Abstract
This study measured glucose uptake in the right ventricle (RV) of patients with pulmonary hypertension and investigated the relationship to hemodynamics and survival. Myocardial 18F-fluorodeoxy-glucose (FDG) uptake was measured using single-photon positron emission tomography (SPECT) in 24 patients with idiopathic pulmonary arterial hypertension (IPAH) and 43 patients with congenital heart disease (CHD). In both IPAH and CHD-PAH, RV FDG uptake (RV/LV ratio) was associated with pulmonary vascular resistance (PVR). A second SPECT scan was performed in nine patients after 6 months treatment with sildenafil. PVR decreased from 1683±426 to 1207±383 dyn s-1 cm-5 (P < 0.05) and cardiac index improved from 2.2±0.2 to 2.8±0.5 L/min/m2 (P < 0.01). RV/LV FDG uptake decreased from 1.28±0.32 before treatment to 0.99±0.23 (P < 0.05). Survival in the IPAH group with a baseline RV/LV FDG uptake greater than the median value of 1.20 was significantly lower than that of the group with RV/LV FDG uptake below 1.20 (log-rank test, P < 0.05). In contrast, baseline RV/LV FDG was of little informative value in CHD. FDG uptake by the RV reflects the severity of PVR in PAH. Increased RV FDG uptake is a marker of poor prognosis in IPAH and is reduced in patients receiving effective therapy. It could prove useful in the early clinical assessment of novel therapies for PAH.
Keywords: pulmonary arterial hypertension, right ventricle, FDG uptake, PET imaging
The response of the right ventricle (RV) to pulmonary arterial hypertension (PAH) is a major determinant of survival.[1–4] Myocardial hypertrophy frequently accompanies PAH. It is thought to be an appropriate reaction to the increased pressure load and necessary to maintain blood flow through increased pulmonary vascular resistance.[4] In addition to hypertrophy, the myocardium changes its energy substrate from fatty acid oxidation to glycolysis.[5–7] This switch in energy substrate is a sign that the myocardium is under stress.
18F-fluoro-2-deoxy-d-glucose (FDG) is a glucose analog that is taken up by viable cardiac myocytes in the same way as glucose, but its subsequent metabolism is blocked and it remains within the myocyte. It is thus a tracer of exogenous glucose uptake and, by inference, of myocardial viability.[8] Myocardial FDG uptake offers a noninvasive measurement of myocardial glucose metabolism. While established for the assessment of LV function, data to date on its value in understanding RV adaptation in pulmonary hypertension are limited.[9–13]
Patients with idiopathic PAH (IPAH) have a worse prognosis than patients with pulmonary hypertension associated with congenital heart disease (PH-CHD). This may be related to better adaptation of the RV in PH-CHD. This study compared RV FDG uptake by SPECT in patients with IPAH and CHD-PAH, and investigated the relationship to the following: (a) hemodynamic and functional parameters, including survival; and (b) the effect of treatment.
MATERIALS AND METHODS
Patient population
Consecutive patients aged over 18 years diagnosed with IPAH and CHD-PAH attending Fu Wai Hospital, Beijing, from April 2007 to March 2009 were invited to participate. Pulmonary hypertension was defined as resting mPAP ≥ 25 mmHg measured by right heart catheterization. Patients with coronary artery disease, cardiomyopathy, primary valvular disease, or systemic arterial hypertension were excluded. Other exclusion criteria were diabetes mellitus or glucose intolerance, based on a fasting plasma glucose concentration of 5.6-7.8 mmol/L or between 7.8-11.1 mmol/L at two hours post-oral glucose load (75 g). The Ethical Committee on Human Research at Fu Wai Hospital approved the study protocol (Project No: 216), and all patients enrolled with written informed consent.
Imaging studies
After a minimum eight hours overnight fast, patients were given a 50 g oral glucose load 30 min before the injection of 185 MBq 18F-fluorodeoxyglucose (18F-FDG). Forty to sixty minutes post-injection, patients underwent SPECT imaging using a dual-head large-field-of-view 5/8-inch crystal SPECT camera (Varicam, GE Healthcare) equipped with ultra-high-energy parallel-hole collimators. The use of energy windows of 18F (511±30% keV) allowed imaging of 18F-FDG. Thirty projection images were acquired over a 180° arc (45° right anterior oblique to 45° left posterior oblique position) at 6° intervals. The acquisition time was 50 sec at each projection. Myocardial images were reconstructed using a standard filtered back projection with a cutoff frequency of 0.35 and order 8 and displayed as series of short-axis, horizontal, and vertical long-axis slices.
In nine patients, a second 18F-FDG SPECT scan was performed after six months treatment with sildenafil (25 mg three times a day).
Data analysis
18F-FDG image data were analyzed semi-quantitatively using short-axis tomogram. Regions of interest (ROI) of 2×2 pixels were drawn over the center of free wall of the RV and left ventricle (LV) myocardium on one apical, one midventricular, and one basal slice, respectively. Each slice was of 1 pixel (4.7 mm) in depth. The arithmetic mean of the mean count for the three slices of each RV was divided by the same for each LV.
Hemodynamic measurements
All patients underwent right heart catheterization with three days of SPECT imaging. The mean pulmonary artery pressure (mPAP), mean right atrial pressure (RAP), and right ventricular end-diastolic pressure (RVEDP) were recorded. The cardiac output was determined by thermodilution, normalized for surface area, and expressed as the cardiac index (CI). Pulmonary vascular resistance (PVR) was calculated as mPAP/cardiac output.
6-min walk test
The 6-minute walk test (6MWT) was performed within three days of SPECT imaging using a standardized protocol, as described previously.[14] Briefly, patients walked along an enclosed, flat 30-m long corridor marked every 5 m. Technicians did not escort but encouraged patients using standardized phases “You are doing well” or “Keep up the good work.” Patients were instructed to walk at their own pace to cover as much ground as possible in six minutes.
Follow-up
Patients were followed up by telephone or letter. Survival was estimated from the date of SPECT imaging study to 31st March 2010.
Statistical analysis
All data are expressed as mean ± SD. Between-group comparisons were done using unpaired Student's t-test or paired Student's t-test where appropriate. Correlations between the two parameters were determined by simple linear regression analysis. In all cases, significance was considered valid at P<0.05. Survival curves were derived using the Kaplan-Meier method, using the median value as a cutoff, and compared using log-rank test.
RESULTS
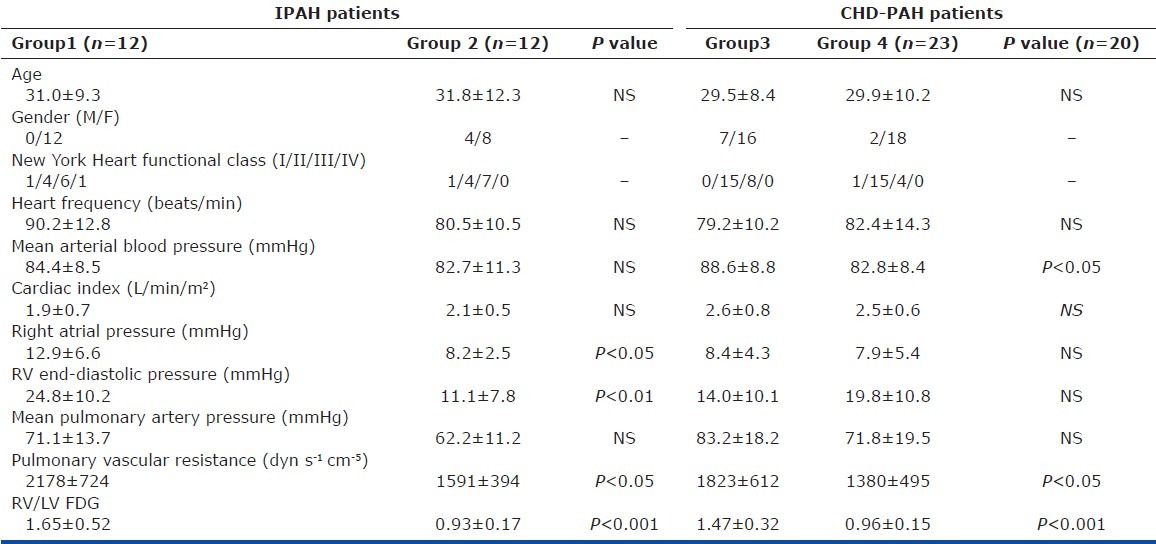
Twenty-four patients (4 men and 20 women; mean age 31.4±10.7 years) with IPAH and 43 patients (9 men and 34 women; mean age 29.7±9.1 years) with CHD-PAH were recruited to the study (12 with atrial septal defect, 21 with ventricular septal defect, and 10 with patent ductus arteriosus). All PH-CHD had undergone surgical correction of their shunt. Table 1 shows the baseline data in each subgroup, based on the most recent clinical classification of pulmonary hypertension.[15] Of these, 47 (17 with IPAH and 30 with CHD-PAH) consented to a 6MWT within three days of radionuclear imaging.
Table 1.
Clinical and hemodynamic characteristics of patients with IPAH and CHD.PAH
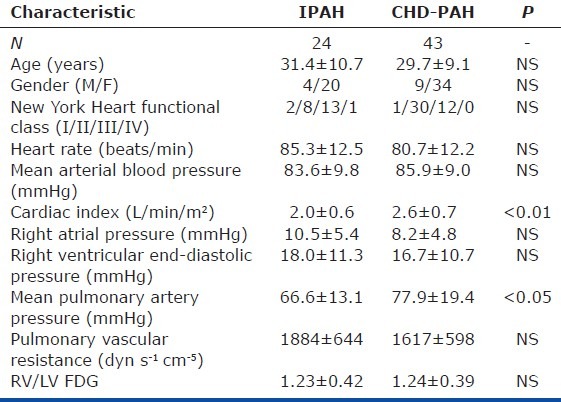
mPAP and CI were significantly higher in CHD-PAH than in IPAH. The mean RV/LV FDG was similar between two groups. Representative midventricular short-axis 18F-FDG SPECT images of two different categories of pulmonary hypertension are shown in Figure 1. The mean plasma glucose levels at the time of SPECT imaging were 8.7±0.9 mmol/L and 8.8±1.0 mmol/L in patients with IPAH and CHD-PAH, respectively.
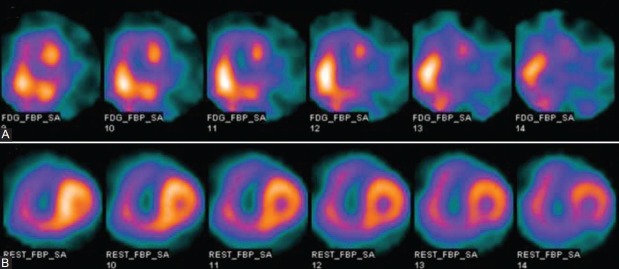
Figure 1.
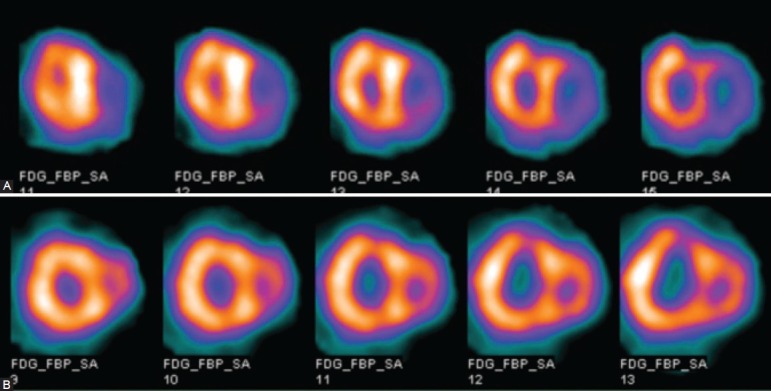
The representative short-axis 18F-FDG SPECT images: (A) The short-axis images of a patient with IPAH, mPAP=86 mmHg, PVR = 2991 dyn s-1 cm-5, RV/LV FDG=1.78. (B) The short-axis images of a patient with CHD-PAH, mPAP=86 mmHg, PVR=1443 dyn s-1 cm-5, RV/LV FDG=1.21.
Relationship of 18F-FDG uptake with pulmonary hemodynamic measurements
In 24 patients with IPAH, RV/LV FDG correlated significantly with mPAP (r = 0.562, P < 0.01), RAP (r = 0.470, P < 0.05), RVEDP (r = 0.611, P < 0.01), and PVR (r = 0.574, P < 0.01). There was significant negative correlation between RV-/LV-FDG and CI (r = -0.503, P < 0.05; Fig. 2).
Figure 2.
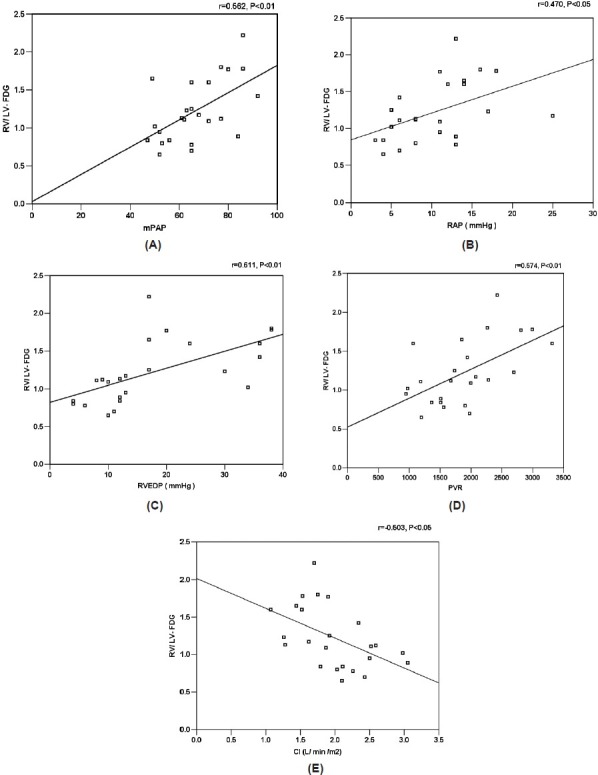
Correlation between RV/LV FDG and mPAP (A), RAP (B), RVEDP (C), PVR (D), and CI (E) in 24 patients with IPAH.
In 43 patients with CHD-PAH, a significant correlation was found between RV/LV FDG and PVR (r = 0.523, P < 0.001), but RV/LV FDG was independent of mPAP, PAR, RVEDP, and CI (Fig. 3).
Figure 3.
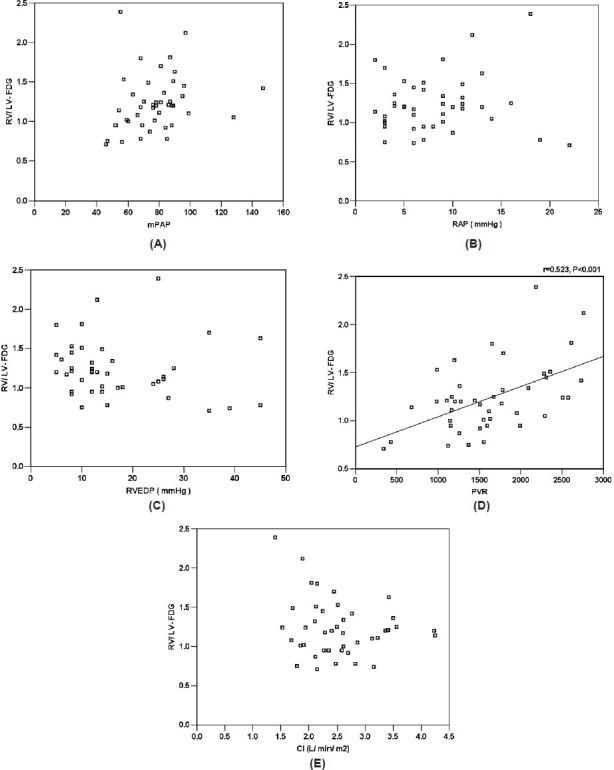
Correlation between RV/LV FDG and mPAP (A), RAP (B), RVEDP (C), PVR (D), and CI (E) in 43 patients with CHD-PAH.
18F-FDG uptake and response to treatment
A second 18F-FDG scan was available in nine patients (three with IPAH and six with CHD-PAH) after they had been treated with sildenafil for six months. Cardiac index improved from 2.2±0.2 to 2.8±0.5 L/min/m2 (P < 0.01), mPAP decreased from 68.7±13.2 to 59.0±15.1 mmHg (P < 0.05), and PVR from 1683±426 to 1207±383 dyn s-1 cm-5 (P < 0.05). RV-/LV-FDG uptake decreased from 1.28±0.32 before treatment to 0.99±0.23 (P<0.05). The mean blood glucose on first and second visits for these patients were 5.1±0.41 mmol/L versus 5.3±0.45 mmol/L fasting (before 50 g oral glucose load), and 8.8±0.8 mmol/L versus 8.9±0.7 mmol/L at the time of SPECT imaging. Representative FDG images of a patient with IPAH before and after six months of treatment are shown in Figure 4.
Figure 4.
Representative short-axis images of a patient with IPAH before (A) and after 6 months of treatment (B). Before treatment the mPAP and PVR were 86 mmHg and 2425 dyn s-1 cm-5, respectively, and 18F-FDG RV uptake significantly increased with a RV/LV FDG value of 2.2. After treatment the mPAP and PVR reduced to 75 mmHg and 1847 dyn s-1 cm-5, respectively, and the RV/LV FDG markedly decreased to 0.9.
Relationship of 18F-FDG uptake with 6MWT
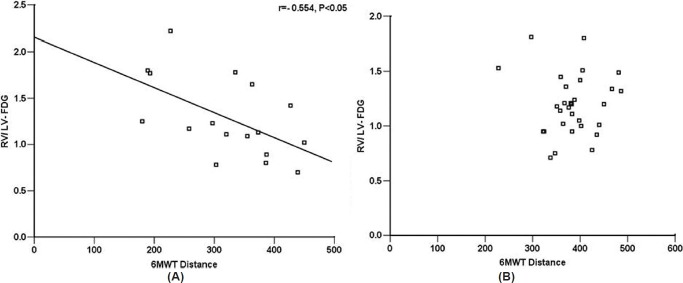
6MWT data were available in 47 patients (17 with IPAH and 30 with CHD-PAH). There was a negative correlation with RV/LV FDG uptake in IPAH patients (r = -0.554, P < 0.05), but that was not evident in CHD-PAH patients (Fig. 5).
Figure 5.
Correlations of RV/LV FDG and 6MWT in 17 patients with IPAH (A) and 30 patients with CHD-PAH (B).
Relationship between 18F-FDG uptake and survival
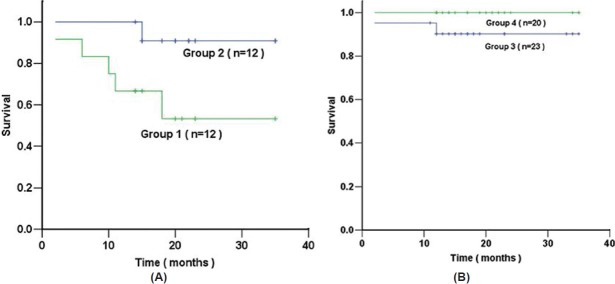
Survival data were obtained in 100% of patients. During 21±8 months of follow-up, eight patients died (six with IPAH and two with CHD-PAH). Right heart failure was the cause of death in all patients. For 24 patients with IPAH, survival in the group with a RV/LV FDG uptake greater than a median cut-off value of 1.20 (Group 1) was significantly lower than that of the group with RV/LV FDG uptake below 1.20 (Group 2; log-rank test, P < 0.05). However, using the same cut-off for 43 patients with CHD-PAH, no difference in survival was found between the group with a RV/LV FDG uptake >1.20 (Group 3) and that of the group with RV/LV FDG uptake <1.20 (Group 4; log-rank test, P > 0.05; Fig. 6). Table 2 shows the comparative clinical and hemodynamic data for two groups of patients with IPAH and two groups of patients with CHD-PAH.
Figure 6.
Kaplan-Meier survival estimates of 24 patients with IPAH (A), survival in the Group 1 (RV/LV FDG ≥ 1.2) was significantly lower than that of Group 2 (RV/LV FDG<1.2; log-rank test, P<0.05). However, using the same cut-off for 43 patients with CHD-PAH (B), no difference in survival was found between Group 3 (RV/LV FDG ≥ 1.2) and Group 4 (RV/LV FDG<1.2; log-rank test, P > 0.05).
Table 2.
Comparative data for IPAH with RV/LV FDG ≥ 1.20 (Group 1) and <1.20 (Group 2) and CHD-PAH with RV/ LV FDG ≥ 1.20 (Group 3) and <1.20 (Group 4)
DISCUSSION
This is the largest study to date of RV FDG uptake in patients with pulmonary hypertension. In both IPAH and CHD-PAH, RV FDG uptake correlated positively with PVR, reducing with reduction in PVR on treatment. Increased RV FDG uptake is a marker of impaired right heart function and poor prognosis in patients with IPAH. In contrast, it was of little informative value in CHD-PAH.
The data support and extend previous observations in patients with PAH.[11] In a small study of 24 patients, 15 with IPAH and 9 with chronic thromboembolism, RV FDG uptake correlated with PVR, mean PAP, right atrial pressure, RV wall stress, and BNP levels in the mixed population. RV FDG uptake was reduced in IPAH patients who responded to epoprostenol therapy at three months. The correlation between RV FDG uptake and RV wall stress supports the idea that increased RV workload was responsible. A more recent study of myocardial FDG uptake in 23 PAH patients using positron emission tomography (PET)[13] has confirmed a correlation with prognostic markers. There are no data comparing IPAH and PH-CHD.
The increase in glucose uptake observed in hypertrophied hearts is an adaptive response and an attempt to preserve ATP supply.[16] It is enabled by increased expression and function of the insulin-independent glucose transporter, GLUT1.[17] A key intermediary signaling molecule in this “metabolic remodeling” is AMP-activated protein kinase (AMPK). AMPK is a heterotrimeric complex comprising α-catalytic and β- and γ-regulatory units that acts as a “low-fuel sensor.” Its activity is increased when cellular high-energy phosphates, including ATP and phosphocreatine, are depleted, although the enzyme is also activated in the hypertrophied heart by energy-state-independent mechanisms. In addition to increasing glucose transport, AMPK activation in ventricular hypertrophy[18] preserves ATP levels by accelerating glycolysis and by inhibiting acetyl CoA carboxylase.[19,20]
RV/LV FDG values might be expected to be higher in the CHD-PAH than the IPAH cohort, given the significantly higher mPAP in the former group. The fact that RV FDG uptake was similar in the two groups suggests better adaptation of the RV in CHD-PAH to the increased workload. This is consistent with studies of the natural history of CHD-PAH, which suggest that CHD-PAH patients have a better clinical outcome. The RV of CHD-PAH patient is exposed to an increased preload from an early age, allowing the RV time to adapt during cardiac development.
In contrast, in IPAH, the need to increase myocardial glucose transport, as measured by RV FDG uptake, is a poor prognostic marker, denoting patients at increased risk of premature death.[21] RV FDG uptake showed a positive correlation with PVR and correlated negatively with the 6MWT, both prognostic markers in IPAH. This may indicate that RV FDG uptake provides additional information in IPAH. Whether RV FDG uptake is an independent prognostic marker cannot be addressed from the current dataset due to the relatively small numbers of patients with IPAH studied.
The main limitation of this study is the spatial resolution of SPECT. We were not able to obtain standardized uptake values (SUV) for RV FDG uptake. PET imaging has advantages in this regard, thereby reducing partial volume effect, and also offers better sensitivity. Both techniques express the results as the RV/LV FDG ratio.[13,21] Arguably, SPECT is more widely available and so our observations have broader utility. The relatively small number of patients studied is also a limitation but the cohort is one of the largest studied to date.
There is unanimous agreement that the RV response to pulmonary hypertension is a major determinant of survival. It is important that new treatments address not only the pulmonary vascular pathology but also the RV in pulmonary hypertension. There is increasing interest in promoting more efficient myocardial ATP production as a therapeutic strategy in PAH by encouraging mitochondrial glucose oxidation. Pyruvate dehydrogenase kinase (PDK) is a key regulator of glucose oxidation. Activated PDK phosphorylates and inhibits pyruvate dehydrogenase (PDH), blocking the entry of pyruvate into the mitochondria, and inhibiting formation of acetyl CoA and retarding the Krebs’ cycle. Studies in animal models of pulmonary hypertension suggest that inhibition of PDK with dichloroacetate increases cardiac contractility ex vivo and in vivo.[10] The morphological and functional response of the heart-to-pressure load can be measured by echocardiography and cardiac magnetic resonance. RV FDG uptake offers additional information on the biochemical adaptation of the RV to increased pressure load and could prove useful in the early assessment of novel therapies for pulmonary hypertension.
ACKNOWLEDGMENTS
Wei Fang conducted the SPECT, collected and analyzed data, and prepared the primary manuscript. Jian-guo leads the clinical project as the Principal Investigator. Lan Zhao and Martin R. Wilkins participated in the data analysis, interpretation, and drafted the manuscript. Chang-ming Xiong, Xin-hai Ni, and Zuo-xiang He, contributed to the acquisition of clinical data.
Footnotes
Source of Support: This study is supported by National Grant (the Eleventh 5-year Plan of Social and Economic Development in China Improved the Diagnosis and Therapy of Pulmonary Hypertension in China, 2006BAI01A07) from the Ministry of Science and Technology, Beijing Municipal Science and Technology Project (Z090507017709032), a project grant from PVRI and Research Fund of capital Medical Development.
Conflict of Interest: None declared.
REFERENCES
- 1.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 2.Sandoval J, Bauerle O, Palomar A, Gómez A, Martínez-Guerra ML, Beltrán M, et al. Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation. 1994;89:1733–44. doi: 10.1161/01.cir.89.4.1733. [DOI] [PubMed] [Google Scholar]
- 3.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–7. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 4.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;24(114):1883–91. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 5.de las Fuentes L, Herrero P, Peterson LR, Kelly DP, Gropler RJ, Dávila-Román VG. Myocardial fatty acid metabolism: independent predictor of left ventricular mass in hypertensive heart disease. Hypertension. 2003;41:83–7. doi: 10.1161/01.hyp.0000047668.48494.39. [DOI] [PubMed] [Google Scholar]
- 6.Nagaya N, Goto Y, Satoh T, Uematsu M, Hamada S, Kuribayashi S, et al. Impaired regional fatty acid uptake and systolic dysfunction in hypertrophied right ventricle. J Nucl Med. 1998;39:1676–80. [PubMed] [Google Scholar]
- 7.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–73. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- 8.Underwood SR. Imaging techniques in the assessment of myocardial hibernation. Eur J Nucl Med Mol Imaging. 2004;31:1209–11. doi: 10.1007/s00259-004-1588-8. [DOI] [PubMed] [Google Scholar]
- 9.Mannting F, Zabrodina YV, Dass C. Significance of increased right ventricular uptake on 99mTc-sestamibi SPECT in patients with coronary artery disease. J Nucl Med. 1999;40:889–94. [PubMed] [Google Scholar]
- 10.Abikhzer Y, Probst S, Rush C. Pulmonary hypertension findings detected by F-18 FDG PET scan. Clin Nucl Med. 2008;33:405–6. doi: 10.1097/RLU.0b013e31817082ad. [DOI] [PubMed] [Google Scholar]
- 11.Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, et al. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol. 2005;45:1849–55. doi: 10.1016/j.jacc.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 12.Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: Resuscitating the hibernating right ventricle. J Mol Med. 2010;88:47–60. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Can MM, Kaymaz C, Tanboga IH, Tokgoz HC, Canpolat N, Turkyilmaz E, et al. Increased right ventricular glucose metabolism in patients with pulmonary arterial hypertension. Clin Nucl Med. 2011;36:743–8. doi: 10.1097/RLU.0b013e3182177389. [DOI] [PubMed] [Google Scholar]
- 14.ATS statement: Guidelines for the six.minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Tian R, Musi N, D’Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–9. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 17.Liao R, Jain M, Cui L, D’Agostino J, Aiello F, Luptak I, et al. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–31. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 18.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: Enemy or ally? J Physiol. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5’-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–20. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 20.Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, et al. Characterization of 5’AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 21.Ramani GV, Gurm G, Dilsizian V, Park MH. Noninvasive assessment of right ventricular function: Will there be resurgence in radionuclide imaging techniques? Curr Cardiol Rep. 2010;12:162–9. doi: 10.1007/s11886-010-0092-y. [DOI] [PubMed] [Google Scholar]