RD, Leinbach RC, Gold HK, Phillips H, McKusick KA, et al. Serial evaluation of right ventricular dysfunction associated with acute inferior myocardial infarction. Am Heart J. 1990;119:816–22. doi: 10.1016/s0002-8703(05)80317-5. [DOI] [PubMed] [Google Scholar]
- 115.Bowers TR, O’Neill WW, Grines C, Pica MC, Safian RD, Goldstein JA. Effect of reperfusion on biventricular function and survival after right ventricular infarction. N Engl J Med. 1998;338:933–40. doi: 10.1056/NEJM199804023381401. [DOI] [PubMed] [Google Scholar]
- 116.San Román JA, Vilacosta I, Rollán MJ, Castillo JA, Alonso J, Durán JM, et al. Right ventricular asynergy during dobutamine-atropine echocardiography. J Am Coll Cardiol. 1997;30:430–5. doi: 10.1016/s0735-1097(97)00152-6. [DOI] [PubMed] [Google Scholar]
- 117.LJ DI. Right Ventricular Infarction. J Intensive Care Med. 1986;1:246–56. [Google Scholar]
- 118.Engström AE, Vis MM, Bouma BJ, van den Brink RB, Baan J, Jr, Claessen BE, et al. Right ventricular dysfunction is an independent predictor for mortality in ST-elevation myocardial infarction patients presenting with cardiogenic shock on admission. Eur J Heart Fail. 2010;12:276–82. doi: 10.1093/eurjhf/hfp204. [DOI] [PubMed] [Google Scholar]
- 119.Oguzhan A, Abaci A, Eryol NK, Topsakal R, Seyfeli E. Colour tissue Doppler echocardiographic evaluation of right ventricular function in patients with right ventricular infarction. Cardiology. 2003;100:41–6. doi: 10.1159/000072391. [DOI] [PubMed] [Google Scholar]
- 120.Antoni ML, Scherptong RW, Atary JZ, Boersma E, Holman ER, van der Wall EE, et al. Prognostic value of right ventricular function in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Circ Cardiovasc Imaging. 2010;3:264–71. doi: 10.1161/CIRCIMAGING.109.914366. [DOI] [PubMed] [Google Scholar]
- 121.Ozdemir K, Altunkeser BB, Icli A, Ozdil H, Gok H. New parameters in identification of right ventricular myocardial infarction and proximal right coronary artery lesion. Chest. 2003;124:219–26. doi: 10.1378/chest.124.1.219. [DOI] [PubMed] [Google Scholar]
- 122.Reitz BA, Wallwork JL, Hunt SA, Pennock JL, Billingham ME, Oyer PE, et al. Heart-lung transplantation: Successful therapy for patients with pulmonary vascular disease. N Engl J Med. 1982;306:557–64. doi: 10.1056/NEJM198203113061001. [DOI] [PubMed] [Google Scholar]
- 123.Dittrich HC, Nicod PH, Chow LC, Chappuis FP, Moser KM, Peterson KL. Early changes of right heart geometry after pulmonary thromboendarterectomy. J Am Coll Cardiol. 1988;11:937–43. doi: 10.1016/s0735-1097(98)90049-3. [DOI] [PubMed] [Google Scholar]
- 124.Chow LC, Dittrich HC, Hoit BD, Moser KM, Nicod PH. Doppler assessment of changes in right-sided cardiac hemodynamics after pulmonary thromboendarterectomy. Am J Cardiol. 1988;61:1092–7. doi: 10.1016/0002-9149(88)90132-4. [DOI] [PubMed] [Google Scholar]
- 125.Katz WE, Gasior TA, Quinlan JJ, Lazar JM, Firestone L, Griffith BP, et al. Immediate effects of lung transplantation on right ventricular morphology and function in patients with variable degrees of pulmonary hypertension. J Am Coll Cardiol. 1996;27:384–91. doi: 10.1016/0735-1097(95)00502-1. [DOI] [PubMed] [Google Scholar]
- 126.Pasque MK, Trulock EP, Cooper JD, Triantafillou AN, Huddleston CB, Rosenbloom M, et al. Single lung transplantation for pulmonary hypertension. Single institution experience in 34 patients. Circulation. 1995;92:2252–8. doi: 10.1161/01.cir.92.8.2252. [DOI] [PubMed] [Google Scholar]
- 127.Toyooka S, Kusano KF, Goto K, Masaomi Y, Oto T, Sano Y, et al. Right but not left ventricular function recovers early after living-donor lobar lung transplantation in patients with pulmonary arterial hypertension. J Thorac Cardiovasc Surg. 2009;138:222–6. doi: 10.1016/j.jtcvs.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 128.Kramer MR, Valantine HA, Marshall SE, Starnes VA, Theodore J. Recovery of the right ventricle after single-lung transplantation in pulmonary hypertension. Am J Cardiol. 1994;73:494–500. doi: 10.1016/0002-9149(94)90681-5. [DOI] [PubMed] [Google Scholar]
- 129.Ritchie M, Waggoner AD, Davila-Roman VG, Barzilai B, Trulock EP, Eisenberg PR. Echocardiographic characterization of the improvement in right ventricular function in patients with severe pulmonary hypertension after single-lung transplantation. J Am Coll Cardiol. 1993;22:1170–4. doi: 10.1016/0735-1097(93)90433-2. [DOI] [PubMed] [Google Scholar]
- 130.Schulman LL, Leibowitz DW, Anandarangam T, DiTullio MR, McGregor CC, Smith CR, et al. Variability of right ventricular functional recovery after lung transplantation. Transplantation. 1996;62:622–5. doi: 10.1097/00007890-199609150-00014. [DOI] [PubMed] [Google Scholar]
- 131.Frist WH, Lorenz CH, Walker ES, Loyd JE, Stewart JR, Graham TP, Jr, et al. MRI complements standard assessment of right ventricular function after lung transplantation. Ann Thorac Surg. 1995;60:268–71. doi: 10.1016/0003-4975(95)00365-r. [DOI] [PubMed] [Google Scholar]
- 132.Piazza G, Goldhaber SZ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2011;364:351–60. doi: 10.1056/NEJMra0910203. [DOI] [PubMed] [Google Scholar]
- 133.Menzel T, Wagner S, Kramm T, Mohr-Kahaly S, Mayer E, Braeuninger S, et al. Pathophysiology of impaired right and left ventricular function in chronic embolic pulmonary hypertension: Changes after pulmonary thromboendarterectomy. Chest. 2000;118:897–903. doi: 10.1378/chest.118.4.897. [DOI] [PubMed] [Google Scholar]
- 134.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 135.Piovella F, D’Armini AM, Barone M, Tapson VF. Chronic thromboembolic pulmonary hypertension. Semin Thromb Hemost. 2006;32:848–55. doi: 10.1055/s-2006-955467. [DOI] [PubMed] [Google Scholar]
- 136.Menzel T, Kramm T, Wagner S, Mohr-Kahaly S, Mayer E, Meyer J. Improvement of tricuspid regurgitation after pulmonary thromboendarterectomy. Ann Thorac Surg. 2002;73:756–61. doi: 10.1016/s0003-4975(01)03573-1. [DOI] [PubMed] [Google Scholar]
- 137.Menzel T, Kramm T, Bruckner A, Mohr-Kahaly S, Mayer E, Meyer J. Quantitative assessment of right ventricular volumes in severe chronic thromboembolic pulmonary hypertension using transthoracic three-dimensional echocardiography: changes due to pulmonary thromboendarterectomy. Eur J Echocardiogr. 2002;3:67–72. doi: 10.1053/euje.2001.0129. [DOI] [PubMed] [Google Scholar]
- 138.Menzel T, Kramm T, Mohr-Kahaly S, Mayer E, Oelert H, Meyer J. Assessment of cardiac performance using Tei indices in patients undergoing pulmonary thromboendarterectomy. Ann Thorac Surg. 2002;73:762–6. doi: 10.1016/s0003-4975(01)03558-5. [DOI] [PubMed] [Google Scholar]
- 139.Reesink HJ, Marcus JT, Tulevski II, Jamieson S, Kloek JJ, Vonk Noordegraaf A, et al. Reverse right ventricular remodeling after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: utility of magnetic resonance imaging to demonstrate restoration of the right ventricle. J Thorac Cardiovasc Surg. 2007;133:58–64. doi: 10.1016/j.jtcvs.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 140.Casaclang-Verzosa G, McCully RB, Oh JK, Miller FA, Jr, McGregor CG. Effects of pulmonary thromboendarterectomy on right-sided echocardiographic parameters in patients with chronic thromboembolic pulmonary hypertension. Mayo Clin Proc. 2006;81:777–82. doi: 10.4065/81.6.777. [DOI] [PubMed] [Google Scholar]
- 141.Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998;339:1522–33. doi: 10.1056/NEJM199811193392107. [DOI] [PubMed] [Google Scholar]
- 142.Frazier OH, Rose EA, Macmanus Q, Burton NA, Lefrak EA, Poirier VL, et al. Multicenter clinical evaluation of the HeartMate 1000 IP left ventricular assist device. Ann Thorac Surg. 1992;53:1080–90. doi: 10.1016/0003-4975(92)90393-i. [DOI] [PubMed] [Google Scholar]
- 143.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 144.Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–84. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 145.Maybaum S, Mancini D, Xydas S, Starling RC, Aaronson K, Pagani FD, et al. Cardiac improvement during mechanical circulatory support: A prospective multicenter study of the LVAD Working Group. Circulation. 2007;115:2497–505. doi: 10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- 146.McCarthy PM, Nakatani S, Vargo R, Kottke-Marchant K, Harasaki H, James KB, et al. Structural and left ventricular histologic changes after implantable LVAD insertion. Ann Thorac Surg. 1995;59:609–13. doi: 10.1016/0003-4975(94)00953-8. [DOI] [PubMed] [Google Scholar]
- 147.Topilsky Y, Maltais S, Oh JK, Atchison FW, Perrault LP, Carrier M, et al. Focused review on transthoracic echocardiographic assessment of patients with continuous axial left ventricular assist devices. Cardiol Res Pract. 2011;2011:187434. doi: 10.4061/2011/187434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Barbone A, Holmes JW, Heerdt PM, The’ AH, Naka Y, Joshi N, et al. Comparison of right and left ventricular responses to left ventricular assist device support in patients with severe heart failure: a primary role of mechanical unloading underlying reverse remodeling. Circulation. 2001;104:670–5. doi: 10.1161/hc3101.093903. [DOI] [PubMed] [Google Scholar]
- 149.Santamore WP, Gray LA., Jr Left ventricular contributions to right ventricular systolic function during LVAD support. Ann Thorac Surg. 1996;61:350–6. doi: 10.1016/0003-4975(95)01056-4. [DOI] [PubMed] [Google Scholar]
- 150.Maeder MT, Leet A, Ross A, Esmore D, Kaye DM. Changes in right ventricular function during continuous-flow left ventricular assist device support [corrected] J Heart Lung Transplant. 2009;28:360–6. doi: 10.1016/j.healun.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 151.Kukucka M, Stepanenko A, Potapov E, Krabatsch T, Redlin M, Mladenow A, et al. Right-to-left ventricular end-diastolic diameter ratio and prediction of right ventricular failure with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2011;30:64–9. doi: 10.1016/j.healun.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 152.Topilsky Y, Hasin T, Oh JK, et al. Echocardiographic variables post LVAD associated with adverse outcome. Circ Cardiovasc Imaging. 2011;4:648–661. doi: 10.1161/CIRCIMAGING.111.965335. [DOI] [PubMed] [Google Scholar]
- 153.Puwanant S, Hamilton KK, Klodell CT, Hill JA, Schofield RS, Cleeton TS, et al. Tricuspid annular motion as a predictor of severe right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant. 2008;27:1102–7. doi: 10.1016/j.healun.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 154.van Wolferen SA, Boonstra A, Marcus JT, Marques KM, Bronzwaer JG, Postmus PE, et al. Right ventricular reverse remodelling after sildenafil in pulmonary arterial hypertension. Heart. 2006;92:1860–1. doi: 10.1136/hrt.2005.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wilkins MR, Paul GA, Strange JW, Tunariu N, Gin-Sing W, Banya WA, et al. Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) study. Am J Respir Crit Care Med. 2005;171:1292–7. doi: 10.1164/rccm.200410-1411OC. [DOI] [PubMed] [Google Scholar]
- 156.Michelakis ED, Tymchak W, Noga M, Webster L, Wu XC, Lien D, et al. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation. 2003;108:2066–9. doi: 10.1161/01.CIR.0000099502.17776.C2. [DOI] [PubMed] [Google Scholar]
- 157.Gan CT, Holverda S, Marcus JT, Paulus WJ, Marques KM, Bronzwaer JG, et al. Right ventricular diastolic dysfunction and the acute effects of sildenafil in pulmonary hypertension patients. Chest. 2007;132:11–7. doi: 10.1378/chest.06-1263. [DOI] [PubMed] [Google Scholar]
- 158.Hemnes AR, Forfia PR, Champion HC. Assessment of pulmonary vasculature and right heart by invasive haemodynamics and echocardiography. Int J Clin Pract Suppl. 2009;162:4–19. doi: 10.1111/j.1742-1241.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 159.Simonneau G, Galiè N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 160.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 161.Roeleveld RJ, Marcus JT, Faes TJ, Gan TJ, Boonstra A, Postmus PE, et al. Interventricular septal configuration at mr imaging and pulmonary arterial pressure in pulmonary hypertension. Radiology. 2005;234:710–7. doi: 10.1148/radiol.2343040151. [DOI] [PubMed] [Google Scholar]
- 162.Yeo TC, Dujardin KS, Tei C, Mahoney DW, McGoon MD, Seward JB. Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. Am J Cardiol. 1998;81:1157–61. doi: 10.1016/s0002-9149(98)00140-4. [DOI] [PubMed] [Google Scholar]
- 163.Arkles JS, Opotowsky AR, Ojeda J, Rogers F, Liu T, Prassana V, et al. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:268–76. doi: 10.1164/rccm.201004-0601OC. [DOI] [PubMed] [Google Scholar]
- 164.Shehata ML, Lossnitzer D, Skrok J, Boyce D, Lechtzin N, Mathai SC, et al. Myocardial delayed enhancement in pulmonary hypertension: Pulmonary hemodynamics, right ventricular function, and remodeling. AJR Am J Roentgenol. 2011;196:87–94. doi: 10.2214/AJR.09.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vogel-Claussen J, Shehata ML, Lossnitzer D, Skrok J, Singh S, Boyce D, et al. Increased right ventricular septomarginal trabeculation mass is a novel marker for pulmonary hypertension: comparison with ventricular mass index and right ventricular mass. Invest Radiol. 2011;46:567–75. doi: 10.1097/RLI.0b013e31821b7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–7. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 167.Vogel-Claussen J, Skrok J, Shehata ML, Singh S, Sibley CT, Boyce DM, et al. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology. 2011;258:119–27. doi: 10.1148/radiol.10100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 169.Horton KD, Meece RW, Hill JC. Assessment of the right ventricle by echocardiography: a primer for cardiac sonographers. J Am Soc Echocardiogr. 2009;22:776–92. doi: 10.1016/j.echo.2009.04.027. quiz 861-2. [DOI] [PubMed] [Google Scholar]
- 170.Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP., Jr Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999;1:7–21. doi: 10.3109/10976649909080829. [DOI] [PubMed] [Google Scholar]
- 171.Francois CJ, Shors SM, Bonow RO, Finn JP. Analysis of cardiopulmonary transit times at contrast material-enhanced MR imaging in patients with heart disease. Radiology. 2003;227:447–52. doi: 10.1148/radiol.2272020366. [DOI] [PubMed] [Google Scholar]
- 172.Shors SM, Cotts WG, Pavlevic-Surjancev B, Francois CJ, Gheorghiade M, Finn JP. Heart Failure: Evaluation of Cardiopulmonary Transit Times with Time-resolved MR Angiography. Radiology. 2003:743–8. doi: 10.1148/radiol.2293021363. [DOI] [PubMed] [Google Scholar]
- 173.Lakoma A, Tuite D, Sheehan J, Weale P, Carr JC. Measurement of pulmonary circulation parameters using time-resolved MR angiography in patients after Ross procedure. AJR Am J Roentgenol. 2010;194:912–9. doi: 10.2214/AJR.09.2897. [DOI] [PubMed] [Google Scholar]
- 174.Polak JF, Holman BL, Wynne J, Colucci WS. Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol. 1983;2:217–24. doi: 10.1016/s0735-1097(83)80156-9. [DOI] [PubMed] [Google Scholar]
- 175.Meluzin J, Spinarová L, Hude P, Krejcí J, Dusek L, Vítovec J, et al. Combined right ventricular systolic and diastolic dysfunction represents a strong determinant of poor prognosis in patients with symptomatic heart failure. Int J Cardiol. 2005;105:164–73. doi: 10.1016/j.ijcard.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 176.Haddad F, Denault AY, Couture P, Cartier R, Pellerin M, Levesque S, et al. Right ventricular myocardial performance index predicts perioperative mortality or circulatory failure in high-risk valvular surgery. J Am Soc Echocardiogr. 2007;20:1065–72. doi: 10.1016/j.echo.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 177.van Wolferen SA, van de Veerdonk MC, Mauritz GJ, Jacobs W, Marcus JT, Marques KM, et al. Clinically significant change in stroke volume in pulmonary hypertension. Chest. 2011;139:1003–9. doi: 10.1378/chest.10-1066. [DOI] [PubMed] [Google Scholar]
- 178.Topilsky Y, Oh JK, Shah DK, Boilson BA, Schirger JA, Kushwaha SS, et al. Echocardiographic predictors of adverse outcomes after continuous left ventricular assist device implantation. JACC Cardiovasc Imaging. 2011;4:211–22. doi: 10.1016/j.jcmg.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 179.Khandaker MH, Espinosa RE, Nishimura RA, Sinak LJ, Hayes SN, Melduni RM, et al. Pericardial disease: diagnosis and management. Mayo Clin Proc. 2010;85:572–93. doi: 10.4065/mcp.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mertens LL, Friedberg MK. Imaging the right ventricle–current state of the art. Nat Rev Cardiol. 2010;7:551–63. doi: 10.1038/nrcardio.2010.118. [DOI] [PubMed] [Google Scholar]
- 181.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–31. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 182.Rutledge JM, Nihill MR, Fraser CD, Smith OE, McMahon CJ, Bezold LI. Outcome of 121 patients with congenitally corrected transposition of the great arteries. Pediatr Cardiol. 2002;23:137–145. doi: 10.1007/s00246-001-0037-8. [DOI] [PubMed] [Google Scholar]


